传统发酵食品臭鳜鱼中分离的苏云金芽孢杆菌(Bacillus thuringiensis 29.118)的毒理学评价
2019-03-08杨培周朱星星操丽丽程杰顺姜绍通
杨培周,朱星星,操丽丽,郑 志,程杰顺,姜绍通
(合肥工业大学食品与生物工程学院,安徽省农产品精深加工省级实验室,安徽 合肥 230009)
Mandarin fish is a fish of high nutritional value with the features of fast growth, tender texture and less fish bone[1]. It is said that two hundred years ago mandarin fish was stored in wooden barrels by local fishermen along the Yangtze River in Xin’an area of Anhui province and shipped to the Huizhou area for sale. To prevent the spoilage of fish, diluted saline water is sprinkled on the body of mandarin fish. After a longdistance transport, fish gives out a kind of special smell of spoilage. After fried and boiled, the taste of fish is flavorful instead of smelly. Up to now, smelly mandarin fish (named Chinese “Chouguiyu”) has become a famous dish around China as an Anhui cooking style.
The surrounding microorganisms are easy to adhere to the body of fish, a part of which gradually proliferate to form a dominant flora. Recently, the potential safety and hazards of fermented fish products have raised serious concerns in public with traditional manufacture technologies. More reports focus on the specif i c metabolites expressed by fermented organisms[2-3],however, few studies were performed about the safety issue of fermented microorganisms. Actually, most of the microorganisms have two-sided features[4]: beneficial to food[5-6];harmful effects on human, animal, and environment[7].Therefore, the toxicological assessments of microorganisms are necessary to in total evaluate the quality of a product[8].
Our previous report has evaluated the safety of Bacillus cereus isolated from Chouguiyu[9]. In addition, a novel bacterium named as Bacillus thuringiensis 29.118 has been isolated from fermented mandarin fish with the traditional fermentation technology, and the proportion of this strain is less than that of the predominant strain in the fermentative microflora. B. thuringiensis is a strain expressing a pesticidal protein widely used in engineered plants[10],such as caterpillars, Lepidoptera pests, black fly larvae.The insecticidal crystal proteins of B. thuringiensis exhibit biological toxicities (endotoxin, exotoxin, and enterotoxin)against pests[11-12]. The safety issues of engineered plants(corn, tobacco, rice and potato) integrated with encoding gene of ð-endotoxins of B. thuringiensis are still being paid attention to by the government and the public. Furthermore,B. thuringiensis is closely linked with Bacillus cereus and other conditional bacteria[13-14]. Recent studies have found that the exotoxin of B. thuringiensis exhibits a certain toxicity to mammalian[15-16]. These reported toxics of B. thuringiensis to mammals increase the concerns of its food safety. The inf l uence of toxic proteins on mammals has led to more fears in the field of food safety recently. However, whether the B. thuringiensis 29.118 strain as a bacterium is safe to mammals is still unclear. Therefore, the toxicological assessment of the B. thuringiensis 29.118 is essential to scientifically evaluate its food safety. In the present study, a series of evaluations were implemented to test its safety. The findings would provide a scientific reference to consumers and manufacturers by toxicological assessments.Meanwhile, the present study would exhibit an important social significance and an economic value for the healthy development of Chouguiyu.
1 Materials and Methods
1.1 The features of the novel B. thuringiensis 29.118 and culture conditions
Mandarin fi sh for the isolation of strain was located at 29oN and 118oE. After a further confirmation, this strain was named as B. thuringiensis 29.118. The content of B. thuringiensis in all the microorganisms is 7.3%. Compared to the B. thuringiensis model strain from NCBI databases,the 16S ribosomal RNA sequences of B. thuringiensis 29.118 exhibited a maximum homologous value of 95% using an indef i nite tropism. Phylogeny test of minimum evolution tree of the B. thuringiensis 29.118 and other bacteria were shown in Fig. 1.
