Outcomes of per oral endoscopic pyloromyotomy in gastroparesis worldwide
2019-03-09ParitMekaroonkamolRushikeshShahQiangCai
Parit Mekaroonkamol, Rushikesh Shah, Qiang Cai
Abstract Per oral endoscopic pyloromyotomy (POP), also known as gastric per-oral endoscopic myotomy (GPOEM), is a novel procedure with promising potential for the treatment of gastroparesis. As more data emerge and the procedure is becoming more recognized in clinical practice, its safety and efficacy need to be carefully evaluated. Appropriate patient selection for favorable clinical success prediction after GPOEM also needs additional research. This review aims to systemically summarize the existing data on clinical outcomes of POP.Symptomatologic responses to the procedure, its adverse effects, procedural techniques, and predictive factors of clinical success are also discussed.
Key words: Gastroparesis; Per oral endoscopic pyloromyotomy; Gastric per-oral endoscopic myotomy; Pyloromyotomy; Outcomes
INTRODUCTION
Gastroparesis is a chronic disabling disease with a complex pathophysiology that is still poorly understood. Its rising prevalence and hospitalization rate are alarmingly concerning[1]. The three main etiology cited for gastroparesis include idiopathic,diabetic related and post-surgical. Gastroparesis clinically manifests as recurrent postprandial nausea and vomiting, early satiety post prandial bloating and upper abdominal pain[2]. In severe cases, it can also lead to weight loss and malnutrition[3].The diagnosis of gastroparesis is suspected by constellation of these clinical symptoms. The diagnosis is further confirmed based on normal upper endoscopy ruling out any structural obstruction and 4 h gastric emptying study proving impaired gastric emptying[2]. Gastroparesis, due to recurrent debilitating nature of disease has significant negative impact on patients’ quality of life, economic burden,and heath care utilization while only with limited therapeutic options[4]. Only few prokinetic medications are available for symptomatic control. Most of which have limited long-term usage due to their side effects or tachyphylaxis[5]. Metoclopramide and domperidone, a D2 dopamine receptor antagonist, are the most widely used but only metoclopramide is Food and Drug Administration (FDA) approved in the United States while domperidone is available in Europe, Canada, Mexico, and New Zealand[5,6]. Metoclopramide also carries a significant risk of extrapyramidal adverse effects, including tardive dyskinesia when taken longer than 12 wk. Other groups of medication, such as 5-HT3 receptor antagonists, phenothiazines, and muscarinic cholinergic receptor antagonist, have been used off-label for symptomatic relieve but they do not have effect of gastric motility. While medications and dietary modification are the first line treatment, approximately 30% of patients do not respond to conservative management[7]. These limitations of medical therapy highlights the need for an alternate therapeutic option[8].
Non-medical treatments of gastroparesis include gastric electric stimulator, surgical pyloroplasty, botulinum toxin injection, and transpyloric stenting. The concept of neurostimulation and pylorus-directed therapies stem from a physiologic knowledge of gastric emptying that involves both gastric motility and pyloric clearance[9]. Gastric motility is driven by the interstitial cells of Cajal, the pacemaker of the gut, which rhythmically generate a slow wave impulse spanning from the greater curvature toward the pylorus. Gastric electrical stimulator was developed based on the principle that the amplitude, frequency, and direction of these electrical activities help control the gastric emptying function[10-12].
Although the efficacy of gastric electrical stimulation was promising in the early open-label studies but their results were not consistently reproduced in subsequent randomized control trials, which raises concern for the true clinical impact of this modality on gastroparesis[7,13-20]. In addition, gastric electrical stimulator requires surgery for placement and has been only approved based on the humanitarian device exception rule, rather than an effective therapy by the United States FDA[21].Implantation and device-associated adverse effects such as pocket infection, sepsis,pulmonary embolism, stroke, or even death have been reported[21].
Even though the pylorus lacks slow-wave impulses, its tone and phasic contraction determines the outlet phase of gastric emptying[11]. Abnormal physiologic characteristics of the pylorus such as narrow cross-sectional area, diameter, increased pressure, distensibility, and prolonged phasic contraction or increased tone have been shown to delay gastric emptying[9,11,22-24]. Other pathophysiology, including antroduodenal hypomotility, impaired fundic accommodation, and pylorospasm, all of which also interact with one another, are believed to play major roles in gastroparesis[25].
Therefore, mechanical disruption of the pyloric muscle is believed to have effects on global gastric emptying as well. This notion of pylorus-directed therapy was strengthened by recent studies on surgical pyloroplasty, transpyloric stenting, and intrapyloric botulinum injection that improved both symptoms score and gastric emptying time[26-33]. However, the efficacy of intrapyloric botulinum injection was not demonstrated in subsequent randomized controlled trials and is no longer recommended by American College of Gastroenterology, while transpyloric stenting is only a temporizing measure and not a long term solution[8,34,35].
