Dbx2 exhibits a tumor-promoting function in hepatocellular carcinoma cell lines via regulating Shh-Gli1 signaling
2019-03-09YanTingHuBeiFangLiPengJunZhangDiWuYanYanLiZhongWuLiLinShenBinDongJingGaoXuZhu
Yan-Ting Hu, Bei-Fang Li, Peng-Jun Zhang, Di Wu, Yan-Yan Li, Zhong-Wu Li, Lin Shen, Bin Dong, Jing Gao,Xu Zhu
Abstract BACKGROUND Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide.HCC patients suffer from a high mortality-to-incidence ratio and low cure rate since we still have no specific and effective treatment. Although tremendous advances have been made in the investigation of HCC, the specific mechanisms of the progression of this disease are still only partially established. Hence, more research is needed to elucidate the underlying potential mechanisms to develop effective strategies for HCC.AIM To determine the role of developing brain homeobox 2 (Dbx2) gene in promoting the development of HCC.METHODS Dbx2 expression in clinical specimens and HCC cell lines was detected by Western blot (WB) and immunohistochemistry. Gain and loss of Dbx2 function assays were performed in vitro and in vivo. Cell viability assays were used to investigate cell growth, flow cytometry was employed to assess cell cycle and apoptosis, and trans-well assays were conducted to evaluate cell migration,invasion, and metastasis. The expression of key molecules in the sonic hedgehog(Shh) signaling was determined by WB.RESULTS Compared to matched adjacent non-tumorous tissues, Dbx2 was overexpressed in 5 HCC cell lines and 76 surgically resected HCC tissues. Dbx2 overexpression was correlated with large tumor size. Both gain and loss of function assays indicated that Dbx2 promoted HCC cell proliferation by facilitating the transition from G1 to S phase, attenuating apoptosis and promoted HCC proliferation,migration, and invasion in vitro and in vivo. Mechanistically, Dbx2 modulated Shh signaling by enhancing FTCH1 and GLi1 expression in HCC cells that overexpressed Dbx2, which was reversed in HCC cells with Dbx2 knockdown.CONCLUSION Our results indicate that Dbx2 is significantly upregulated in HCC tissues and plays significant roles in proliferation and metastasis of HCC cells by activating the Shh pathway.
Key words: Developing brain homeobox 2; Hepatocellular carcinoma; Sonic Hedgehog pathway; Expression; Tumor tissues
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide[1]. HCC patients suffer from a high mortality-to-incidence ratio and low cure rate since we still have no specific and effective treatment[2]. Although tremendous advances have been made in the investigation of HCC, the specific mechanisms of the progression of this disease are still only partially established. Hence, more research is needed to elucidate the underlying potential mechanisms to develop effective strategies for HCC.
The developing brain homeobox (Dbx) gene was first discovered in an embryonic telencephalon of mouse in 1992[3,4]. Dbx2 gene has been reported in mammals and zebrafish. Dbx1 and Dbx2 genes encode a family of homeodomain transcription factors of the H2.0 class that define an intermediate spinal progenitor domain. They are expressed within the p0, p1, and pD6 progenitor domains in vertebrate neural tube[5]. Studies on Dbx2 functions have come to focus on its important roles in neural patterning and differentiation[6-9]. More importantly, Dbx2 participates in neural tube differentiation of mouse embryonic stem cells or regulation of the sonic hedgehog(Shh) signaling pathway, while the detailed underlying mechanisms remain uncovering[10]. We performed an analysis using the cancer genome atlas (TCGA) and found that the 3-yr survival rate was significantly lower in glioma patients with high Dbx2 expression than in patients with low Dbx2 expression (P < 0.01). Another study has identified Dbx1, a homology of Dbx2, as a novel candidate biomarker gene in breast carcinogenesis[11]. In a previous study, we found that the methylation level of Dbx2 was significantly lower in 31 early-stage HCC patients than in 27 healthy controls[12]. These studies indicated that Dbx2 may play an important role in tumor progression. Dbx2 was significantly upregulated in HCC in this study (Figure 1A, B),indicating that Dbx2 is involved in hepatocellular carcinogenesis. Until now, there has been no document to report the role of Dbx2 in malignant cancer. We determined to further investigate the effects of Dbx2 on HCC proliferation and metastasis in vitro and in vivo.
The Shh signaling pathway plays crucial roles in embryonic patterning and adult tissue homeostasis[13,14]. It was reported that the Shh signaling pathway was activated aberrantly in tumorigenesis and development of gastrointestinal, pancreatic, breast,lung, ovarian cancers and so on[15-18]. Some studies concluded that Shh signaling pathway was involved in invasion and metastasis in various malignant tumors[19-24].Hyperactivation of the Shh pathway in different kinds of malignant cancers including HCC induced uncontrolled progression of cancer characteristics, such as proliferation,migration, and invasion[25,26]. An antibody and several small-molecule antagonists targeting the Shh pathway are now under development due to the important role of Shh in cancer progression[27]. Scarcely any meaningful outcome could be obtained in clinical trials, although tremendous efforts have been made. This is mainly because of an insufficient understanding of the mechanisms of tumor occurrence and progression[28]. Therefore, it is indispensable to reveal relevant molecular mechanisms of Shh pathways in hepatocarcinogenesis.
Understanding how Dbx2 plays a role in HCC may help us acquaint potential molecular mechanisms of hepatocellular carcinogenesis and progression and facilitate newly therapeutically targeted strategies for preventing or retarding HCC progression. In this study, we evaluated Dbx2 expression in matched surgically resected HCC tissues and adjacent non-tumor tissues and investigated the biological effects of Dbx2 and Shh pathway in HCC cells to explore the possible molecular mechanisms involved.
MATERIALS AND METHODS
Patients and specimen collection
Hepatocellular carcinoma cell lines
Five HCC cell lines, HepG2, Li-7, Huh7, Huh7.5.1, and SMMC-7721, and one hepatic epithelial cell line, LO2, were used. These cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL, Carlsbad, CA, United States)supplemented with 10% fetal bovine serum (PBS) (Gibco BRL, Carlsbad, CA, United States), and incubated in a 37 °C incubator with 5% CO2.
Lentiviral vector transduction of hepatocellular carcinoma cells
Lentiviruses carrying shRNA targeting human Dbx2 lentiviral vectors and lentiviruses carrying overexpressing lentiviral vectors were purchased (GenePharma Co., Shanghai, China). Cells (1.5 × 105/well) transiently transduced with lentiviral vector (at a 20 nM final concentration) were seeded in 24-well plates and collected 24 h post transfection. HCC cells with stable depleted expression or overexpression of endogenous Dbx2 were selected by culturing in medium with puromycin (0.8 μg/mL). The efficiency of transduction was confirmed by Western blot assay.
Cell viability assay
HCC cells with stable overexpression or knockdown of Dbx2 and corresponding control cells (1 × 104/well) were incubated in 96-well plates. Cell viability was measured using a cell counting kit 8 assay (Dojindo, Shanghai, China) according to the manufacturer’s protocols. Absorbance at 490 nm was measured using a plate reader once a day after 1-5 d of cell culture. Results were calculated by comparing OD490 to baseline.
Colony formation assay
HCC cells with stable overexpression or knockdown of Dbx2 and corresponding control cells (5 × 103/well) were incubated in a 6-well plate for 2 wk. The number of colonies stained by 5% crystal violet was counted. All experiments were performed in triplicate.