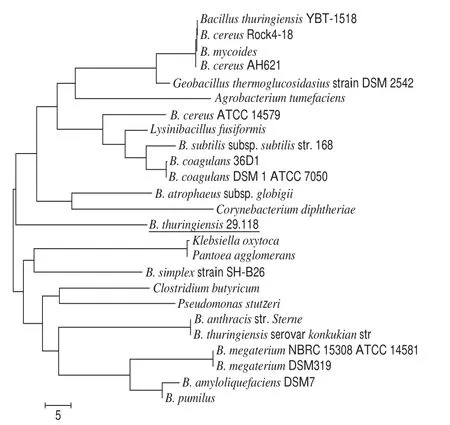
Fig.1 Phylogenetic tree of B. thuringiensis 29.118 and other bacteria based on 16S rRNA sequences
The result showed that B. thuringiensis 29.118 was different from other existing strains at the level of genetics.After investigating its cell morphology, biochemical analysis[17]and molecular identification[18], the isolated strain was confirmed as a novel B. thuringiensis, and this strain was preserved at -80 ℃ in frozen pipes loaded with a cryoprotective agent of 20% (V/V) glycerol. Before use, the strain was inoculated and activated, then the activated strain was cultivated at 37 ℃ in a lysogeny broth with agitation at 180 r/min for 48 h. The cell was collected by a centrifugation speed of 8 000 × g at 4 ℃for 10 min. The dry power of the bacterium cell was obtained through vacuum freeze-drying.
1.2 Main reagents
Salmonella typhimurium strains of TA97, TA98, TA100 and TA102 were provided by Moltox Company (Shanghai,China); the phenobarbital/benzoflavone (10%)-induced rat liver S-9 was provided by Beijing Wise Taikang Biotechnology Co. Ltd.; eosin, hematoxylin and Giemsa dye were provided by Nanjing Jian Cheng Bioengineering Institute; mitomycin C, 4-nitro-o-phenylenediamine was purchased from Shanghai Alfa Aesar Chemical Co. Ltd.;2-aminof l uorene and 1,8-dihydroxyanthraquinone were from Tianjin Kmaels Biological Science and Technology Co. Ltd.;cyclophosphamide was from Shanghai Fortuneibo-Tech Co.Ltd.; the new-born calf serum was obtained from Shanghai Thermo Fisher Scientif i c.
1.3 Experimental animals and feed
With a body weight of (20.0 ± 2.0) g, Kunming mice of specific-pathogen-free (SPF) grade were used. The animal and their feed were from Changzhou Cavins Laboratory Animal Co. Ltd., with a Certificate of Conformity (No.:SCXK 2011-0003). Experimental studies involving mice in this study followed the guidelines established by Guide for the Care and Use of Laboratory Animals (revised by Institute of Laboratory Animal Resources, National Academy of Sciences, Washington D.C. 1996). The animal room was controlled under a given condition at a temperature of(24 ± 2) ℃, a relative humidity of (55 ± 5)%, and a 12 h light/dark cycle. Mice were fed with standard feed and provided drinking water ad libitum during the 1-week acclimatization. The feed was withdrawn 4 h before a test.
1.4 Acute oral toxicity test
Acute oral toxicity of B. thuringiensis 29.118 was evaluated by following the Organization for Economic Cooperation and Development (OECD) Guideline No. 425.Twenty healthy mice were divided into two groups consisting of 10 males and 10 females each. The initial doses were 2 000 mg/kg mb, and the tested group and the control group were respectively treated with the strain and normal saline by oral gavage. The survived mice were continuously observed for two weeks. The daily general behavior was recorded, and the poisoning-related symptoms and the time of death were also recorded in detail. At the end of a test period, all survived mice were sacrif i ced by euthanasia with a concentration of 1%pentobarbital sodium according to a dose of 50 mg/kg mb,and then the bodies were subjected to a gross pathological examination. Median lethal dose (LD50) was calculated by AOT425 StatPgm program.