With the significant advancement in the field of submucosal endoscopy in the last few years, per oral endoscopic pyloromyotomy (POP), also known as gastric per oral endoscopic myotomy (GPOEM), has emerged as a pylorus-directed therapy for gastroparesis. The procedure was conceptually originated from POEM, a similar endoscopic procedure for treatment of achalasia. They both use submucosal tunneling technique to reach and dissect the target muscles, lower esophageal sphincter in achalasia and pyloric ring in gastroparesis[36]. The submucosal space offers a safe conduit to carry out pyloromyotomy via endoscopy, rather than surgery[37-39]. Due to its minimally-invasive nature and its promising outcomes, this novel procedure has quickly gained popularity worldwide. The first human case of POP was performed by Khashab et al[39]in 2013 without any adverse event and demonstrated significant clinical improvement at 12-wk follow up. Subsequently in 2014, the first human case of POP was performed successfully in Europe and later in 2015, a case series of 7 patients was published on POP outcomes[37]. Since then, many single-center and a few multicenter studies have been published reporting short term outcomes on POP in gastroparesis patients, mostly in retrospective fashion (Table 1)[39-42]. The reported symptomatic responses and clinical outcomes have been very promising[2,37,40,43-48]. This review aims to examine current evidence on clinical outcome of POP.
PROCEDURAL TECHNIQUES
The procedural steps of POP follow the established sequence of POEM (Figure 1) as:(1) Mucosotomy to create an entry to submucosal plane; (2) submucosal tunnel is created using submucosal dissection technique; (3) myotomy of the targeted muscle;and (4) mucosal defect closure. However, POP is generally considered more technical demanding than POEM for a few reasons: (1) The direction of the submucosal tunnel is curved, as opposed to a straight tube in esophagus; (2) there is more movement in the procedural field from antral contractility, compared to a aperistaltic esophagus; (3)identification of the targeted muscle, the pyloric ring in POP, is more difficult than identifying lower esophageal sphincter in POEM due to both aforementioned reasons rendering risk for tunnel deviation from the desired axis; (4) the wall of duodenal bulb is much thinner than gastric cardia, increasing risk for perforation; and (5) the maneuverability of the scope is more limited due to an inevitable loop in the stomach.
In addition, due to its novelty, there is a lack of standardized technique for POP.Extent and depth of pyloromyotomy may vary depending on endoscopist’s preference and how well the pyloric ring can be identified in the submucosal tunnel.Most report suggested performing the procedure in supine position as it will be easier to orient the scope direction but left lateral decubitus position may be required when a large gastric loop is present[49]. Prolonged period of clear liquid diet for 2-3 d both before and after POP was recommended as a routine pre- and post-procedure protocol to maximize visualization and reduce the risk of procedure-related infection[2,49]. Generous irrigation should be exercised to clean the stomach content and mucosotomy site. Gentamycin rinse was advocated by some centers[47]. Other technical variations among endoscopists include site of mucosal entry (lesser vs greater curve), mucosotomy closure tools (clips vs suture), depth of pyloromyotomy,and the need for fluoroscopy[2,45,50-52]. Though general anesthesia is recommended in all studies but conscious sedation in endoscopy suite can be safely and successfully performed as well[49]. Intravenous antibiotic prophylaxis is routinely administered though there is no high-quality evidence to support the practice. Proper antibiotic,dosing, and duration are still not yet refined. How much impact on these minor variations have on clinical response is also not known.
In the early studies, mucosal entry was performed mainly on the greater curve or anterior wall of the stomach and full thickness myotomy was reported[37,53,54].However, subsequent studies verified that submucosal tunnel can be safely and effectively performed regardless of the site of mucosal entry and selective circular myotomy can achieve clinical success without the perforation risk in full thickness myotomy[41,47,48,55,56]. While mucosotomy on the greater curve makes the scope more in a neutral position and allows greater maneuverability, performing a mucosal entry on the lesser curve has its own advantages that are: (1) Shorter scope length to mucosotomy site by minimizing the gastric loop; (2) shorter length of the submucosal tunnel, reducing the risk of tunneling in the wrong direction; and (3) the procedural field is not a dependent area when the patient is on supine position, therefore blood and food would not interfere with the endoscopic visualization[48,49,55].
Though the type of endoscopic knife and injectant used during POP have been heterogeneous in various reports, which included triangle-tip knife (KD-640 L,Olympus, Tokyo, Japan), hybrid knife (ERBE, Germany), a hook knife (KD-620LR;Olympus, Japan), mixed methylene blue/indigo carmine with hypertonic saline,normal saline, or hydroxy-ethyl starch, but common devices that are considered mandatory are silicone-base transparent over-the-scope cap and carbon dioxide for insufflation during the procedure. The cap facilitates submucosal entry, creates a working space in the submucosal tunnel, and also helps with hemostasis from small vessels in the tunnel. Due to submucosal nature of the procedure, pneumoperitoneum can occur. Carbon dioxide, which is absorbed 160 times faster than nitrogen gas in room air is essential to minimize this risk[57]. For hemostasis, soft coagulation mode(ERBE, Germany) for ablation of small vessels with a diameter less than 5 mm and coag-grasper (FD-411QR; Olympus, Japan) for bleeding control from a large vessel are generally used[49,55,56].