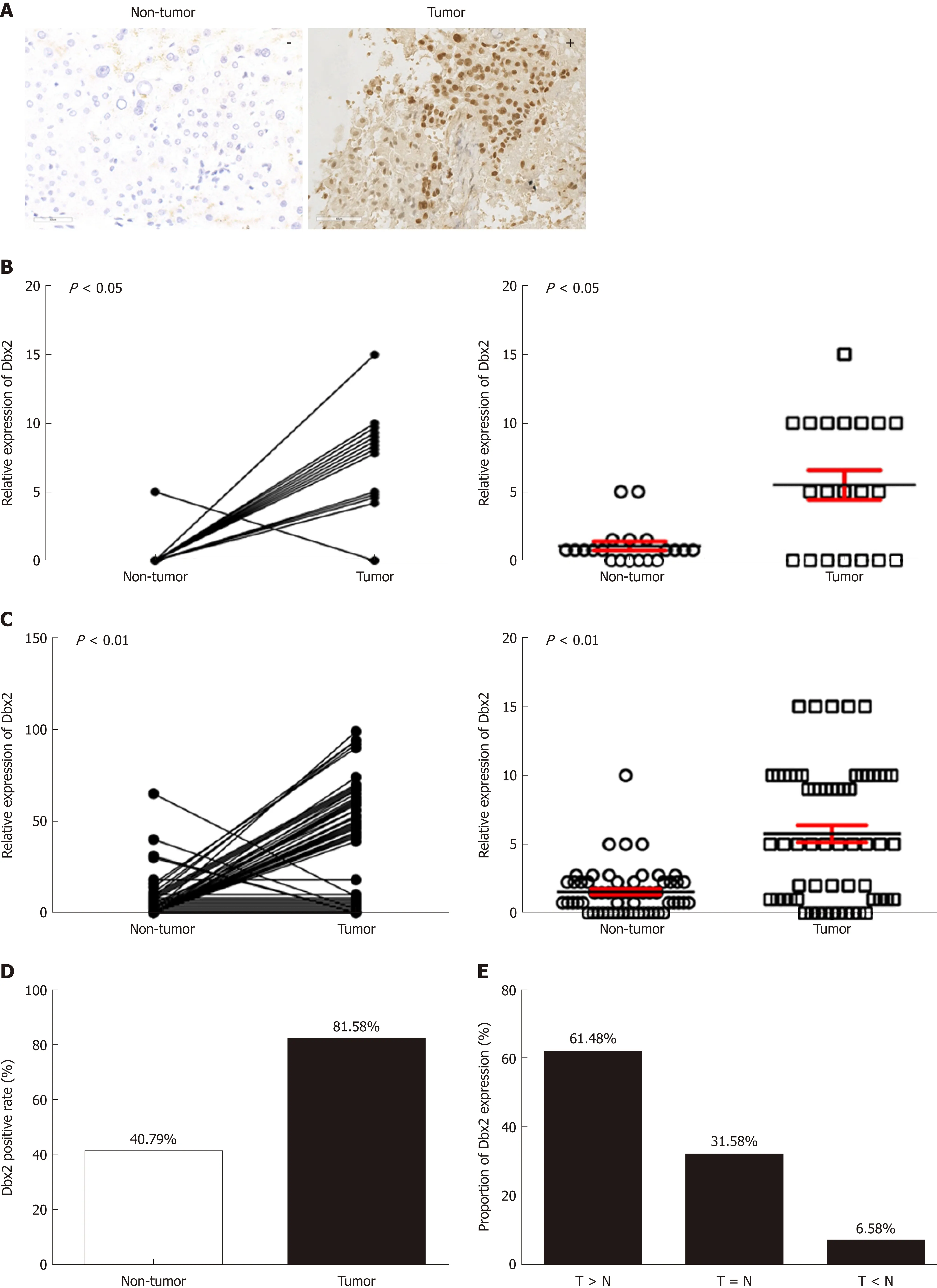
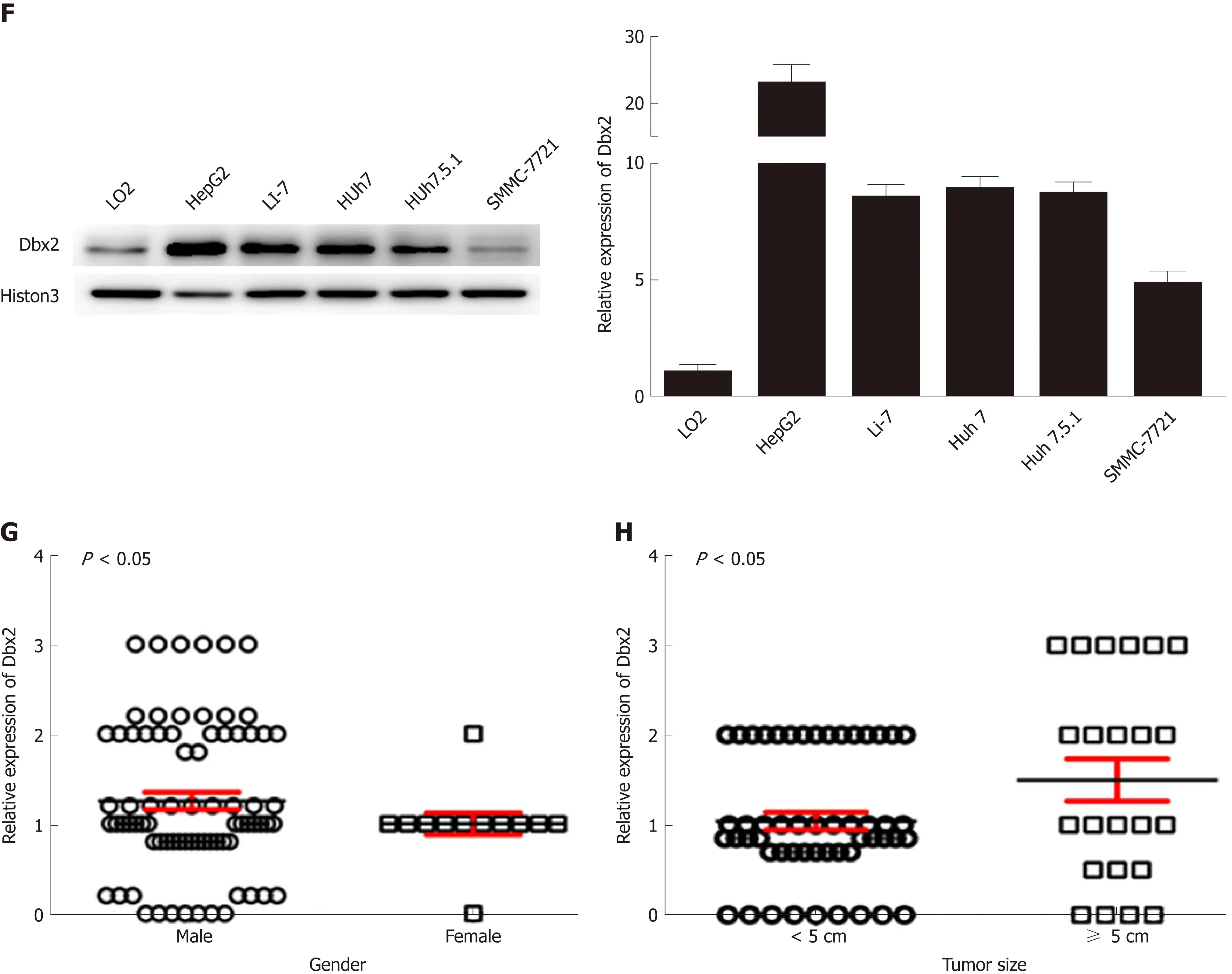
Figure 1 Developing brain homeobox 2 expression is frequently up-regulated in tumor tissues than in non-tumor tissues from patients with hepatocellular carcinoma. A: Positive developing brain homeobox 2 (Dbx2) expression in tumor tissues and negative Dbx2 expression in non-tumor tissues as revealed by immunohistochemistry (×400); B and C: Dbx2 expression was significantly upregulated in tumor tissues compared with their matched adjacent non-tumor tissues as revealed by immunohistochemistry; D and E: Relative Dbx2 expression in tumor tissues to paired non-tumor tissues; F: There was more Dbx2 expression in hepatocellular carcinoma (HCC) cells than in normal hepatic epithelial LO2 cells; G and H: Dbx2 expression was significantly related with gender and tumor size.