1.5 Genetic toxicity experiment
1.5.1 Bacterial reverse mutation assay
Four S. typhimurium histidine-deficient test strains were TA97, TA98, TA100, and TA102. The S-9 rat liver homogenate was used for the metabolic activation system.Bacterial reverse mutation assay was conducted in accordance with the methodology provided by OECD Guideline No. 471.Five doses (8, 40, 200, 1 000, and 5 000 μg per plate) were tested based on the result of an acute oral toxicological test.The untreated control, solvent control (sterile water), and positive control were prepared concurrently. S. typhimurium and B. thuringiensis were incubated with or without an S-9 mixture at 37 ℃ for 48 h. The tested strain-specif i c positive controls were 4-nitro-o-phenylenediamine for TA97 and TA98 without S-9, sodium azide for TA100, mitomycin C for TA102, and 2-aminofluorene for TA97, TA98, and TA100 with S-9, and 1,8-dihydroxyanthraquinone for TA102 without S-9 at 20, 1.5, 2.5, 10, 50 μg per plate, respectively[19-20].The positive results were determined as a 2-fold increase compared to the solvent control or a dose-dependent increase in revertant colony counts.
1.5.2 Bone marrow cell micronucleus assay
Bone marrow cell micronucleus assay was performed in accordance with OECD Guideline No. 474. Fifty mice were randomly divided into five groups: low dose(500 mg/kg mb), middle dose (1 000 mg/kg mb), high dose(2 000 mg/kg mb), the positive control (cyclophosphamide,40 mg/kg mb) and the negative control (normal saline). Each group contained 10 mice with half males and half females.The mice were orally administered using the sample twice within a 24 h interval, and sacrif i ced by euthanasia 6 h after the last treatment. After clamping abdominal fur, stripping of the femurs, cutting off one end of the femur, fl ushing slowly with a newborn calf serum of 1 mL, mixing the solutions evenly, the cell suspension was obtained. One drop of this cell suspension was smeared on a clean slide, air-dried, fi xed with 95% methanol, and then stained with 5% Giemsa. One thousand polychromatic erythrocytes (PCEs) were examined,and the number of observed micronuclei in the PCEs was recorded for each mouse by using an optical microscope. The proportion of micronuclei was expressed as a per mille (-).
1.5.3 Sperm deformity test
The sperm deformity test was performed according to the previous report[21]. Twenty-f i ve adult male mice were in total used in this test. The design and dosage were the same as above. The mice were treated once daily for consecutive 5 days, then were sacrif i ced by euthanasia on day 30 after the last treatment. The mice were dissected, gently stripped of the bilateral epididymis, cleaned adipose tissue, cut into pieces in 1 mL normal saline, and then filtered by lens-wipingpapers. One drop of sperm suspension was smeared on a clean slide. After air-dried, fi xed with methanol and stained with 2% eosin, the sample was microscopically examined.The spermatozoa with morphological abnormalities were identified from 1 000 sperms of each animal. The rate of malformation was calculated and expressed as a percentage.The negative control and positive control were investigated by using normal saline and 40 mg/kg mbcyclophosphamide,respectively.
1.6 Thirty-day feed test
Forty mice were randomly divided into four groups of 5 males and 5 females each. The control group was administered normal saline, while the doses of three treatment groups were 500, 1 000, and 2 000 mg/kg mbonce daily for 30 days. During the period of treatment,the daily behavior, daily and weekly body weights were recorded. After treatment, the mice were put in a cage with food provided for 16 h with free access to water intake,anesthetized with 1% sodium pentobarbital solution, then blood samples were quickly collected from the tail veins and placed in coagulating tubes. After the mice were sacrif i ced,both organs and tissues were harvested and microscopically observed. The liver tissue and colon tissue were isolated and soaked into 10% neutral formalin, embedded in paraff i n wax,cut into 5 μm sections, stained with hematoxylin and eosin(H&E), and examined microscopically[22]. The blood was centrifuged to obtain the serum at a speed of 2 120 × g at 4 ℃ for 10 min. A concentration of 10% liver homogenate was prepared and centrifuged to obtain the supernatant fraction with a centrifugation speed of 2 120 × g at 0 ℃ for 10 min. Serum analyses were performed to measure aspartate aminotransferase (AST), alanine aminotransferase (ALT),fasting glucose (GLU), triglyceride (TG) and blood urea nitrogen (BUN) using specif i c kits. The process was abided by the manufacturer’s instructions. The liver homogenate concentrations of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and malondialdehyde (MDA) were also determined.