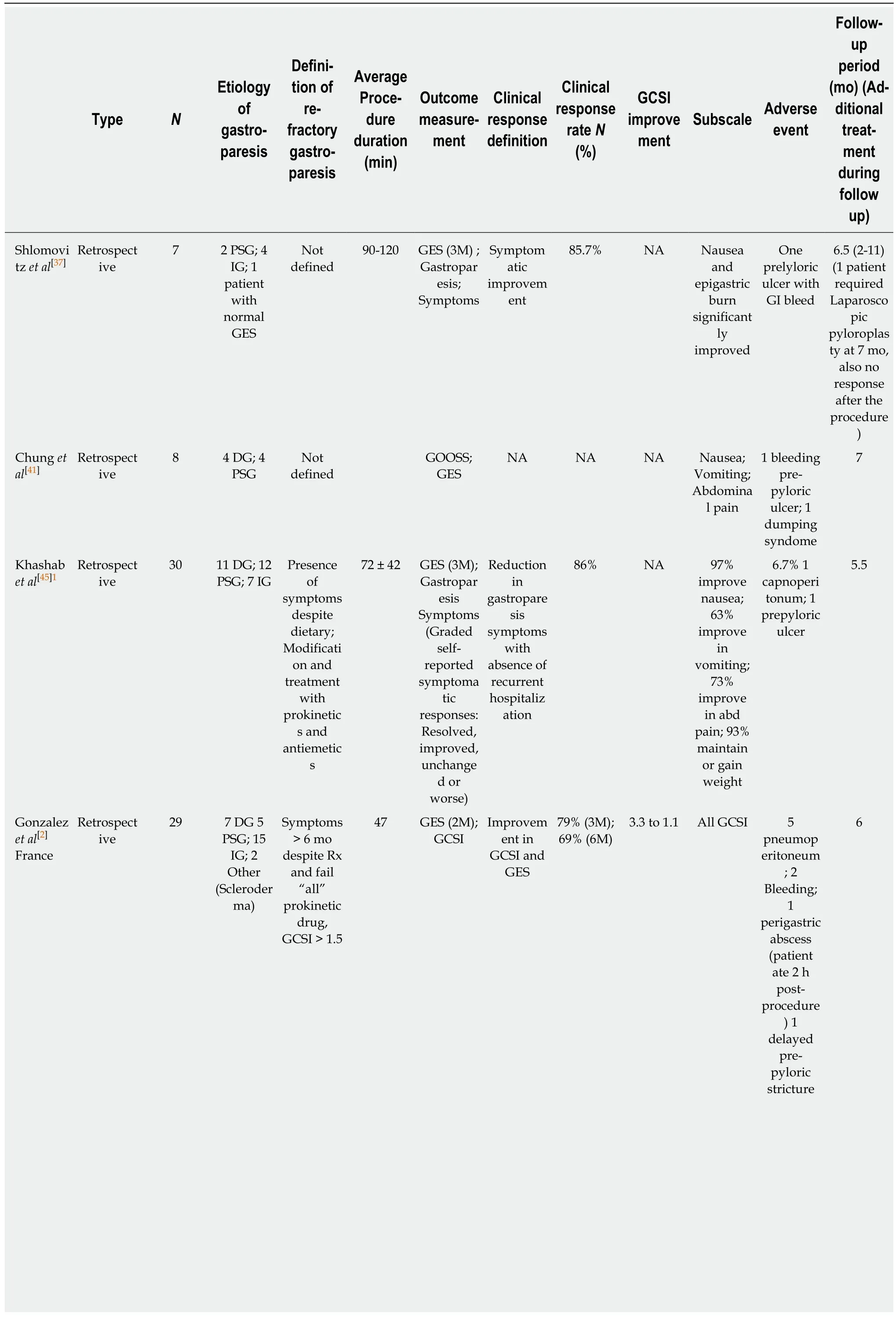
Table 1 Clinical outcomes of per oral endoscopic pyloromyotomy
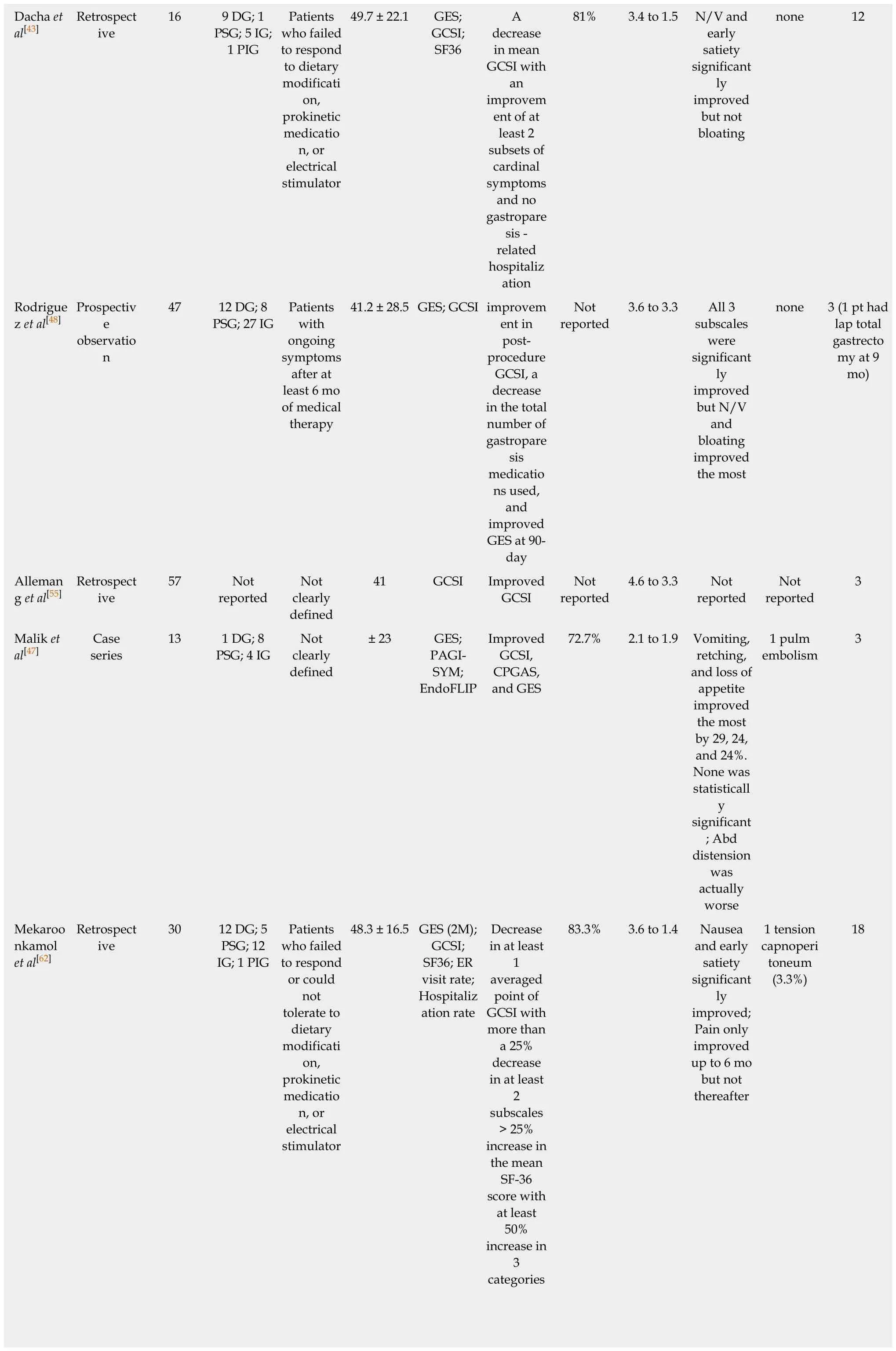
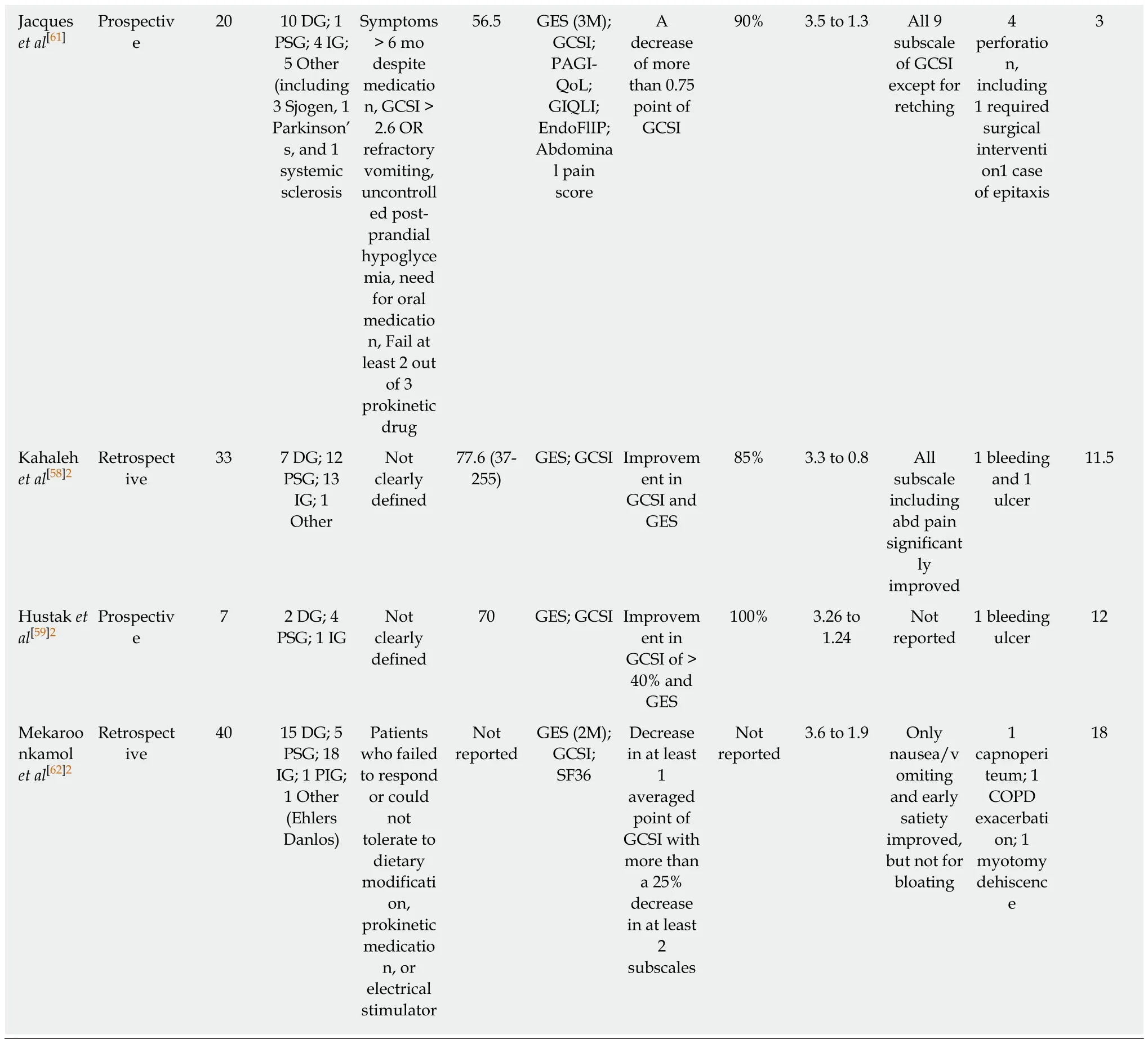
1Multicenter trial, two centers in the United States were involved.2Abstract only publications.DG: Diabetic gastroparesis; PSG: Post-surgical gastroparesis; IG: Idiopathic gastroparesis; PIG: Post-infectious gastroparesis; GES: Gastric emptying scintigraphy; GCSI: Gastroparesis cardinal symptoms index; GOOSS: Gastric outlet obstruction scoring system; SF36: Short form 36; PAGI-SYM: Patient Assessment of Gastrointestinal Symptoms; N/V: Nausea and vomiting; EndoFLIP: Endoscopic functional luminal imaging probe; CPGAS: Clinical Patient Grading Assessment Score; COPD: Chronic obstructive pulmonary disease.
OUTCOME OF PER ORAL ENDOSCOPIC PYLOROMYOTOMY
Studied population
Since Kawai and colleagues proved that the concept of pyloromyotomy can be performed endoscopically using submucosal technique similar to that of POEM procedure in 2014[42], multiple centers have performed POP for patients with refractory gastroparesis. Most studies have been reported from The United States and France with some reports from Korea, Brazil, Australia, India, Venezuela, Mexico, and Czech