Cell cycle assay
The paired cells were prepared as described above and fixed with 70% ethanol for 24 h at 4 °C. Cells were washed with PBS and stained with 50 μg/mL propidium iodide(PI) (BD Biosciences, NY, United States) for 30 min at room temperature in the dark.Cell cycle was assessed with an FACS scan machine (BD Biosciences, NY, United States) and data were analyzed using ModFit 3.0 software (BD Biosciences, NY,United States).
Cell apoptosis assay
For apoptotic analysis, cells were stained via a V-allophycocyanin (V-APC) and PI staining kit (BD Biosciences, NY, United States) according to the manufacturer’s instructions, followed by flow cytometry within 1 h. Cell apoptosis was analyzed with WinMDI 2.9 software (BD Biosciences, NY, United States).
Cell migration assay
The paired cells were incubated (5 × 103/well) in a 6-well plate. Cell migration was assessed with a wound-healing assay. The confluent cell surface was scratched with a pipette tip and the width of two flanks of the wound was recorded once a day for 3 d.
“Mom,” I said as patiently as I could, “you have to learn to ride a bike if you want to ride with me in the Bike-a-Thon, and it’s only two weeks away.”
Cell invasion assay
The paired cells were suspended in serum-free medium at a density of 2 × 105cells/mL. Here, 24-well plates and Matrigel invasion assays (BD Biosciences,Erembodegem, Belgium) were used. Cells (2 × 104) were load into the upper chamber,and 500 μL DMEM and 20% FBS were added to the lower chamber. Cells that passed through the membrane after 24-h incubation were fixed with methanol for 10 min and stained with crystal violet for 10 min. Then the stained cells were counted in five randomly selected microscopic views.
Western blot analysis
Briefly, total proteins extracted from cell pellets were lysed with CytoBuster Protein Extraction Reagent (Merck Millipore, Darmstadt, Germany) and measured using a BCA Protein Assay Kit (Beyotime Biotechnology, Jiangsu, China). About 20 to 50 μg protein of each sample was separated by 8%–15% SDS-PAGE and transferred to nitrocellulose membranes (Sartorius Stedim Biotech, Gottingen, Germany). The membranes were incubated with primary antibody at 4 °C for more than 12 h and then with secondary antibody at room temperature for 1 h. Proteins were visualized with ECL Plus Western Blot Detection Reagents (LOT16327B4, Millipore, United States). We conducted Western blot to evaluate the expression of markers with anti-Histon3 antibody (4499), anti-N-cadherin antibody (13116), anti-E-cadherin antibody(3195), anti-Vimentin antibody (5741), anti-CDK2 antibody (2546), anti-CDK4 antibody (12790), anti-CDK6 antibody (3136), anti-Cyclin D1 antibody (2978), anti-Cyclin A antibody (4656), anti-Cyclin E antibody (20808), anti-p21 antibody (2947),anti-p27 antibody (3686), anti-Bax antibody (5023), anti-bcl-2 antibody (15071), anti-Survivin antibody (2808), anti-Shh antibody (2207), anti-PTCH1 antibody (2468), anti-PTCH2 antibody (2470), anti-SUFU antibody (2522), anti-GLI1 antibody (3538), anticleaved caspase-9 antibody (7237), anti-cleaved caspase-8 antibody (9496), and anticleaved caspase-3 antibody (9664) purchased from Cell Signaling Technology.
In vivo tumorigenicity
HCC cells with stable overexpression or knockdown of Dbx2 and corresponding control cells (2 × 106/well) were injected subcutaneously into the dorsal right flanks of 6-wk-old female NOD/SCID mice (n = 5/group). Tumor size and mouse weight were measured every 3 d until animal sacrifice or experiment ending. Tumor volume was calculated using the following formula: V = (L × W2)/2 (V, volume; L, length of tumor; W, width of tumor). All experiments were manipulated in accordance with the guidelines of Peking University Cancer Hospital Animal Care Commission.
Immunohistochemical staining for Dbx2
Four-micrometer-thick FFPE sections were deparaffinized and rehydrated, followed by antigen retrieval in EDTA (pH = 9, ZLI-9069, Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China). After treatment with endogenous peroxidase, the sections were incubated with primary anti-Dbx2 monoclonal antibody (1:800, PA5-34391, Thermo, NY, United States) at 4 °C overnight, followed by incubation with relevant IgG-HRP conjugate (PV-6000, Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China) and visualization using a 3,3’-diaminobenzidine kit(GK347011, GeneTech, Shanghai, China) according to the manufacturer’s instructions.
Statistical analysis
All statistical analyses were calculated with SPSS 21.0 software (SPSS Inc. Chicago, IL,United States). The χ2-test was used to analyze the relationships between Dbx2 expression and clinical characteristics. The Mann-Whitney U-test was used to compare the difference in Dbx2 expression between tumor and non-tumor tissues.The differences in cell or tumor proliferation, and metastatic ability between two groups were compared by repeated measures analysis of variance and the χ2-test.
RESULTS
Clinicopathological characteristics of the HCC patients
A total of 76 HCC samples were collected in this study. The clinicopathological data including gender, age, tumor size, differentiation, lymph node metastasis, HBV and HCV infection, and serum alpha-fetoprotein (AFP) level are described in Table 1. Men accounted for 84.21% of this cohort (64/76) and the median age of the 76 HCC patients was 56 years (range, 26-76 years).
Dbx2 is significantly up-regulated in HCC
Dbx2 expression was detected in all 76 HCC samples and matched adjacent nontumor tissues using immunohistochemistry. The Dbx2 positive rate in tumors(81.58%, 62/76) was higher than that in adjacent non-tumor tissues (40.79%, 31/76; P< 0.01) (Figure 1B-D). The proportion of tumor tissues with higher expression thantheir respective adjacent non-tumor tissues was 61.84% (47/76), and the proportion with lower expression was 6.58% (5/76) (Figure 1E). Compared with normal hepatic epithelial LO2 cells, Dbx2 expression was up-regulated in HepG2, Li-7, Huh7,Huh7.5.1, and SMMC-7721 cells (Figure 1F), which suggested that Dbx2 may function as an oncogene in HCC.