1.7 Statistical analysis
The results were expressed as the mean ± standard deviation. All the data were analyzed by using the statistical package for the social sciences (SPSS) version 17.0 software.A single factor t-test was used to determine the difference between groups. P < 0.05 and P < 0.01 meant the levels of signif i cance and extreme signif i cance.
2 Results and Analysis
2.1 Acute oral toxicity
As an efficient technique to assess the identification of hazard and the management of risk, acute oral toxicity offers insight into the mechanisms of action by obtaining information on the biological activity of a specif i c chemical.In the present study, the symptom of action retardation with a 12 h administration was observed in a male mouse treated with a dose of 3 000 mg/kg mb. Two male mice showed the formation of eye secretions after a 24 h administration.After a 48 h administration, the female mice treated with a dose of 4 000 mg/kg mbshowed a decrease of the intake amount of food. The poisoned symptoms of the necropsy of mice were distinguished according to the pathematology.Both the slight swelling and congestion occurred in the intestinal mucosa, liver and kidney. Atrophy occurred in the thoracic gland. However, all the mice survived at a dose of 2 000 mg/kg mbafter 14-day of acute oral toxicity. There was no visible abnormality in the visceral organs of mice necropsies. The results of the analysis indicated that the dose of 2 000 mg/kg mbcould not cause distinct poisoned symptoms of mice.
The oral LD50of mice was higher than 4 000 mg/kg mbwith B. thuringiensis 29.118 strain as the material. The results indicated that the dose of 2 000 mg/kg mbdid not cause acute oral toxicity in mice. The LD50value leads to death in 50% of the investigated animals in a given period, which is regarded as the basis of the chemical toxic determination. However,LD50has its limitation, which is not absolutely suitable for all the specific compounds in terms of accuracy, and this parameter ignores the clinical symptoms. For acute oral toxicity related to food, the clinical symptoms directly express the action effect of intake. Therefore, in the present study,clinical symptoms were also observed as important references.
2.2 Bacterial reverse mutation assay
The toxicity assessments of B. thuringiensis for mammals have aroused great public concerns recently. The bacterial reverse mutation assay is used to assess the safety of specific chemicals as an effective method of mutagenic evaluation. In the present study, the mutagenic effects of the B. thuringiensis 29.118 were evaluated by the bacterial reverse mutation assays of four S. typhimurium strains(TA97, TA98, TA100 and TA102). The experimental results showed that no distinct evidence of mutagenicity was found; compared to the solvent control, there were no signif i cant differences in the numbers of revertants with four S. typhimurium strains following treatment with concentration gradients (8, 40, 200, 1 000 and 5 000 μg per plate) (Table 1).The experiment of positive control meant the tested results were reliable, and the given gradients of concentration could be confirmed that no mutagenic evidence was found using the bacterial reverse mutational assay. Each group with and without S-9 rat liver had signif i cant differences compared to the positive chemical.