Republic[2,37,40,43,45,46,48,49,54-56,58,59]. This may be in part due to high prevalence of the disease with only one approved medication for symptomatic treatment in the United States. However, as more data on its safety and efficacy emerges, it can be anticipated the procedure will become available in other centers with expertise in submucosal endoscopy as well.
Based on an electronic search of PubMed, Medline, Cochrane and Scopus databases for articles containing the terms “Endoscopic pyloromyotomy”, “POP”, or “GPOEM”between January 2013 and September 2018, there have been 13 publications on clinical outcome of POP, including 3 abstract-only publications as described in Table 1. Three case reports were excluded as the data on their clinical outcome was not available[40,54,60]. All published study, except for a study by Jacques et al[61]were retrospective studies.
Across all publications, 291 patients underwent POP[2,37,41,43,45,47,48,55,56,58,59,61,62]. Etiology of gastroparesis were diabetes (n = 69), post-surgery (n = 61), idiopathic (n = 93), postinfection (n = 1), and other causes (n = 10), which included systemic sclerosis, Sjogren syndrome, and Ehlers Danlos syndrome as described in Table 1. The inclusion criteria of all study were similar, which was patients with refractory gastroparesis, except for Two small studies where responses to medical therapy was not mentioned in the inclusion criteria and assumingly all patients with gastroparesis were included[37,41].There were also minor differences in how each group defined “refractory”. While most studies required only presence of symptoms despite dietary and prokinetics treatment, Gonzalez et al[2]and Jacques et al[61]set more strict inclusion criteria. Both studies required persistent gastroparetic symptoms of longer than 6 mo while on medical therapy. Gonzalez et al[2]required gastroparesis cardinal symptoms index(GCSI) of > 1.5 after all prokinetic medications, while Jacques et al[61]required GCSI of> 2.6 after failing at least 2 out of 3 prokinetics. Studies from Dacha et al[43]and Mekaroonkamol et al[56]were conducted by the same group from Emory University,United States. They excluded patients whose predominant symptom was abdominal pain[43,56]. Using the previous pylorus-directed therapy to predict response of POP,Rodriguez et al[48]included only those who had symptomatic improvement after intrapyloric botulinum injection. This heterogeneity among inclusion criteria needs to be considered when comparing outcomes between each study.