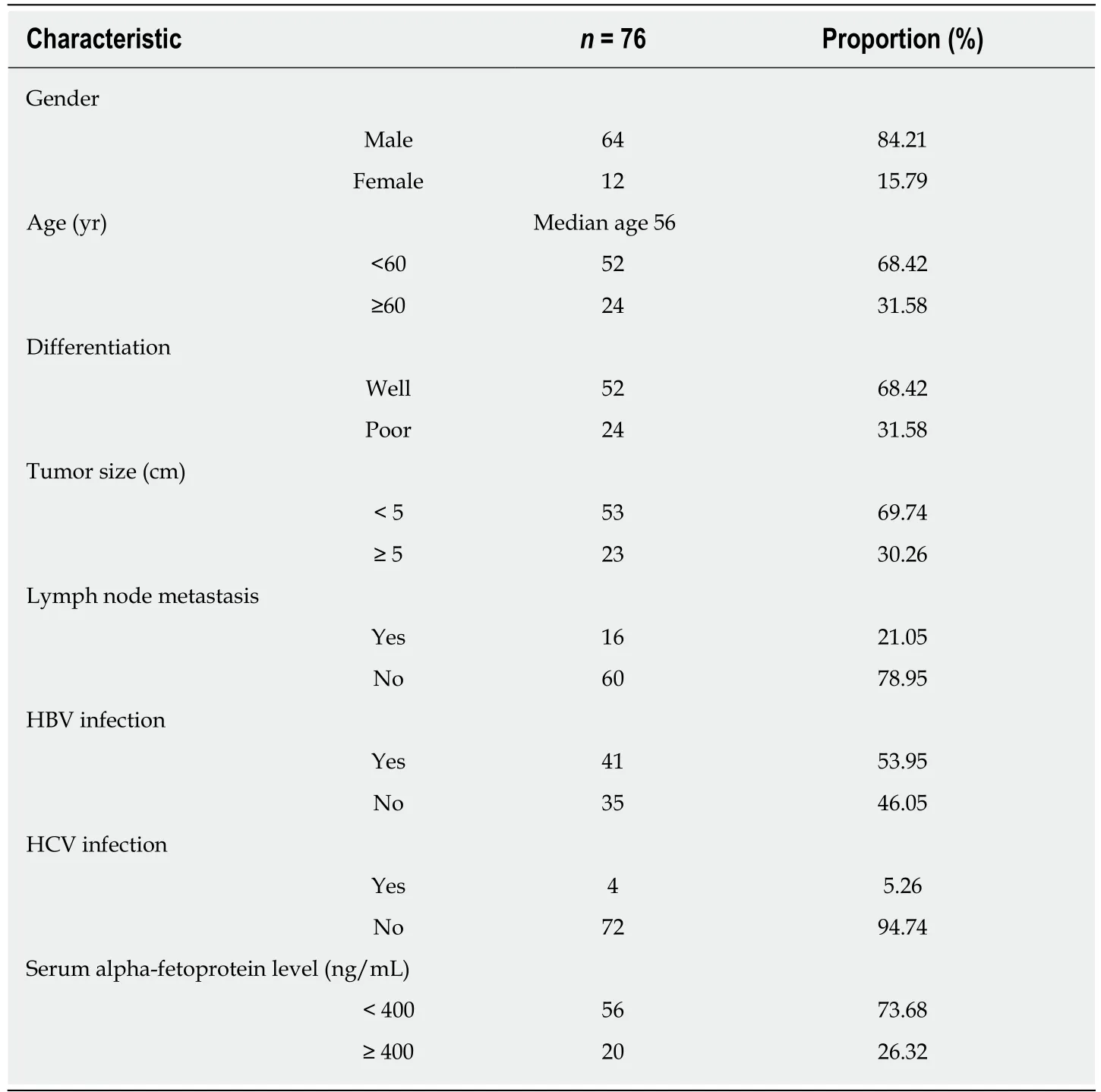
Table 1 Clinicopathological characteristics of hepatocellular carcinoma patients included in the study
Correlation of Dbx2 expression with clinical characteristics
To understand the potential mechanism of Dbx2 in HCC, we analyzed Dbx2 expression stratified by different characteristics. As shown in Table 2, Dbx2 expression was found to be significantly closely related to gender (P = 0.038) and tumor size (P = 0.025) (Figure 1G, H), but unrelated to age, differentiation, lymph node metastasis, HBV infection, HCV infection, or serum AFP level. Based on our data, we estimated that Dbx2 expression might be related to T stage of HCC and a poor prognosis.
Dbx2 promotes the proliferation of HCC cells in vitro and in vivo
Gain and loss of function experiments were conducted to assess the biological behavior of Dbx2 in vitro and in vivo. Cell viability assays showed that ectopic Dbx2 expression (Figure 2A) promoted the growth of SMMC-7721 and Huh7 cells.However, knockdown of Dbx2 inhibited HepG2 and Huh7 cell growth (P < 0.05;Figure 2B). These results were in accord with those obtained in colony formation assays (P < 0.01; Figure 2C).
SMMC-7721 cells with stable Dbx2 expression and control cells were injected subcutaneously into NOD/SCID mice. The results indicated that compared with the control group, tumor growth was significantly faster in the Dbx2 overexpression group (P < 0.05; Figure 2D). Meanwhile, tumor growth was inhibited in in vivo xenografts generated using HepG2 cells with stable knockdown of Dbx2 expression(P < 0.05; Figure 2D).
Dbx2 brings the cell cycle into the S phase
We conducted cell cycle assay to explore the mechanisms that might be responsiblefor the growth-promoting effect of Dbx2 in HCC cells. The percentage of S phase cells was significantly greater in SMMC-7721 and Huh7 cells that exhibited ectopic expression of Dbx2 (P < 0.05; Figure 3A). Consistently, the expression of related cell cycle molecules was changed by up-regulated Dbx2 expression in SMMC-7721 and Huh7 cells; the expression of cyclinA, p21, and p27 was downregulated, while cyclindependent kinase (CDK2, CDK4, and CDK6), cyclin B, and cyclin D were upregulated(Figure 3B). However, knockdown of Dbx2 in HepG2 and Huh7 cells induced arrest in G1 phase and the expression of cell cycle regulators was concomitantly changed(Figure 3A, B).
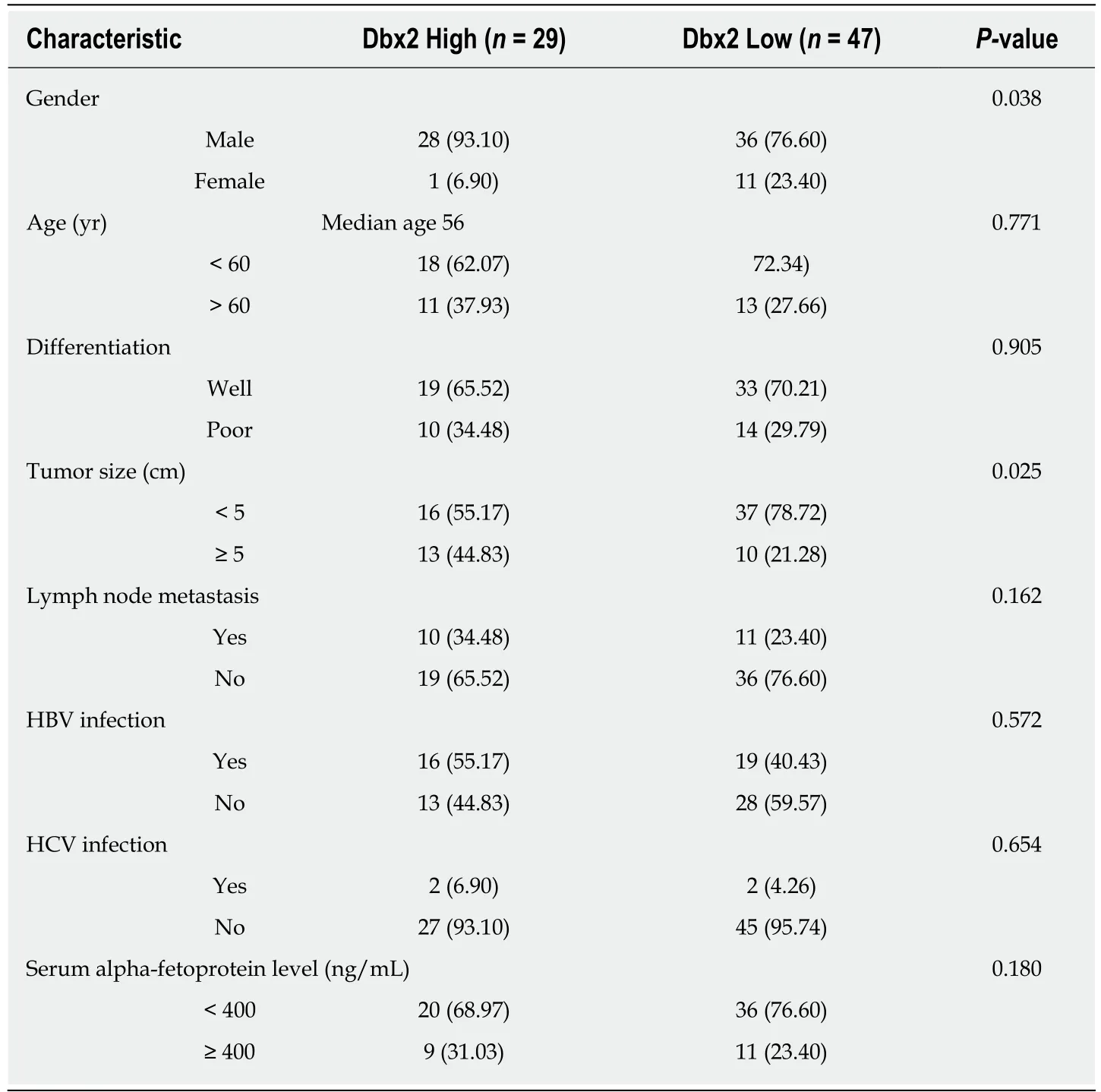
Table 2 Correlation between developing brain homeobox 2 expression and characteristics of hepatocellular carcinoma n (%)
Dbx2 attenuates apoptosis in HCC cells by inactivating the caspase-dependent pathway
To explore the mechanisms that may underlie the ability of Dbx2 to promote growth in HCC cells, the rate of cellular apoptosis was detected using V-APC and PI staining by flow cytometry. As shown in Figure 3C, compared with control cells, the proportions of apoptotic cells in SMMC7721 and Huh7 cell lines were significantly decreased after transfection with Dbx2 (P < 0.01). We then conducted Western blot analysis to evaluate the expression of molecules of the caspase-dependent pathway.As shown in Figure 3D, the levels of activated caspase-9, activated caspase-8,activated caspase-3, and nuclear poly ADP-ribose polymerase were lower in the Dbx2-transfected SMMC-7721 and Huh7 cells than in the control cells, but those of the anti-apoptosis proteins Bcl2 and survivin were higher. However, the proportions of apoptotic cells in HepG2 and Huh7 cells with knockdown of Dbx2 were higher (P <0.05; Figure 3C) and the expression of apoptosis-related regulators changed accordingly (Figure 3D).
Dbx2 promotes migration and invasion in HCC cells
We performed wound healing assay, transwell migration assay, and Matrigel invasion assay to assess the possible role of Dbx2 in HCC cells. Our results indicated that, relative to control cells, overexpression of Dbx2 promoted migration and invasion of SMMC-7721 and Huh7 cells (P < 0.05). However, knockdown of Dbx2 in HepG2 cells and Huh7 cells rendered cell migration and invasion less pronounced than in control cells (P < 0.05; Figure 4A, B). Consistent changes were observed in epithelial-mesenchymal transition proteins (Figure 4C).
