两个含双膦配体和[2,3-f]吡嗪并[1,10]菲咯啉的Cuガ配合物的合成、结构及光谱学性质
2019-04-12卢延磊赵宇萌匡晓楠李中峰辛秀兰杨玉平金琼花张江威
卢延磊 朱 宁 赵宇萌 林 森 匡晓楠 李中峰 辛秀兰 杨玉平 金琼花*, 张江威
(1首都师范大学化学系,北京 100048)(2北京工商大学食品学院,北京 100048)(3中央民族大学理学院,北京 100081)(4中国科学院大连化学物理研究所催化基础国家重点实验室金催化中心,大连 116023)
0 Introduction
Currently,there are plentiful scientific researches in luminescent metal complexes used in climate science,materials science.The Cuガcomplexes[1-10]are extensively studied due to the high relative abundance and environmental friendliness of copper[5,11-13]in comparison to some precious metals,such as Os,Pt,Ir[14-15].Especially heteroleptic Cuガ complexes,such as copperガ-diimine-phosphine complexes[16-18], attract wide interest in recent years.
1,10-phenanthroline(1,10-phen),on account of its peculiar conjugacy of heteroaromatic,can easily coordinate with metal ion forming stable metal complexes[19].1,10-phen and its derivatives with unique rigidity structures often show metal-to-ligand charge transfer(MLCT)characteristics owing to the lowerπ*orbital energy.In addition,they are also optically active ligands used to synthesize luminescent complexes[20].
Following the earlier studies on heteroleptic copperガcomplexes bearing 1,10-phen derivatives and diphosphine ligands[18,21], we chose[2,3-f]pyrazino[1,10]phenanthroline(dpq)as diimine ligand,1,2-bis(diphenylphosphino)benzene (dppBz) and 1,2-bis(diphenylphosphino)ethane (dppe) as diphosphine ligands(Scheme 1)to prepare two new complexes.The relevant synthesis routes are summarized in Scheme 2.We describe herein the synthesis,structure characterization and spectroscopic properties of the two new Cuガcomplexes with dpq ligand[Cu(dppBz)(dpq)]ClO4(1)and[Cu(dppe)(dpq)]ClO4(2)(Scheme 2).Complexes 1 and 2 have been isolated and characterized by X-ray diffraction,elemental analysis,infrared spectroscopy,absorption spectra,1H NMR and31P NMR spectroscopy,terahertz (THz)time-domain absorption spectroscopy,and emission property of 2 is also studied.Single-crystal X-ray diffraction analysis reveals that a 1D hollow tube-like structure is formed by C-H…πintermolecular forces and hydrogen bonds in complex 1,while two units are linked together via one π-π stacking force and two C-H … π intermolecular forces in complex 2.The terahertz spectra of the two complexes were measured by the ultrashort pulses of coherent terahertz radiation(0.1~4 THz,3~133 cm-1).Terahertz spectra is a vibrational spectros-copy that is used to probe the vibrational modes in the far-infrared and sub-millimeter region of the electro-magnetic spectrum,which is pretty useful in chara-cterizing the structures and studying the functions of polarity complexes[22].

Scheme 1 Chemical structures of ligands dppBz,dppe and dpq
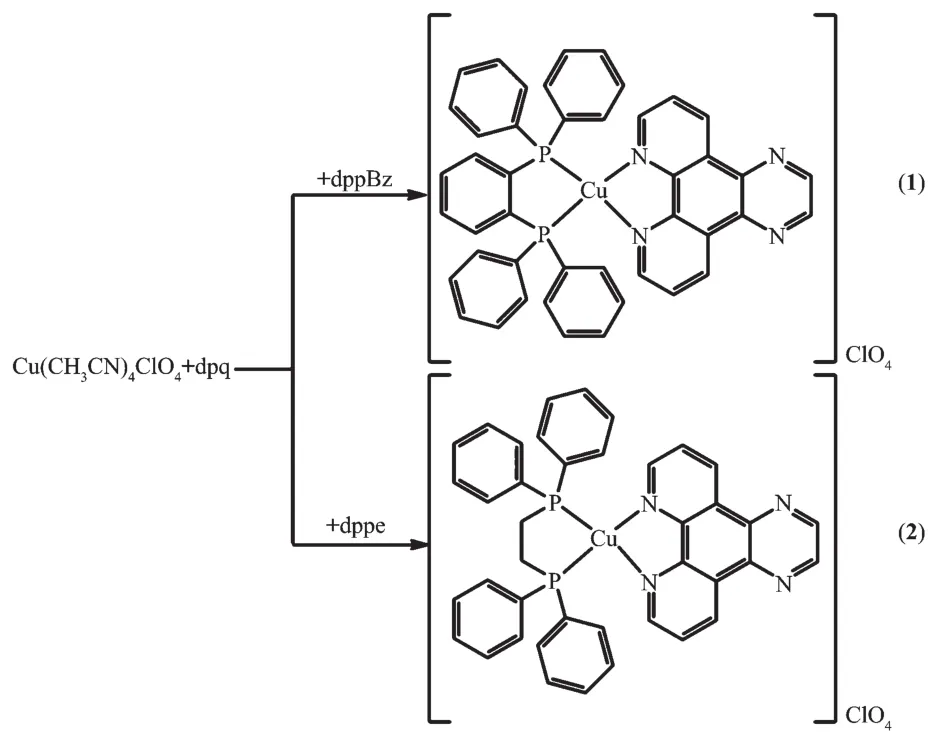
Scheme 2 Synthetic routes for complexes 1 and 2
1 Experimental
1.1 Materials and measurements
All commercially available starting materials were used as received,and solvents were used without any purification.FT-IR spectra (KBr pellets)were measured on a Perkin-Elmer Infrared spectrometer.Room-temperature fluorescence spectra were measured on F-4500 FLSpectrophotometer.C,H and Nelemental analysis were carried out on an Elementar Vario MICROCUBE(Germany)elemental analyzer.1H NMR and31P NMR were recorded at room temperature with a Bruker DPX 600 spectrometer.The THz absorption spectra were recorded on the THz time domain device of Minzu University of China,based on photoconductive switches for generation and electro-optical crystal detection of the far-infrared light,effective frequency in a range of 0.2~2.8 THz[23-24].