Outcome measurements
Despite some difference on baseline characteristics of studied population, the clinical outcomes among published data have consistently suggested high clinical response rate ranging from 69%-100%. However, outcome measurements and follow-up duration are quite heterogeneous across all studies (Table 1). Gastric emptying scintigraphy (GES) was included as one of the parameters to measure clinical success in almost all of the studies even though it may not be the best tool to evaluate clinical success as it has been shown to have poor correlation with clinical symptoms[63-66]. We believe that clinical success of POP in gastroparesis cannot be solely based upon GES.Most studies have also included objective scoring system to track improvement in clinical symptoms. Different symptomatology were evaluated. Khashab et al[45]used patient-report score to evaluate nausea, vomiting, and abdominal pain, while Shlomovitz et al[37]used a questionnaire-based scoring system to evaluate gastroparesis-related symptoms including epigastric burning and pain. Although GCSI was most commonly used scoring system, but other validated tools such as standardized short form 36 (SF36) and Patient Assessment of Gastrointestinal Symptoms (PAGI-SYM) were also used[43,47,48,56,58]. Due to significant heterogeneity in outcome measurements, not all clinical endpoints are comparable across all studies.
Technical success
Although POP is more technically demanding compared to its predecessor POEM procedure due to number of aforementioned reasons, including how well pyloric ring can be identified, complete pyloromyotomy was achieved in all patients reported in published studies (100% technical success rate) with procedural time ranging from 40-120 min[43,45,47,48,51,52]. However, it is noteworthy that only highly skilled endoscopist who had extensive experience with POEM and submucosal endoscopy performed the procedures. This number may also be subjected to reporting bias and publication bias.Trainees participated in a few studies with varying degree of hands-on involvement depending on the endoscopist’s discretion[43,56]. The wide range of procedural time was possibly related to the learning curve of the procedure, difficulty in submucosal tunneling, and time needed to identify pyloric ring.
Clinical success
One of the challenges in evaluating clinical success of any gastroparesis treatment is the lack of validated objective measurements that correlate well with clinical symptoms. GCSI was the main outcome measurement in most studies with other patient-report scoring systems utilized as note above[43,47,48,56,58]. Although recall bias could not be completely eliminated, clinical response rate of POP has been very encouraging with significantly improved symptoms and quality of life ranging from 73%-100% at up to 18-mo follow-up period[37,43,45,47,56]. All studies but one showed significant drop in total GCSI after POP[43,48,61,67]. The symptoms that improve the most were nausea and vomiting, while bloating and abdominal distension were not consistently improved among existing studies[37,43,45,47,48,56,58]. Although Rodriguez et al[48]and Kahaleh et al[58]reported significant improvement in bloating in their patient population after POP, but other studies could not reproduce the results with one study even showed worsened abdominal distension after the procedure[43,45,47,56].
Abdominal pain is a common symptom in gastroparesis with some patients even have pain-predominant disease. However, pain is not one of the cardinal symptoms included in GCSI. Many studies evaluated this component by using indirect surrogate such as PAGI-DYM, SF36, or direct questioning. Improvement in abdominal pain has been reported in 56%-73% of patients after POP but the follow-up period in these studies were only up to 11.5 mo[45,48,58]. One study showed that the improvement in pain did not sustain and lasted for only 6 mo[56]. While Long term data is required to better understand the effect of POP on pain symptoms in gastroparesis.
Improvement in post procedure gastric emptying quantified by GES was included as the only objective parameter to measure clinical success in most studies. A 4-h study with retention percentage of more than 10%-20% at 4-h was generally required.GES was repeated 2-3 mo after POP in most studies. However, there was significant variability in post-procedure improvement in gastric retention. Post procedure GES was completed in as low as 34% to 100% of the studied patients[48,56,61]. Hustak et al[59]and Jacques et al[61]reported GES improvement in 100% patients, while Mekaroonkamol et al[56]reported GES improvement in 78% of study subjects and normalized GES in 48% patients. A few studies reported significant improvement in GES at 2-h retention but statistical significant improvement was not observed at 4-h[2,37,47]. Relatively smaller sample size in these studies may have contributed to this discrepancy. Interestingly, irrespective of the degree of improvement in GES, clinical response measured in terms of GCSI remains > 70% in all studies[2,37,43,47,48,56,61].
Despite the difference in each symptom responses to POP, overall quality of life of the patients with gastroparesis was shown to have improved after the procedure in 70%-78% of the patients[43,47,56]. Out of 8 aspects of quality of life assessed in SF36, the domains showing significant improvement included vitality, general health, social functioning, and metal health In addition, frequency in emergency room visits,gastroparesis-related hospitalization rate, and anti-emetic medication requirement have significantly reduced post-POP, when compared to the control group[43,56]. The mean length of hospital stay was 1-3.3 d[43,45,47,48]. Majority of the patients were able to tolerate oral diet and significantly gained weight[43,45,48].