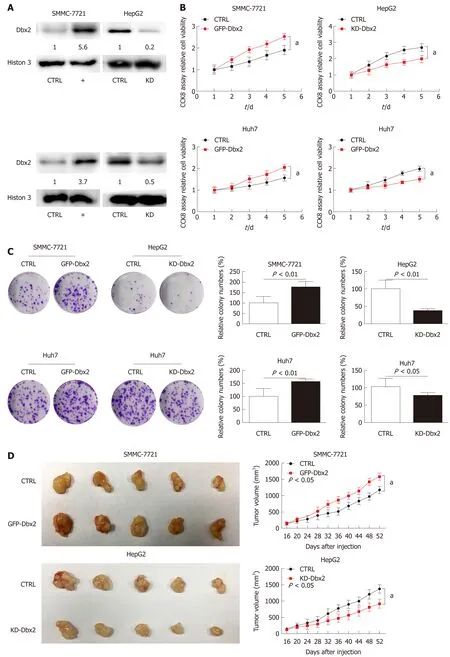
Figure 2 Developing brain homeobox 2 promotes cell growth in vitro and in vivo. A-C: Ectopic expression of developing brain homeobox 2 (Dbx2) in SMMC-7721 and Huh7 cell lines promoted cell growth as indicated by a cell viability assay and colony formation assay. The results were reversed in HepG2 and Huh7 cell lines with knockdown of Dbx2; D: Ectopic Dbx2 expression in SMMC-7721 cells promoted tumor growth and knockdown of Dbx2 in HepG2 cells inhibited tumor growth in NOD/SCID mice. aP < 0.05; bP < 0.01.
Dbx2 activates Shh-Gli1 signaling in HCC
It was confirmed that Dbx2 plays a vital role in the development of neurons mainly through the Shh-Gli1 signaling pathway, but the role of this pathway in HCC is still not clear. Our data showed that in SMMC-7721 cells and Huh7 cells, Shh signaling was activated by up-regulation of Dbx2 and was significantly repressed in HepG2 and Huh7 cells with knockdown of Dbx2 (Figure 5A). These findings indicated that Dbx2 could activate the Shh-Gli1 signaling pathway in HCC.
DISCUSSION
The evolutionarily conserved Dbx proteins were reported to play important roles in the development of the vertebrate central nervous system. Dbx genes (Dbx1 and Dbx2) have been reported to encode a family of homeodomain transcription factors in mammals. Dbx2 is highly expressed during neuronal development and regulates differentiation of interneurons in the human brain and spinal cord. Elevated Dbx2 expression promotes age-related phenotypes in young adult neural stem/progenitor cells, including the reduced proliferation and the altered differentiation in neural stem/progenitor cell cultures. Depleting Dbx2 in aged neural stem/progenitor cells caused the reverse gene expression changes. It has been reported that Dbx1, a homology of Dbx2, could serve as a novel candidate biomarker gene in breast carcinogenesis[11]. High Dbx2 expression was associated with a shorter 3-yr survival in glioma patients by the cancer genome atlas (TCGA) analysis in another study of us.However, little was known about the role of Dbx2 in HCC pathogenesis. We have found that the methylation level of Dbx2 was significantly lower in 31 early-stage HCC patients than in 27 healthy controls in our previous study[12]. We suggested that Dbx2 may play an important role in HCC development and metastasis. In our study,we showed that Dbx2 was more often elevated in HCC tissues relative to adjacent non-tumorous tissues by immunohistochemistry. Overexpression of Dbx2 was demonstrated by Western blot analysis in HCC cell lines against normal hepatic epithelial LO2 cells. We also detected more Dbx2 overexpression in 76 HCC tissue samples than in matched adjacent non-tumor tissues by immunohistochemistry assay.What is more, high levels of Dbx2 expression were statistically significantly associated with gender and tumor size. Compared to non-tumors tissues, our data showed that aberrant upregulation of Dbx2 was found in HCC tissues.
We further investigated Dbx2 expression in several cell lines, including LO2,HepG2, Li-7, Huh7, Huh7.5.1, and SMMC-7721. Significant ectopic Dbx2 expression was found in cancer cell lines by Western blot (Figure 2A). Gain and loss of function experiments were employed to assess the effect of Dbx2 on the biological behavior of cell lines. Overexpression of Dbx2 promoted cancer cell growth in SMMC-7721 and Huh7 cells. HepG2 and Huh7 cell growth was inhibited by knockdown of Dbx2 (P <0.05; Figure 2B). These results were also confirmed by colony formation assay (P <0.01; Figure 2C). SMMC-7721 cells with stable Dbx2 expression were injected subcutaneously into NOD/SCID mice. Compared with the control group, tumor growth was significantly faster in the Dbx2 overexpression group (P < 0.05; Figure 2D). Meanwhile, the tumor growth was inhibited in in vivo xenografts with stable Dbx2 knockdown (P < 0.05; Figure 2D). We concluded that Dbx2 expression was closely correlated with HCC progression.