1.2 Synthesis of[Cu(dppBz)(dpq)]ClO4(1)
Complex 1 was prepared by the reaction of[Cu(CH3CN)4]ClO4(0.0654 g,0.2mmol),dppBz(0.089 3 g,0.2 mmol)and dpq (0.046 4 g,0.2 mmol)in the mixed solvents of 5 mL CH2Cl2and 5 mL CH3OH.The mixture was stirred for 6 hours and filtered.Yellowish crystals were obtained from the filtrate after standing at room temperature for 4~5 days.Yield:67%.Element analysis Calcd.for C44H32CuN4P2ClO4(%):C,62.78;H,3.83;N,6.66.Found(%):C,62.54;H,3.80;N,6.54.IR data(cm-1,KBr pellets):2 918w,1 632m,1 436m,1 402m,1 385m,1 121s,1 094vs,814w,736m,695m,622w,521m,440w.1H NMR(600 MHz,DMSO-d6,298 K):δ7.26~7.76(m,CHbenzenefromdppBz),8.06~9.55(m,heterocyclic hydrogen from dpq,including solvent signals);31PNMR(600 MHz,DMSO-d6,298 K):δ-3.53(s,phosphorus from dppBz).
1.3 Synthesis of[Cu(dppe)(dpq)]ClO4(2)
Complex 2 was prepared in a manner similar to the described for 1 except that dppe (0.079 7 g,0.2 mmol)was used instead of dppBz.Orange-red crystals of 2 were obtained from the filtrate after standing at the room temperature for several weeks.Yield:63%.Element analysis Calcd.for C40H32CuN4P2ClO4(%):C,60.53;H,4.06;N,7.06.Found(%):C,60.37;H,4.07;N,6.87.IR data(cm-1,KBr pellets):1 628m,1 435m,1 403m,1 386m,1 121s,1 094vs,815w,738m,698m,623w,520m,440w.1H NMR(600 MHz,DMSO-d6,298 K):δ7.37~7.47 (m,CHbenzenefromdppe),8.17~9.63(m,heterocyclic hydrogen from dpq,including solvent signals);31PNMR(600 MHz,DMSO-d6,298 K):δ-3.53(s,phosphorus from dppe).
1.4 Structure determination