The physiologic changes in gastric motility that led to these observed differences in clinical responses of each symptom is not yet known. However, it has been hypothesized that the affected location in gastric dysmotiliy may play a role when categorizing gastroparesis into two different subtypes: (1) Fundic-predominant(proximal retention), characterized by bloating, early satiety, and abdominal pain,which are the result of visceral hypersensitivity and impaired relaxation of the fundus post-prandially[68-70]; and (2) Antral-predominant (distal retention) gastroparesis which is a result of pyloric dysfunction and impaired antral contraction causing delayed emptying from distal stomach and manifest with nausea/vomiting. It is expected that gastroparesis with distal retention from pyloric dysfunction would respond better to POP[43,71]. However, differentiating these 2 subtypes objectively remains difficult creating yet another challenge in appropriate patient selection for the procedure.Endoscopic functional luminal imaging probe (EndoFLIP) is a system which was first described in clinical practice in 2007. Impedance planimetry (IP) is a technique which allows to study the relationship of cross sectional area and pressure during volume distention in GI lumen. EndoFLIP uses multi-detector IP system to produce 3-dimentioanl images of any distensible organ within GI tract[72]. EndoFLIP recordings allow dynamic imaging of sphincter distention with a cylindrical balloon of variable diameter with instant cross sectional area measurement along with direct calculations of pyloric sphincter pressures[73]. While EndoFLIP has shown to have widely useful in esophageal disorder, its use across other gastrointestinal motility disorder has been increasing as well[72]. EndoFLIP directed therapy could have a significant role here in future but it currently remains under investigation. Considering these conflicting data on symptomatic response and the fact that most patients with gastroparesis have mixed symptoms, our practice is to advise the patient that not all symptoms will respond equally with nausea/vomiting has more likelihood of improvement than bloating and pain. The decision to proceed with the procedure should always be individualized.
Two cases were reported to undergo subsequent surgical intervention after no response to POP. One had laparoscopic total gastrectomy at 9 mo after POP[48], while the other underwent laparoscopic pyloroplasty 7 mo after the procedure, which also did not yield any significant clinical improvement[37]. The result was not surprising as both interventions offer the same therapeutic mechanism. However, one study reported a repeat POP in a patient who initially improved but had gastroparesis symptoms recurred 24 mo after the index procedure. The patient had significant clinical response even after a repeat pyloromyotomy[62].
Adverse events
Post-procedural hemorrhage, pyloric ulcer, and tension capnoperitoneum have been reported as serious adverse events of POP with complication rate ranging from 0-6.7%[43,45,47,48,58].
Bleeding:Bleeding has been reported as an adverse event by multiple studies[2,49,58,59].All peri-procedural bleeding were controlled endoscopically and/or medically (with proton pump inhibitors) without any further interventions. Many studies have also reported pyloric ulcer after the procedure[37,45,49]. These ulcers at the incision site may be the cause of GI bleeding. Causal relationship was not established between ulcer the source of bleeding in published studies except Hustak et al[59]and Chung et al[49]where bleeding was attributed to the ulcer.
Perforation:Perforation has been reported in a recent study by Jacques et al[61]. While capnoperitoneum/pneumoperitoneum has been reported by a few previous studies.Despite high rate of perforation in animal studies[53,74,75], incidence of procedure-related perforation in humans was rare. This can be explained by the difference in separability of gastric muscle layers between human and porcine model. Recently,Jacques et al[61]reported 20% rate of perforation (4/20 patients, only 1 required surgical intervention, while others were managed conservatively). The reason for such high rate of perforation in this study remains unknown but full-thickness myotomy approach as well as extension of myotomy into duodenal side in a retrograde manner could have contributed. Allemang et al[55]recommends against extending myotomy into duodenal side to minimize risk of perforation. At our center, we performed selective circular pyloromyotomy as the pyloric ring without duodenal extension to minimize the risk of perforation[43,56].
Capnoperitoneum/pneumoperitoneum:In contrast to perforation, capnoperitoneum/pneumoperitoneum is encountered with reported incidence rate ranging from 0-17%[2,32,56]. Most cases are managed with either conservative treatment and resolved on its own. If severe, affecting patient ventilation or hemodynamics, it can be treated with needle decompression as described by Gonzalez et al[2]and Mekaroonkamol et al[56]At our institute, needle decompression kit is in the procedure room and both physician and trainee staff are trained in needle decompression for tension capnoperitoneum if required during or after the procedure. There has not been any significant morbidity or mortality reported till date as a consequence of capnoperitoneum/pneumoperitoneum.
Other reported adverse events:One case of post-procedure pulmonary embolism in the setting of known hypercoagulable state and prior thrombotic event was reported[47]. There was one reported death but it was not procedural-related. Other adverse events included one case of post-procedure dysphagia and one case of pneumonia[37,48]. Regarding infection risk, there was one reported case of peri-gastric intraperitoneal abscess, which was successfully treated with antibiotics alone[2].
Surgical pyloroplasty with or without gastric pacemaker has shown successful outcomes in gastroparesis. A study comparing outcomes of POP with surgical pyloroplasty showed POP has average shorter operative time, less intraprocedural blood loss and less length of stay. Overall complication rates as well as need for post procedure intensive care unit admissions were also significantly lower for POP arm[76].However, data in this area remains limited and till date there is no randomized controlled trial comparing surgical outcomes with endoscopic pyloromyotomy.
Predictive factors
The existing data on safety and efficacy of endoscopic pyloromyotomy in gastroparesis is limited by its small size and the retrospective nature of published studies, making it difficult to determine its validity. There is still significant lack of clarity on selecting appropriate patients for this intervention. At our center, we offer POP to the patients with refractory gastroparesis who have failed or not a candidate for medical treatment (prokinetic agents), who are not on narcotics regularly and who do not have pain predominant disease due to concern for overlapping functional pain,which is unlikely to respond to pylorus-directed therapy.