To explore the mechanisms of the growth-promoting effect of Dbx2 in cell lines, we further performed cell cycle analysis. The percentage of S phase cells was significantly greater in SMMC-7721 and Huh7 cells that exhibited ectopic expression of Dbx2 (P <0.05; Figure 3A). G1/S transition was promoted in HCC cells after Dbx2 overexpression; conversely, knockdown of Dbx2 inhibited HCC proliferation and induced G1 phase arrest. As we know, unlimited proliferation is one of important characteristics of cancer[29,30]. Subsequently, we further found the expression levels of cell cycle-related proteins to be consistent with the progression of the cell cycle.Additionally, studies on apoptosis demonstrated that overexpression of Dbx2 could attenuate apoptosis while knockdown of Dbx2 expression could activate apoptosis through the caspase pathway. We also elucidated that Dbx2 promoted cell migration and invasion by wound healing assay and transwell migration assay. The functions were reversed after Dbx2 knockdown. In a word, our data suggested that Dbx2 plays a crucial role in liver cancer cell proliferation, migration, and invasion.
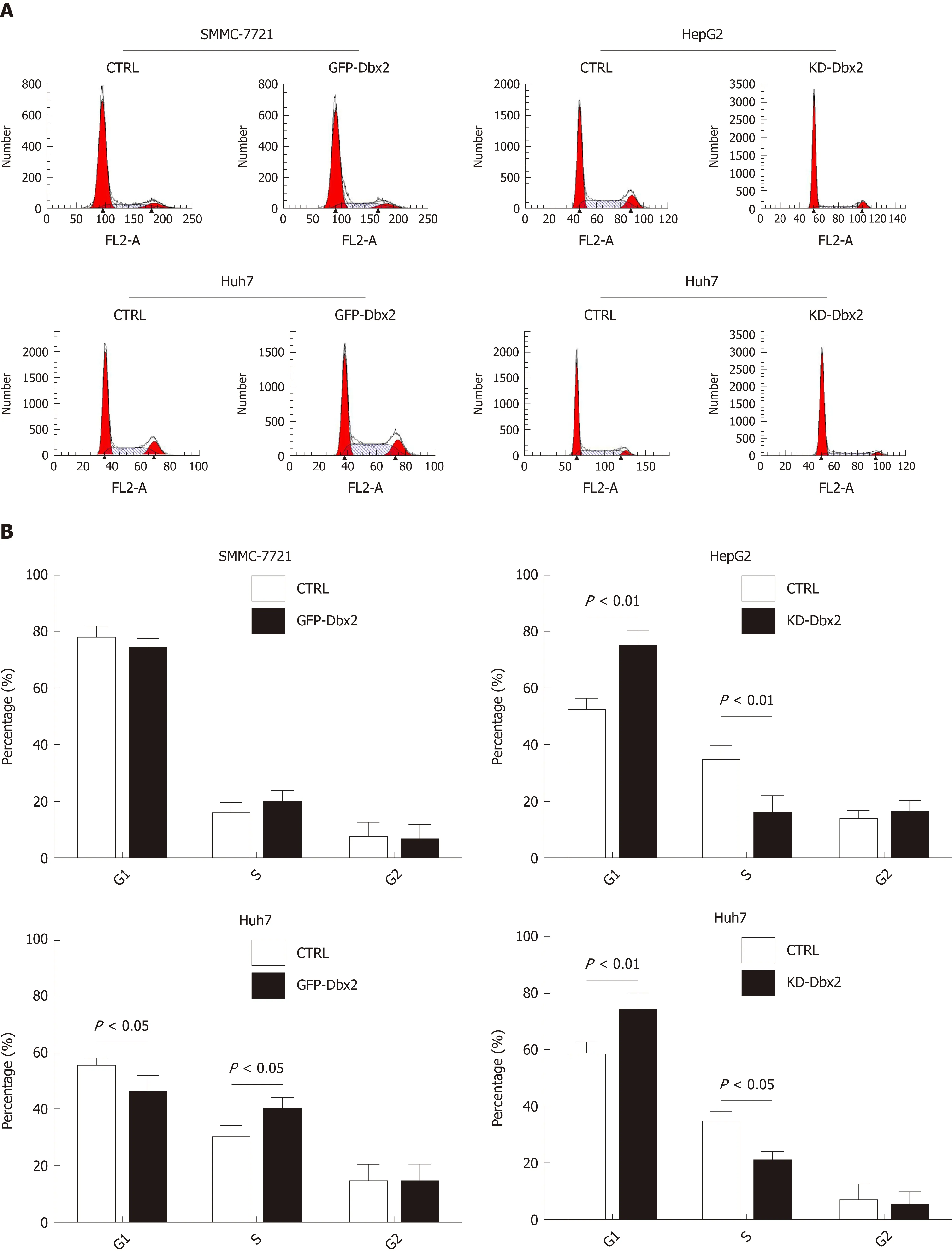
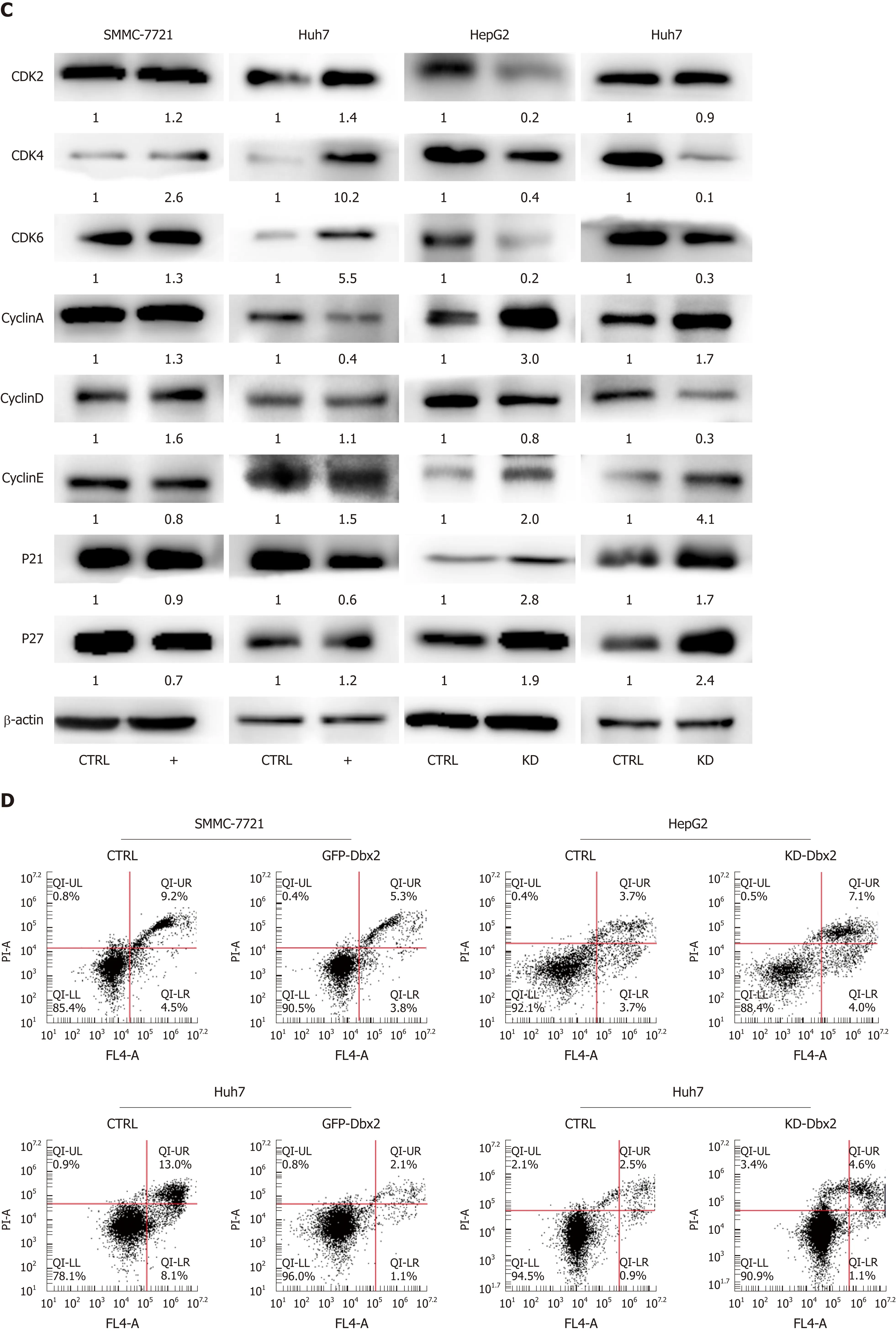
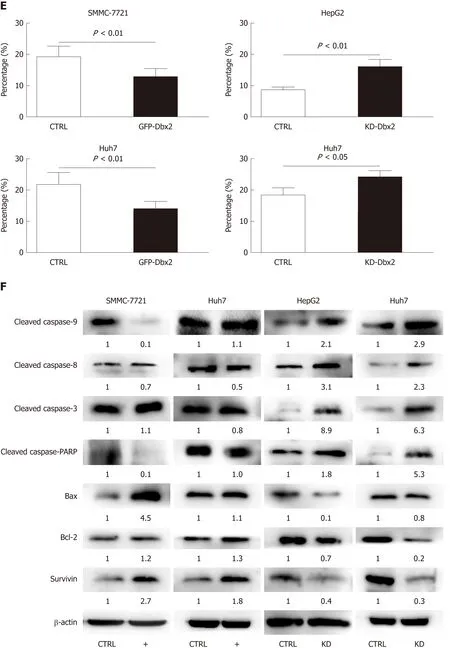
Figure 3 Developing brain homeobox 2 regulates cell cycle and inhibits cell apoptosis in hepatocellular carcinoma cells. A and B: Ectopic expression of developing brain homeobox 2 (Dbx2) induced cell cycle into S phase; C: Ectopic expression of Dbx2 regulated cell cycle protein expression; D and E: Ectopic expression of Dbx2 inhibited cell apoptosis; F: Ectopic expression of Dbx2 downregulated apoptotic protein expression. The results were reversed after Dbx2 knockdown.
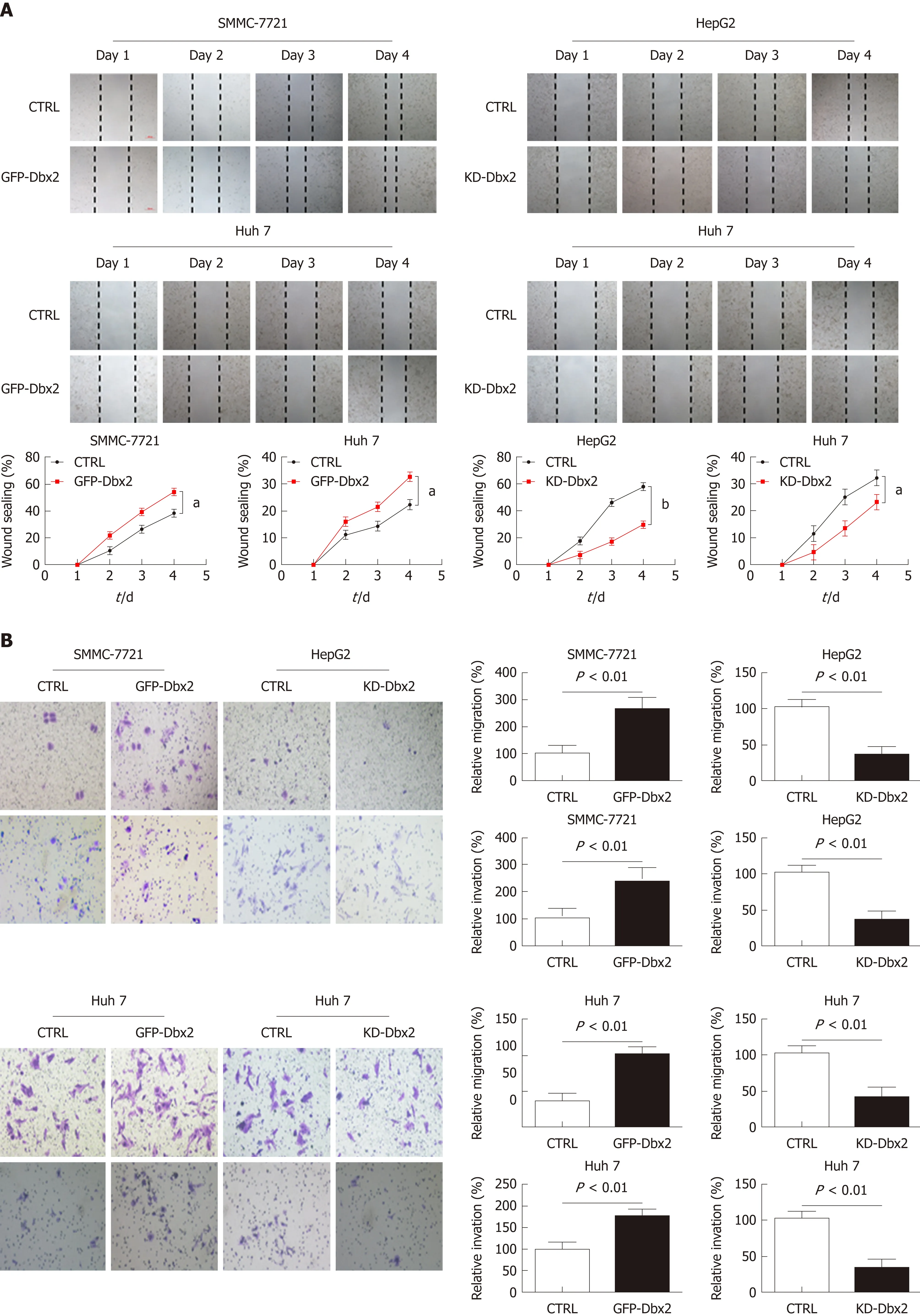
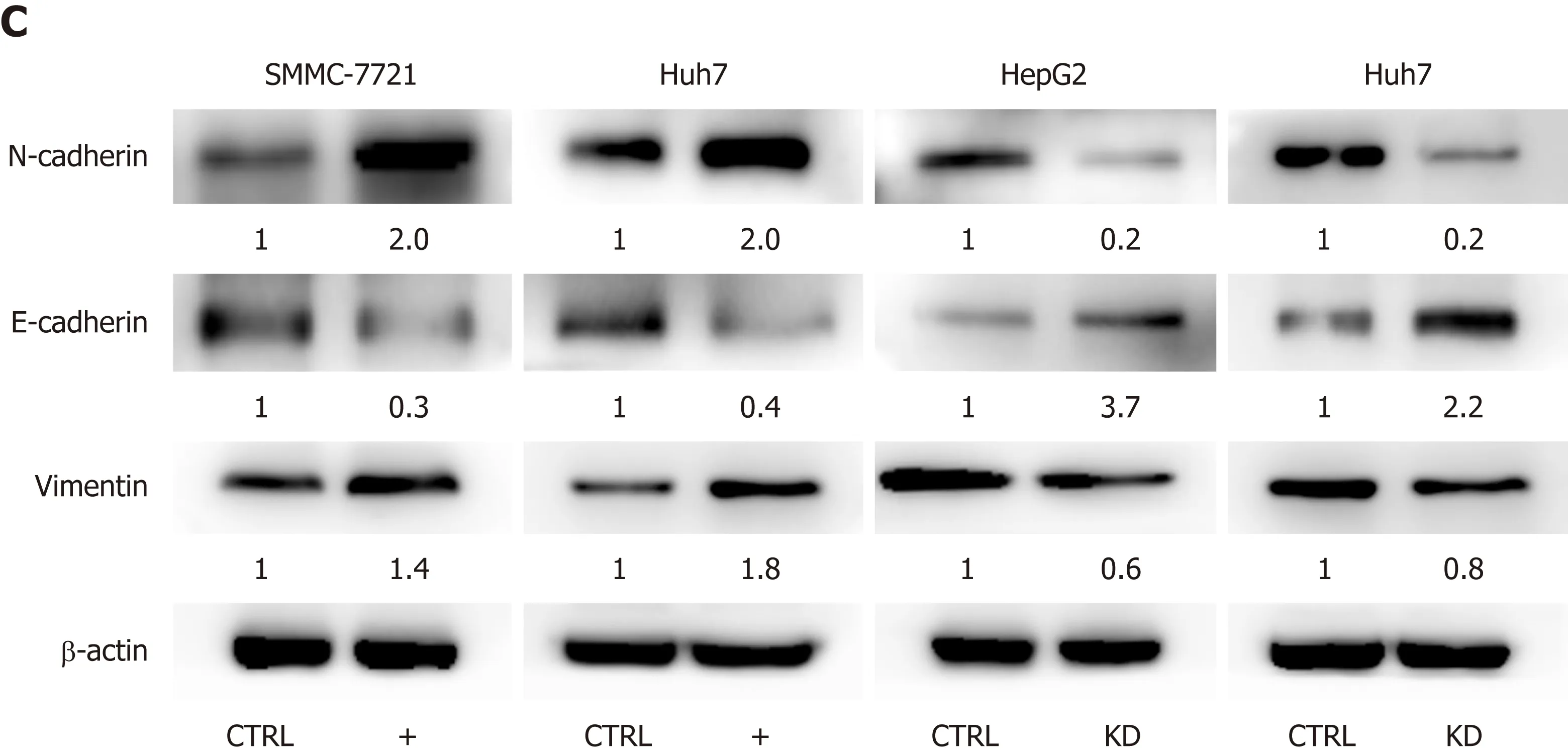
Figure 4 Developing brain homeobox 2 promotes cell migration and invasion in hepatocellular carcinoma cells. A: Wound healing assay; B: Transwell migration and Matrigel invasion assay demonstrated that developing brain homeobox 2 (Dbx2) promoted cell migration and invasion. The effects were reversed after Dbx2 knockdown; C: Effect of Dbx2 on expression of several epithelial-mesenchymal transition related proteins detected by Western blot analysis. aP < 0.05; bP <0.01.