Singlecrystalsof thetitlecomplexeswere mounted on a Bruker Smart 1000 CCD diffractometer equipped with a graphite-monochromated Mo Kα (λ=0.071 073 nm)radiation at 298(2)K.Semi-empirical absorption corrections were applied using SABABS program[25].All the structures were solved by direct methods using SHELXS program of the SHELXTL-97 package and refined with SHELXL-97[26-27].Metal atom centers were located from the E-maps and other non-hydrogen atoms werelocated in successive difference Fourier syntheses.The final refinements were performed by full matrix least-squares methods with anisotropic thermal parameters for non-hydrogen atoms on F2.The hydrogen atoms were generated geometrically and refined with displacement parameters riding on the concerned atoms.
Crystallographic data and experimental details for structural analysis are summarized in Table 1,and selected bond lengths and angles of complexes 1~2 are summarized in Table 2.
CCDC:1874580,1;1874581,2.
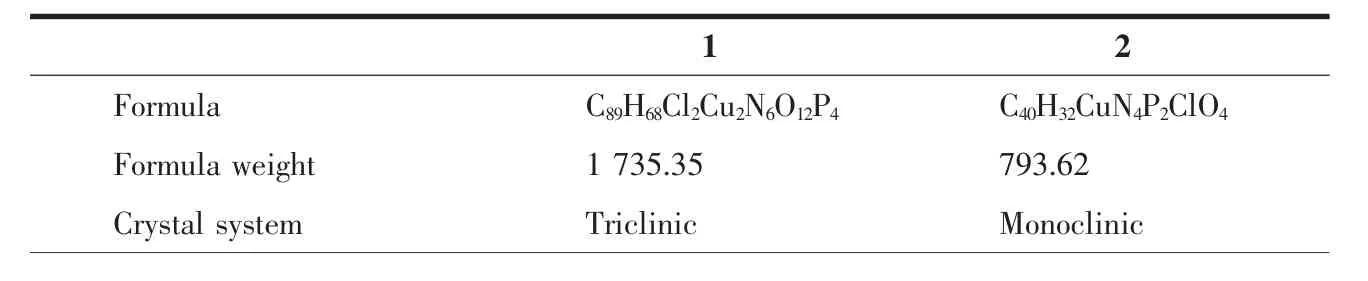
Table 1 Crystallographic data for complexes 1 and 2
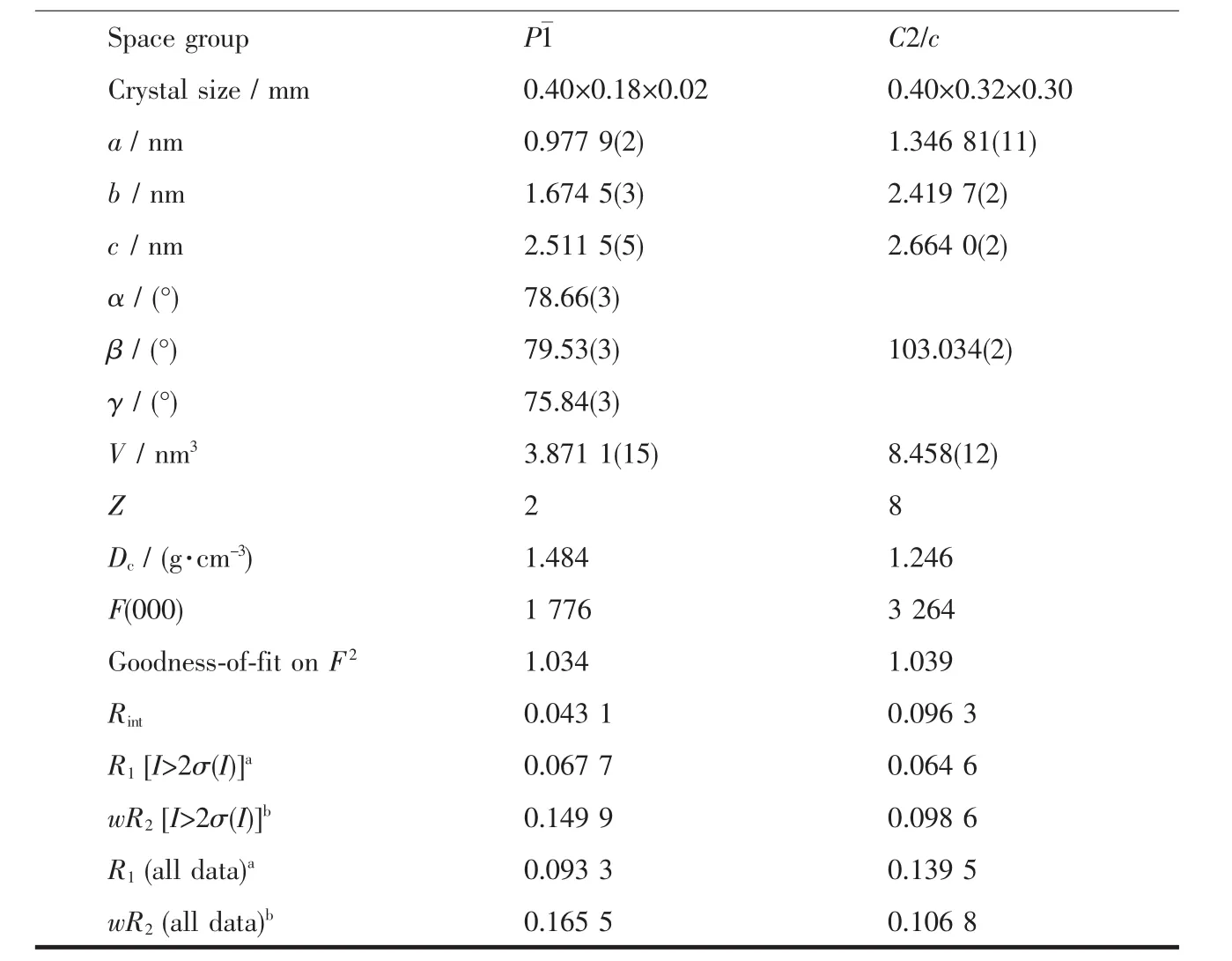
Continued Table 1
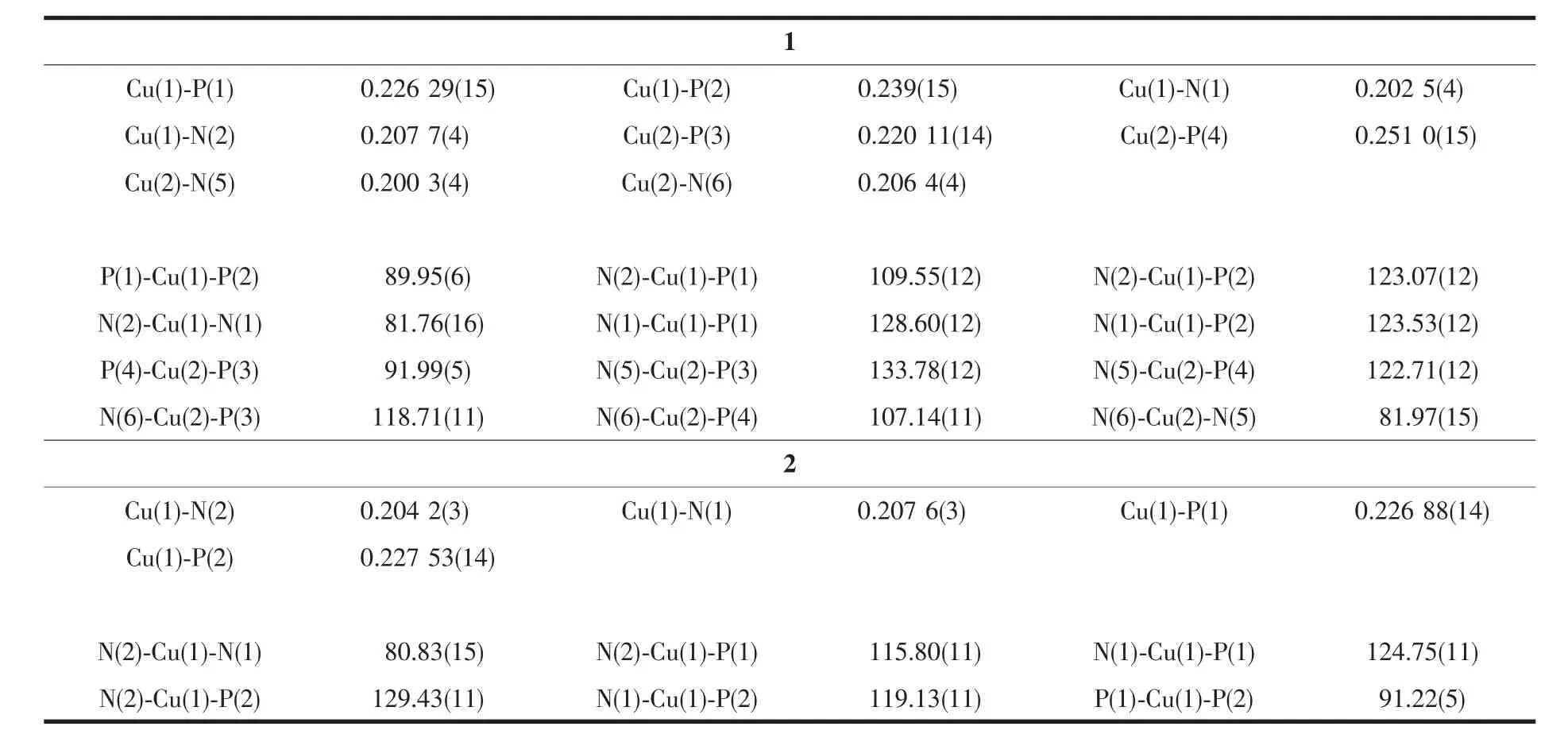
Table 2 Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2
2 Results and discussion
2.1 Infrared spectroscopy and NMR spectra
The infrared spectra of complexes 1 and 2 showed that the absorption peaks around 1 436~1 386 cm-1are put down to C-C absorbing vibration of the phenyl rings in diphosphine ligands,and the middle absorption peaks around 1 350 cm-1are due to C-H bending vibration in diimine ligands.In addition to the absorption of chemical bonds on the cation skeleton,there were also characteristic absorption peaks of counter ions,such as the absorption of the Cl-O stretch vibration around 1 093 cm-1[28].