Multiple clinical parameters were evaluated as potential predictive factors of POP.Gonzalez et al[2]reported diabetes and female gender as predictors of poorer outcomes, but this was not shown in subsequent studies[48,61,62]. Outcomes of POP between diabetic vs non-diabetic gastroparesis remain conflicting. Jacques et al[61]showed favorable outcomes in diabetic gastroparesis post POP with the use of EndoFLIP. Pyloric physiology after POP including pyloric pressure, pyloric distensibility as well as pyloric diameter was shown to have improved more in diabetic patients as compared to non-diabetic cohort[61]. In contrast, Rodriguez et al[48]showed best response of POP were achieved in idiopathic and post-surgical gastroparesis while diabetic gastroparesis patients with advanced macrovascular changes such as nephropathy had worse outcomes. Mekaroonkamol et al[56]performed a comparative analysis between diabetic and non-diabetic cohort. Multivariate linear regression models did not show a significant association between etiology of gastroparesis and clinical improvement, rather there was a significant correlation between the duration of disease and a decrease in GCSI. Exact impact of diabetes and etiology of gastroparesis on outcomes of POP remain unclear at this point
Certain characteristics of the pylorus such as its diameter, cross-sectional area,distensibility, and compliance are known to relate to severity of gastroparesis symptoms[22,23,77]; for example, decreased pyloric diameter and cross-sectional area is associated with post-prandial fullness and early satiety[22]. Such association can explain the clinical response of POP and suggested the possible utility of pyloric measurements as a predicting tool for the procedure.
The factors predicting favorable response to POP remains unknown. Few studies have used EndoFLIP as a surrogate marker to assess pyloric sphincter indices in assessing response to POP. Malik et al[47]showed that while average pyloric pressure decreases, cross-sectional area as well as pyloric diameter increase significantly after POP. The only parameter associated with clinical success was increased crosssectional area after POP, which is consistent with a prior study by the same group that found the cross sectional area to have an inverse correlation with symptoms of gastroparesis[22]. However, only a few patients (4/9) had a complete EndoFLIP measurement in this study. In contrast, the study by Jacques et al[61]showed with EndoFLIP use, increase in pyloric channel diameter and distensibility index was most marked in diabetic patients as well as distensibility index < 9.2 mm2/mmHg was associated with favorable outcomes after POP. Hence, the approach of using pyloric physiologic measurements to predict outcome of POP appear to be physiologically sound, further studies to validate its use are warranted.
As Malik et al[47]showed prior response to intrapyloric botulinum injection can be a good predictor for clinical success after POP, Rodriguez et al[48]took a similar approach by selecting patients for POP based on their response to intrapyloric botulinum injection, which is the least invasive pylorus directed therapy prior to subjecting patients to either endoscopic or surgical pyloroplasty. Although the study had significant improvement in post procedure GCSI and GES, the clinical response rate was similar to other studies where clinical improvement from intrapyloric botulinum injection was not used as an inclusion criteria. There was also no direct comparison of patients who received intrapyloric botulinum injection vs those who didn’t. Hence, it is difficult to draw any clinical conclusion on the benefit of pre-POP botulinum toxin injection, especially when 2 large randomized control trials did not demonstrate the benefit of this intervention in gastroparesis treatment and there was also a theoretical risk of submucosal fibrosis from such injection, potentially complicating a subsequent POP.
Since POP is relatively newer intervention, appropriate learning curve for POP is not yet well defined. Recently study by Suresh et al[78]looked into learning curve for POP and suggested about 18 procedures were required to achieve procedural efficiency (defined as < 60 min) and continued improvement in efficiency as furthermore procedures were performed. However, outcomes of procedure based on endoscopist’s experience were not assessed in this study. When compared to data about learning curve for laparoscopic pyloromyotomy, about 30 cases are required to develop procedural efficiency however outcomes did not differ based on operator’s experience[79].
CONCLUSION
POP has shown a promising outcome as a minimally invasive option for treatment of refractory gastroparesis. In experienced hands in high volume center, it is technically feasible with a low risk of adverse events. It significantly reduces gastroparesis symptoms at least in up to 18-mo period with nausea/vomiting being the mostresponsive symptoms. In addition, it can improve quality of life and reduce hospitalization rate. Predictors of clinical outcomes and utility of pyloric physiologic measurements need to be further investigated. While initial data has shown promising results, future large multicenter trials with sham group comparison will be helpful in further assessing outcomes of POP as a standard of care approach for gastroparesis.
杂志排行
World Journal of Gastroenterology的其它文章
- Current and future pharmacological therapies for managing cirrhosis and its complications
- Dbx2 exhibits a tumor-promoting function in hepatocellular carcinoma cell lines via regulating Shh-Gli1 signaling
- Dynamic changes of key metabolites during liver fibrosis in rats
- Procyanidin B2 protects against diet-induced obesity and nonalcoholic fatty liver disease via the modulation of the gut microbiota in rabbits
- Triggers of histologically suspected drug-induced colitis
- Women on the liver transplantation waitlist are at increased risk of hospitalization compared to men