Shh signaling plays an important role in embryonic development and regulation of cellular functions including proliferation, survival, stemness, and differentiation. The phenotype and proliferation of cancer cells are controlled by regulating the Shh pathway via interaction with Shh[31-33]. Aberrant activation of Shh signaling has been demonstrated in HCC. Activation of the Shh pathway is initiated by the binding of Shh ligands to their receptor, and leads to the activation of Smo. Smo activates the Gli family of transcription factors that regulate the expression of Shh target genes including Ptch1 and Gli1. For this reason, we determined whether Dbx2 regulates the Shh pathway and cell proliferation by interacting with Shh. Our data clearly showed that Dbx2 interacted with Shh in SMMC-7721 and Huh7 cell lines. Loss of Dbx2 resulted in significant repression of the Shh pathway during short-term Shh stimulation by regulating Ptch1 and Gli1 (Figure 5B). These results indicated that Dbx2 promoted cell proliferation, presumably by activating the Shh signaling pathway. Our findings provide evidence that Dbx2 plays an important role in cell proliferation by activation of the Shh signaling pathway.
In conclusion, this study shows that Dbx2 is upregulated in HCC cell lines and tissue samples. Gain and loss of function experiments of Dbx2 demonstrated that ectopic Dbx2 expression could promote HCC cell line proliferation, migration, and invasion in vitro by regulating the Shh signaling pathway, and accelerate tumor growth in in vivo xenografts.
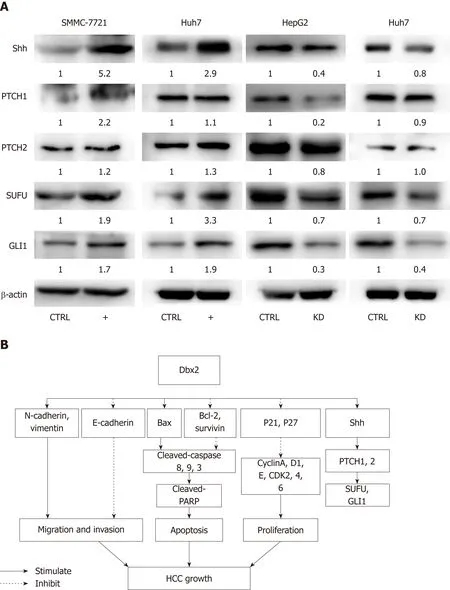
Figure 5 Developing brain homeobox 2 activates the Shh-Gli1 signaling pathway in hepatocellular carcinoma. A: Ectopic expression of developing brain homeobox 2 (Dbx2) upregulates the expression of Shh, PTCH1, PTCH2, SUFU, and GLI1 proteins, which are downregulated after Dbx2 knockdown; B: Proposed scheme of molecular basis for gain and loss of function of Dbx2 in HCC according to our results.