2.2 Description of crystal structures
Complex 1 is a dimer composed of one asymmetric unit containing two[Cu(dppBz)(dpq)]ClO4..Single-crystal X-ray diffraction analysis reveals that complex 1 crystalizes in triclinic system with P1 space group.The molecular structure is shown in Fig.1.Selected bond distances and bond angles for complex are shown in Table 2.Each Cuガion is fourcoordinated and attach to four atoms (two atoms are from chelating dppBz ligand and the other two are from chelating dpq ligand),forming the cation[Cu(dppBz)(dpq)]+,while the ClO4-anion plays the role of balancing the charge.The geometry around each Cuガcenter is distorted tetrahedral configuration because the angles in a range of 81.97(15)°~133.78(12)°.The bonds Cu-N(P)in a range of 0.200 3(4)~0.262 9(15)nm are close to those in analogous complexes[29-30].Single X-ray diffraction reveals that[Cu(dppBz)(dpq)]+units form a 1D hollow tube-like structure through two hydrogen bonds:C(23)-H(23)…N(3)(C(23)…N(3)0.253 nm)and C(88)-H(88)…N(7)(C(88)…N(7)0.260 nm),as well as three C-H…πinteractions.Just like in the reported complexes[31],C-H…πinteractions play a critical role in structural orientation(Fig.2,Table 3).
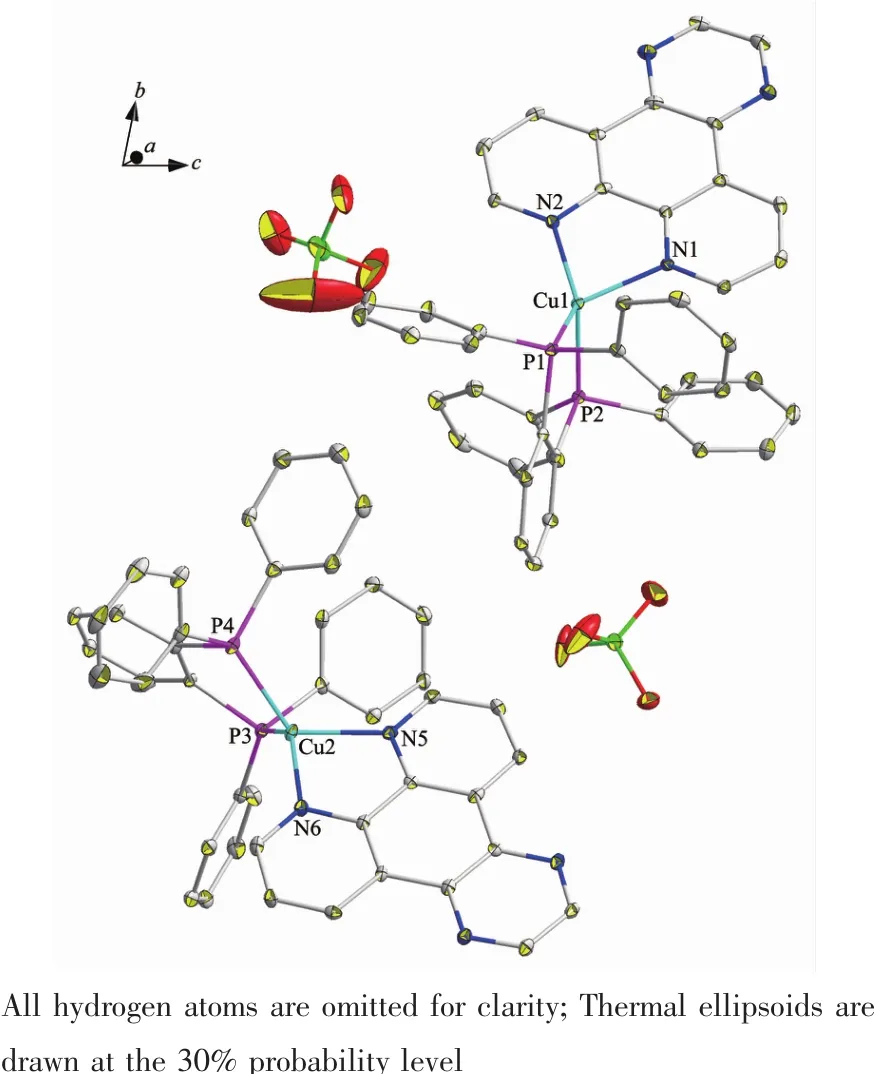
Fig.1 Molecular structure of complex 1

Fig.2 One-dimensional infinite chain of complex 1
Single-crystal X-ray diffraction analysis reveals that complex 2 crystallizes in monoclinic system with space group C2/c.As shown in Fig.3,it can be seen that the two N atoms of dpq ligand and two Patoms of dppe ligand chelate to Cuガ,forming a mononuclear complex bearing a distorted tetrahedral configuration centered on copperガ.In complex 2,the average bond distances of Cu-P(N)is 0.216 6 nm,which is larger than that in the reported complexes bearing dppe ligand[32].Two neighboring molecules in complex 2 are connected together byπ…πstacking(0.395 nm)and C-H…πinteractions(Fig.4,Table 3).