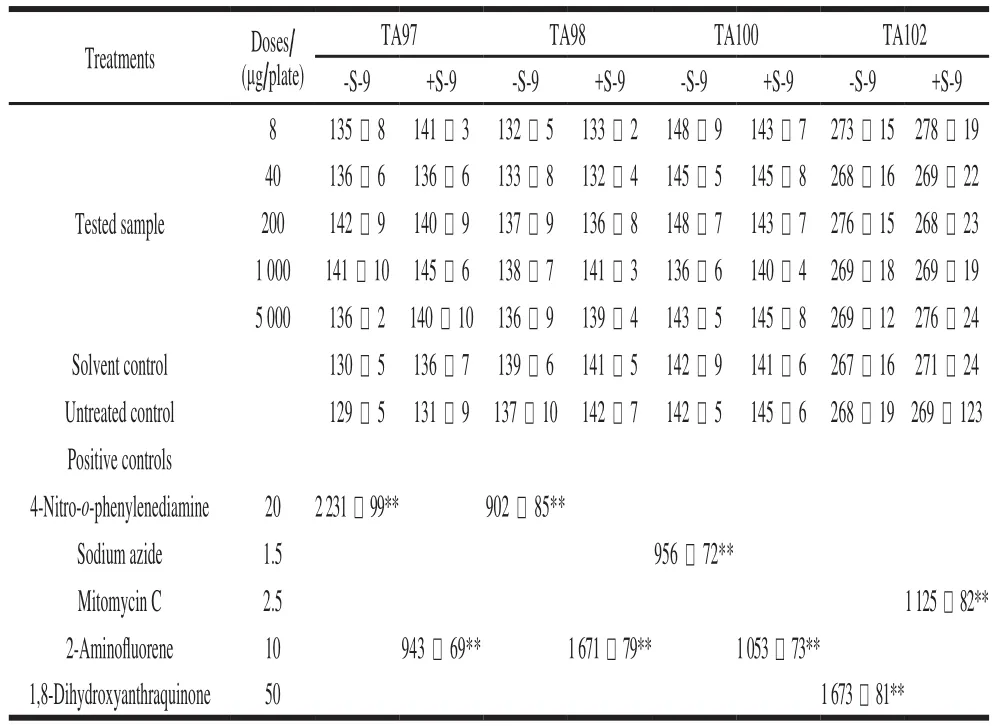
Table1 Toxicity assessment of B. thuringiensis 29.118 to mice by bacterial reverse mutation assay
2.3 Bone marrow cell micronucleus assay
To determine the extent of chromosomal damage in vivo,bone marrow cell micronucleus assay was used to evaluate the safety of B. thuringiensis 29.118 on mice. Compared to the negative control, there was no significant difference in the micronucleus rate among the treatment groups (P >0.05) (Table 2). Both the male mice and the female mice exhibited the same trend based on the analysis of difference.The difference of micronucleus rate between positive control and low-dose existed statistically significant (P < 0.01).No significant difference was found among the low-dose,medium-dose and high-dose.
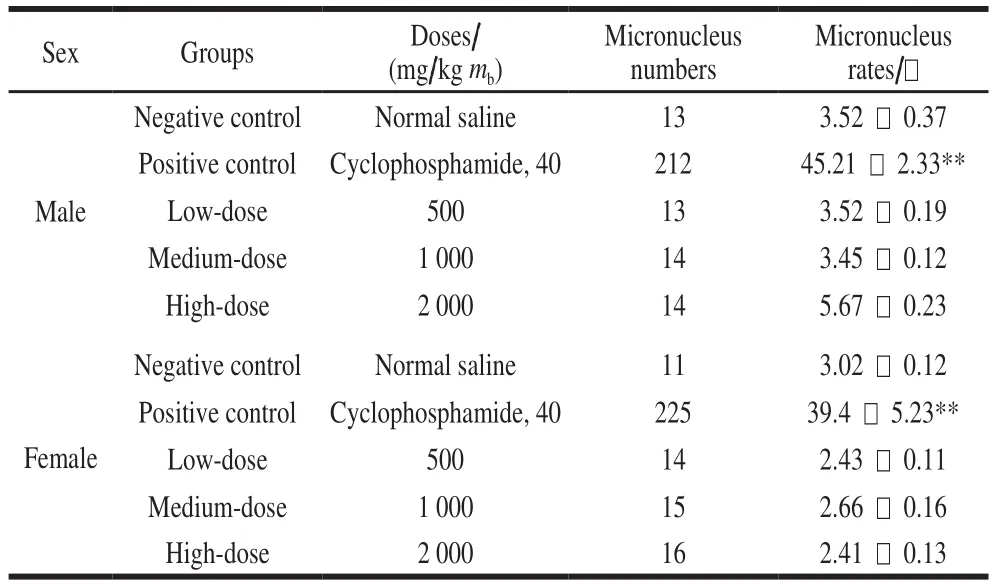
Table2 Toxicity assessment of B. thuringiensis 29.118 to mice by bone marrow micronucleus assay
2.4 Sperm deformity test
In this study, sperm abnormality test was used to investigate that whether B. thuringiensis 29.118 had a reproductive toxicity to the male mice. The head of sperm changed into a red color by dyeing with a concentration of 2% eosin. The result showed different types of sperm deformity including normal sperm, non-hook