ARTICLE HIGHLIGHTS
Research background
Great efforts have been made in exploring the mechanism of hepatocellular carcinoma (HCC),but the details of the HCC pathogenesis are still only partially established. Developing brain homeobox 2 (Dbx2) is frequently upregulated in tumor tissues, while there has been no experimental evidence regarding the function of Dbx2 in HCC.
Research motivation
Investigation of Dbx2 functions may suggest potential molecular mechanisms of hepatocellular carcinogenesis and progression, and further offer the potential for developing novel therapeutic strategies for HCC treatment.
Research objectives
We measured Dbx2 expression in HCC tissues and matched non-tumor tissues and investigated biological functions and the possible molecular mechanisms of Dbx2 in HCC.
Research methods
We detected Dbx2 expression in HCC samples and adjacent non-tumor tissues by immunohistochemistry. The biological behavior of Dbx2 in vitro and in vivo was then assessed by overexpression and knockdown of the Dbx2 gene.
Research results
Dbx2 was upregulated in HCC tissues, which was related to tumor size. Dbx2 had a role of promoting proliferation and metastasis by activating sonic hedgehog (Shh) signaling in HCC.
Research conclusions
Dbx2 was overexpressed in HCC cell lines and tissues, which could promote HCC progression through the Shh signal pathway.
Research perspectives
Dbx2 might serve as a tumor promoter and to be a potential therapeutic target in the future.
杂志排行
World Journal of Gastroenterology的其它文章
- Current and future pharmacological therapies for managing cirrhosis and its complications
- Outcomes of per oral endoscopic pyloromyotomy in gastroparesis worldwide
- Dynamic changes of key metabolites during liver fibrosis in rats
- Procyanidin B2 protects against diet-induced obesity and nonalcoholic fatty liver disease via the modulation of the gut microbiota in rabbits
- Triggers of histologically suspected drug-induced colitis
- Women on the liver transplantation waitlist are at increased risk of hospitalization compared to men