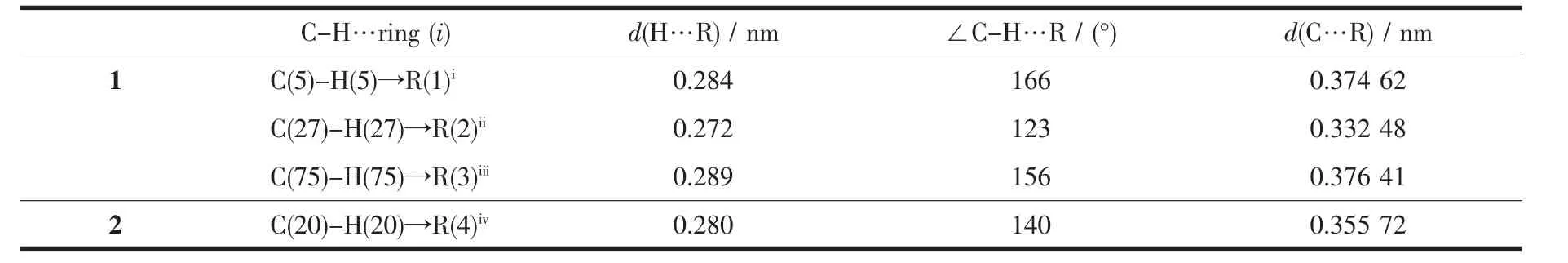
Table 3 Intermolecular C-H…πinteractions in complexes 1 and 2
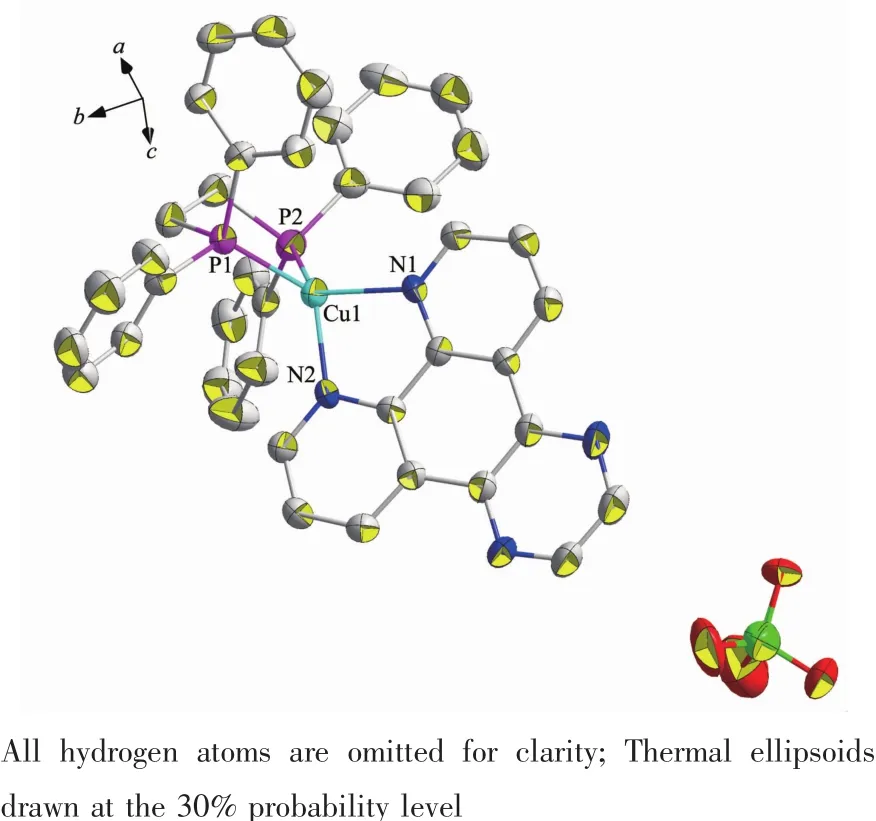
Fig.3 Molecular structure of complex 2
2.3 Fluorescence spectra
At room temperature,the solid-state excitation and emission spectra of complex 1 and 2 weremeasured(Fig.5).We cannot get the excitation and emission data of complex 1.The photophysical parameters of complex 2 are summarized in Table 4.The ligand dppe exhibited fluorescence signal at 434 nm with an excitation maximum at 310 nm[33-34].Complex 2 exhibited yellow-orange light emission when it was excited with UV light,and the emission maxima at 298 K was observed at 563 nm withλmax=366 nm.The fluorescence spectrum at 298 K was broad without vibronic progressions,indicating that the excited state of the emission has a charge-transfer characteristic at ambient temperature[35].According to the analysis of the absorption spectrum (2.4 Absorption spectra),the luminescence is attributed to metal-to-ligand charge transfer.