sperm, chubby head sperm, banana-shaped sperm (Fig. 2) were distinguished according to their shapes. The statistic results of malformation types and numbers were shown in Table 3. The percentages of malformation of the control, low-dose, medium-dose,high-dose, and the positive respectively were 6.9, 7.2, 8.5, 7.9 and 32.3. The sperm deformity rate was signif i cantly different between positive control and negative control (P < 0.01).There was no significant difference between the treatment and the control groups (P > 0.05). Therefore, the tested B. thuringiensis did not lead to the deformity of sperm under the given conditions at the level of statistics.
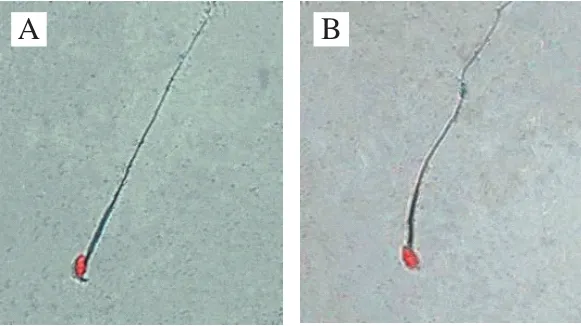
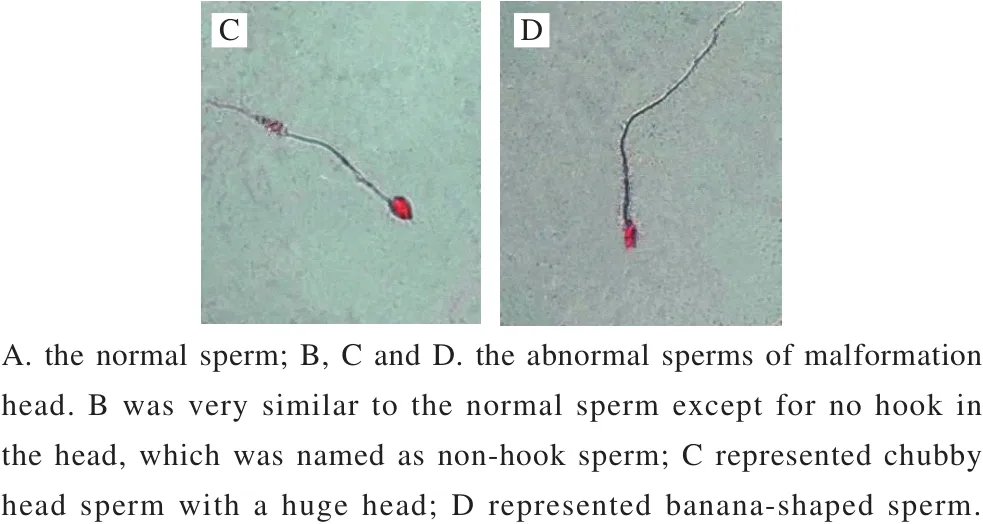
Fig.2 Morphological observation of sperms of mice (500 ×)
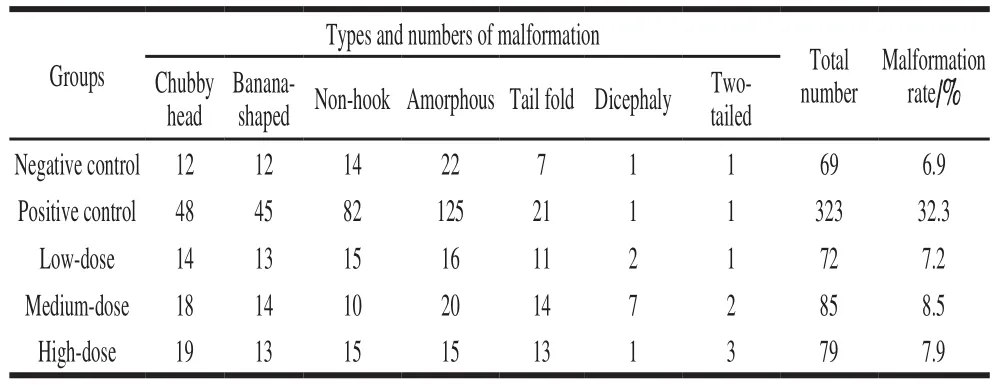
Table3 Sperm deformity in mice caused by B. thuringiensis 29.118
2.5 Thirty-day feed test
All the mice survived the 30-day feed test, and no distinct clinical signs of toxicity were found. Initial weights of both male mice and female mice were about 22 g. The fi nal weights of the control and three treatment groups reached 38 g after a 4-week feed. The body weight of the treatment mice did not show significant differences compared to the control. The growth and development of mice for all the given doses showed well (Table 4) (P > 0.05). Seven items of serum (AST, ALT, GLU, TG, T-SOD, GSH-Px and BUN)and three liver homogenate items (GSH-Px, T-SOD and MDA) were measured to analyze the biochemical indexes.There were no signif i cant differences between the treatment and the control for the tested parameters (P > 0.05). The cell injury of the liver was observed by H&E staining. The homogenate MDA of liver from the high-dose group was significantly different compared to the control (P < 0.05).The sections of the liver from the control indicated that the healthy liver cell had the basic features of intact organization structure, clear cell boundary and the bright dyeing. No distinct clinical symptoms of toxicity were found after 30 days feed, and no pathological symptoms were also found in the autopsy. The cells of degeneration and necrotic inf l ammation were not observed (Fig. 3A). The dose resulted in the formation of hemocyte cell on the wall of hepatic sinusoid. Distinct space was found among the cells from high-dose groups (Fig. 3B).Therefore, the cell walls of all three treatment groups still kept intact, and the signif i cant pathological symptoms are not found.