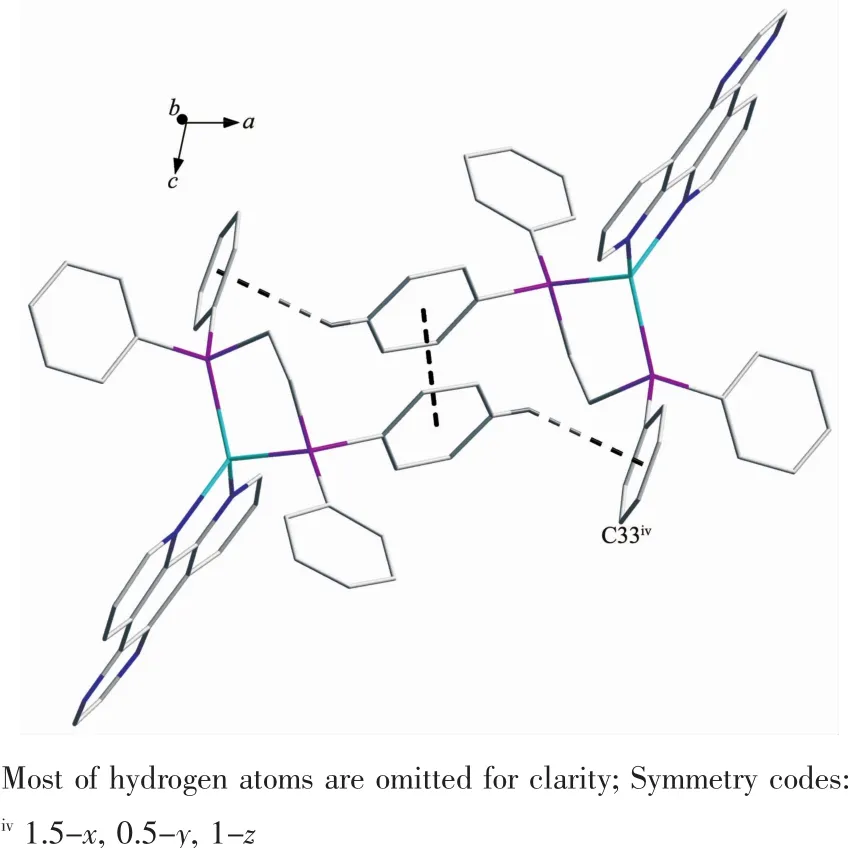
Fig.4 Two neighboringmolecules in complex 2 connected together byπ…πstacking and C-H…π interactions
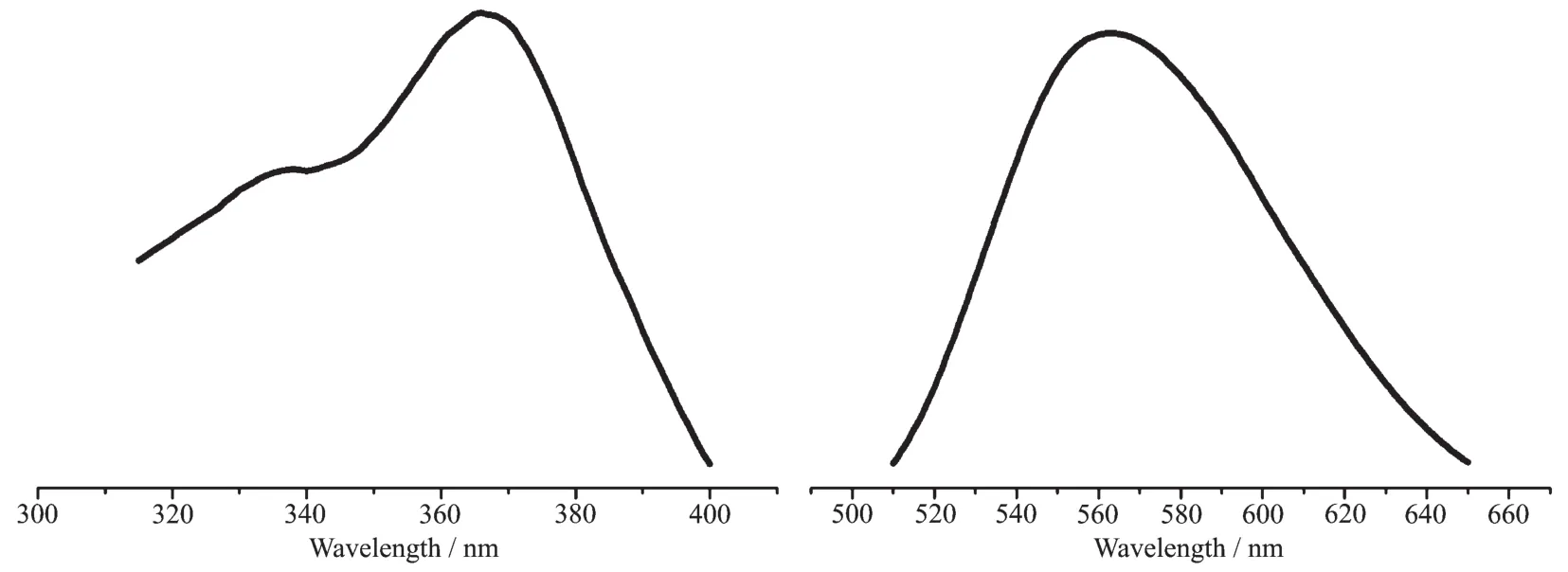
Fig.5 Solid-state excitation(left)and emission(right)spectra of complex 2 at 298 K

Table 4 Luminescence properties of complex 2 in solid state at 298 K
2.4 Absorption spectra

Fig.6 Absorption spectra of complexes 1 and 2 in CH2Cl2
Absorption spectra of complexes 1 and 2 were obtained(Fig.6),in spite of that the fluorescence data of complex 1 were not gained.From the absorption spectra,the following conclusions can be drawn.On the one hand,the complexes had strong UV absorption peaks in a range of 280~290 nm.The absorption peaks in this range show the intraligand charge transfer(ILCT)characteristics[36]of complexes 1 and 2.On the other hand,low-energy UV absorption peaks around 400~450 nm can be attributed to metal-toligand charge transfer (MLCT),which are consistent with reported heteroleptic copperガcomplexes[37].
2.5 Terahertz(THz)time-domain absorption spectroscopy
The terahertz (THz)time-domain absorption spectra of the diphosphine ligands dppBz,dppe,the diimine ligand dpq and complexes 1~2 were measured in a range 0.2~2.8 THz (Fig.7 and Fig.8)at room temperature.The relevant data are summarized in Table 5.
The results show that the types of ligands as well as the structures of complexes both have a significant impact on the absorption peaks of the terahertz timedomain absorption spectroscopy.It′s worth noting that the newly formed terahertz spectra peaks for the complexes(0.67,0.73 THz for 1,and 0.53,0.67,0.74,0.87 THz for 2)in a range of 0.40~0.90 THz are related to the coordination of copperガ.As shown in Fig.7,Fig.8 and Table 5,the terahertz spectra peaks of ligands are shown at 0.27,0.79,2.17,2.43 THz for dppBz,0.79,0.93,1.00,1.46,1.67,1.79,1.90,2.40 THz for dppe,and 0.32,1.00,1.38,1.44,1.70,1.79,2.14,2.40,2.46,2.78 THz for dpq.By comparing the THz absorption spectra of the complexes with those of the ligands,we can note that the peaks of ligands disappeared or moved after reaction.Therefore,terahertz time-domain absorption spectroscopy is a sensitive method for distinguishing and determining the weeny difference in complexes.
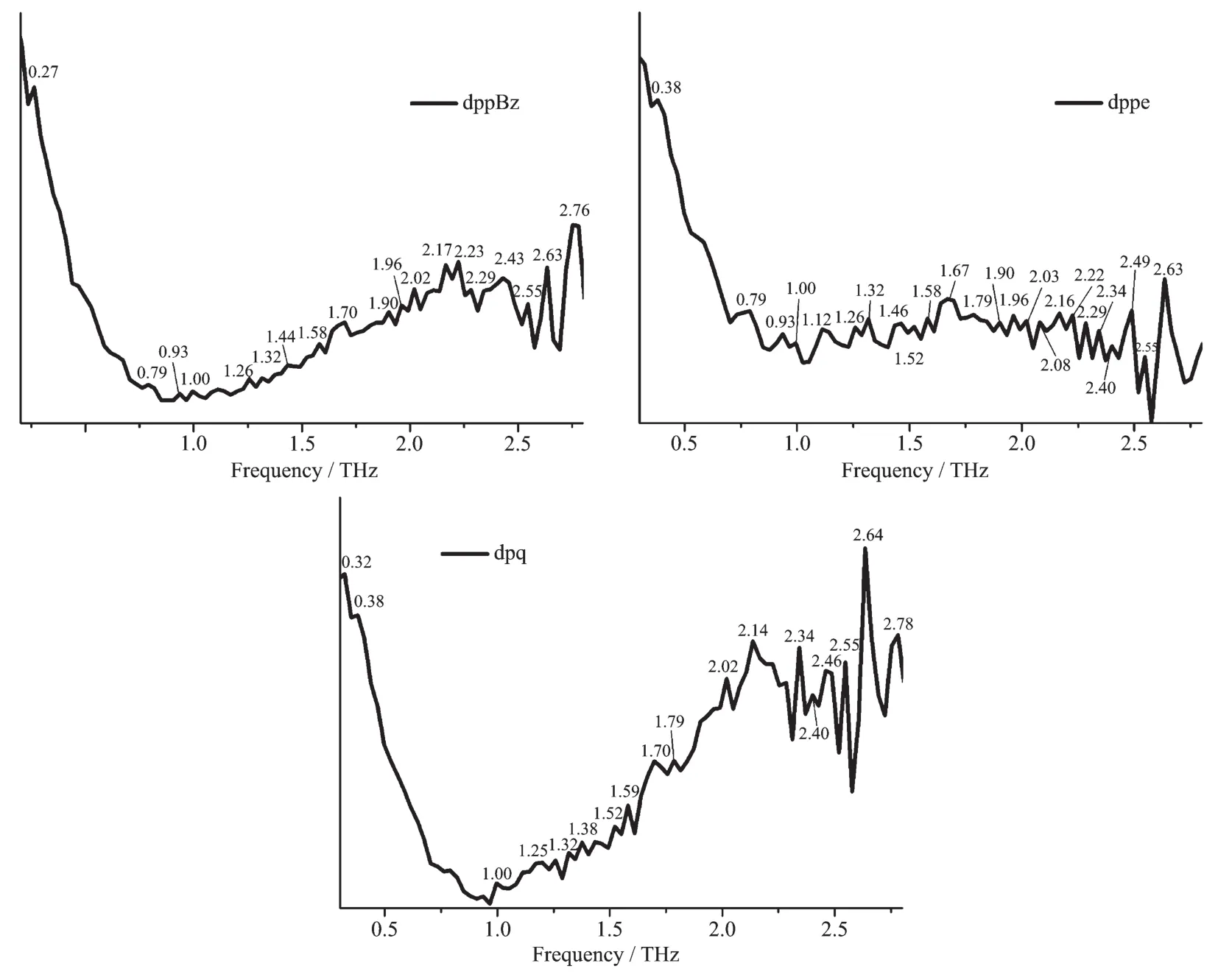
Fig.7 THz absorption spectra of ligands:dppBz,dppe,dpq
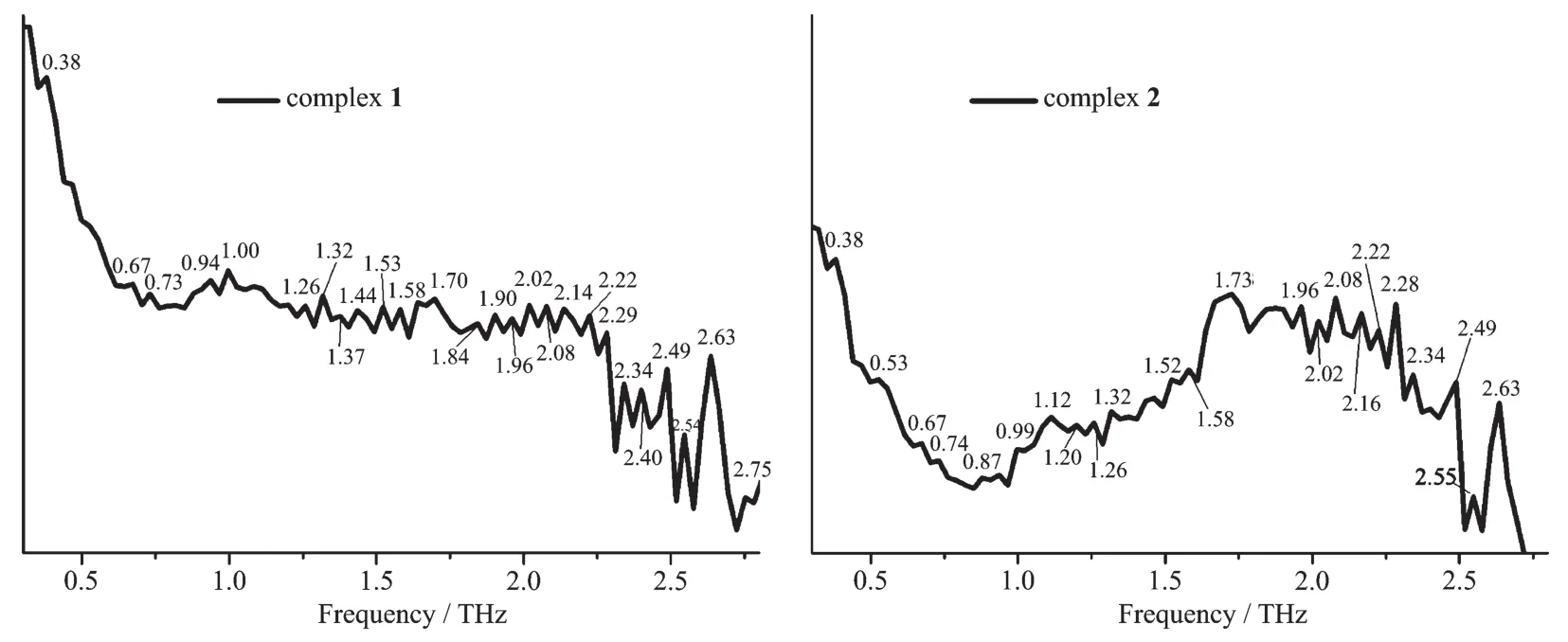
Fig.8 THz absorption spectra of complexes 1 and 2
3 Conclusions
Two functional heteroleptic Cuガ complexes containing diphosphine ligands and 1,10-phen derivative[2,3-f]pyrazino[1,10]phenanthroline have been synthesized and characterized by X-ray diffraction,elemental analysis,infrared spectroscopy,absorption spectra,NMR spectra,fluorescence spectra,terahertz(THz)time-domain absorption spectroscopy.Single X-ray diffraction reveals that complex 1 is a dimercomposed of one asymmetric unit,containing two[Cu(dppBz)(dpq)]ClO4,with the unit cells packing into a 1D hollow tube-like structure by hydrogen bonds and C-H…πintermolecular forces.Complex 2 is a simple mononuclear structure.Two neighboring molecules of 2 are connected together byπ…πstacking and C-H…πinteractions.Absorption spectrum reveals that the luminescence of complexes is derived from metal-to-ligand charge transfer(MLCT).Terahertz time-domain absorption spectroscopy can help identify the tiny differences of structures of complexes.
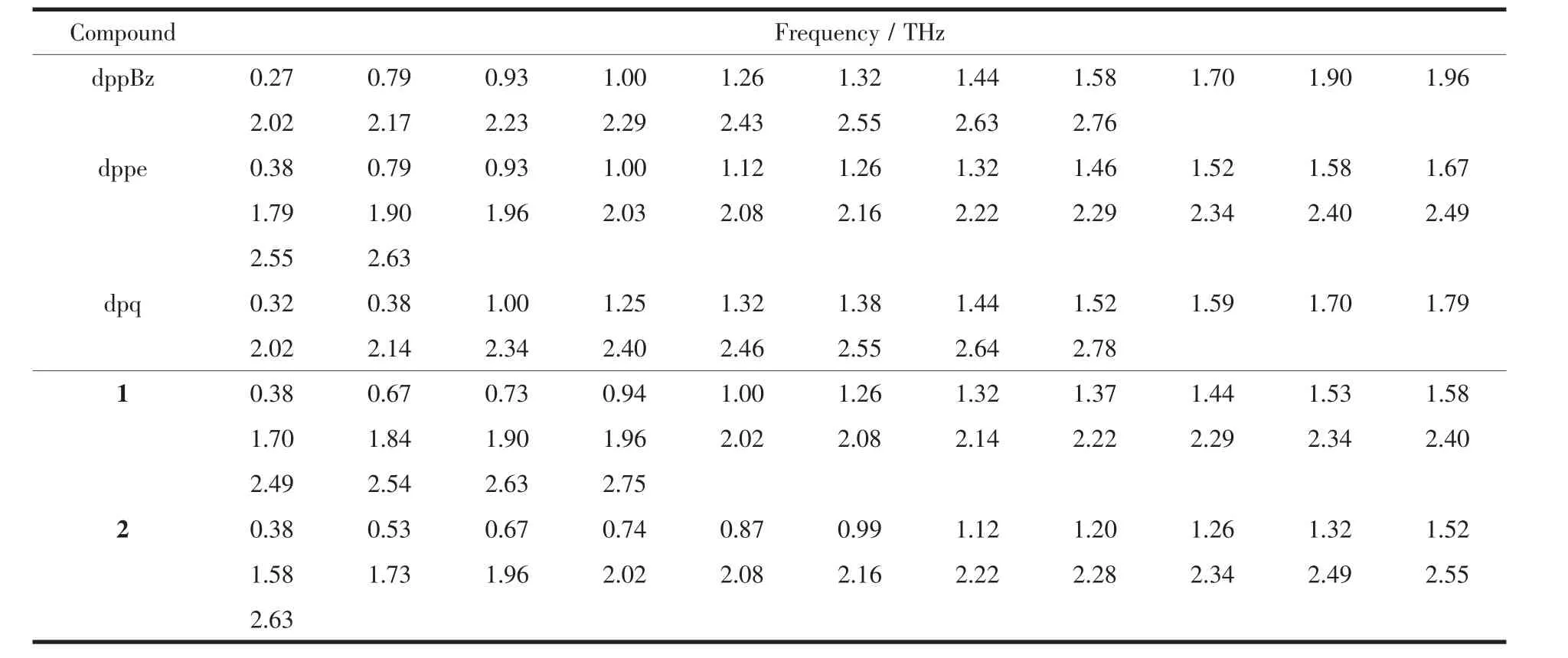
Table 5 Terahertz spectra peaks of the ligands and complexes 1~2