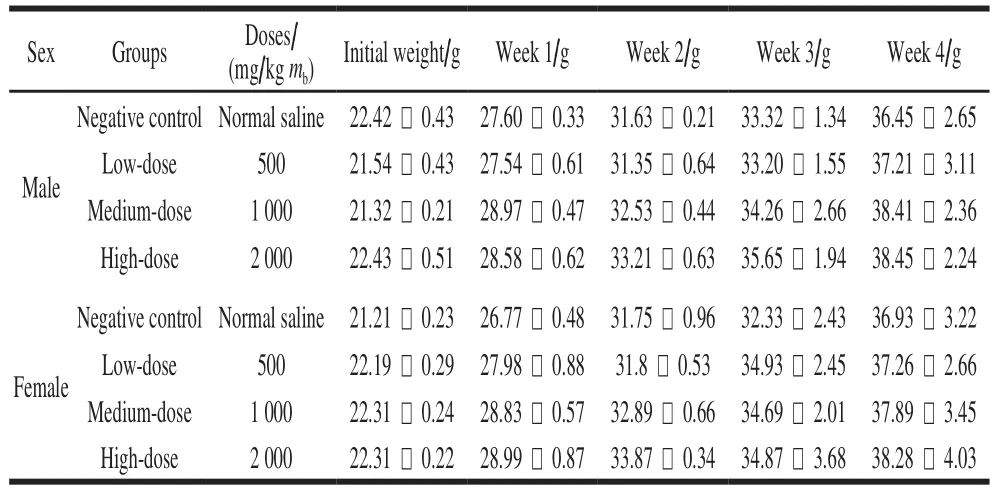
Table4 Body mass gain in mice after 30 days of feeding
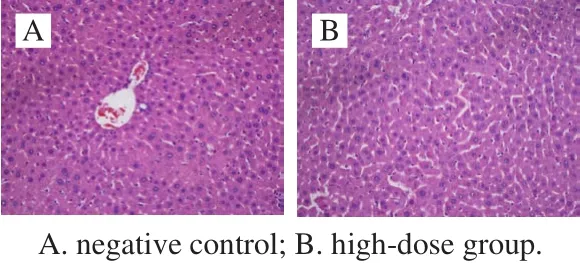
Fig.3 Histological micrographs of liver tissue sections after 30 days of feeding (200 ×)
2.6 Micro-structural observation of the colon
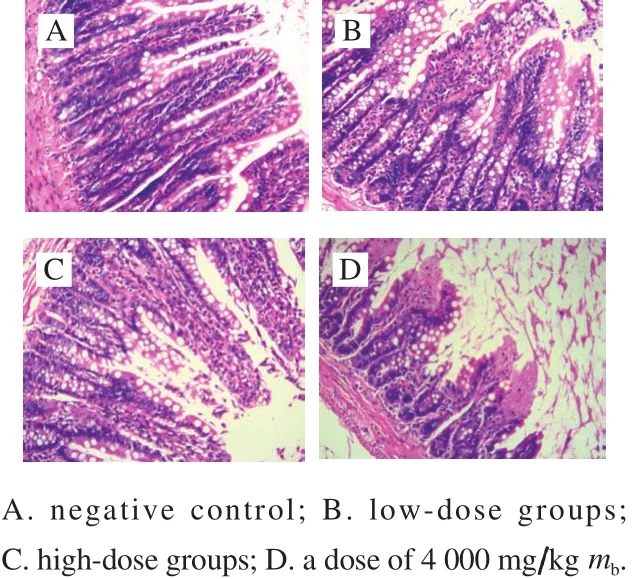
Fig.4 Histological micrographs of colon tissue sections (100 ×)
The tissue slices of colon with H&E staining were used to investigate whether there existed the pathologic symptoms. The intestinal mucosa structures of colon from the control group and the treatment groups were observed under a microscope with an amplification of 100-fold(Fig. 4). No distinct pathological symptoms were found in the colon tissues of mice with dosages of 500 and 2 000 mg/kg mb(Fig. 4B, C), and the muscular coat and mucosal structure of each group still kept intact. The intestinal glands were arranged neatly, and the colonic intestinal epithelial cells were also intact. The structures of low dose groups and high dose groups were similar to the control. However, a dose of 4 000 mg/kg mbled to significant change of the structures of colonic intestinal epithelial cells (Fig. 4D). The results indicated that with the doses of 500 and 2 000 mg/kg mb,all the intestinal mucosa of the treatment groups kept intact.There was no signif i cant difference in the thickness of colonic mucosal between the control and the treatments. Therefore,B. thuringiensis 29.118 had no significant damage to the colon of mice with a dose of 2 000 mg/kg mb.
3 Discussion
The toxicological extents of B. thuringiensis mainly reflect in two different levels: the toxicity of dosage and toxicological crystal proteins produced by B. thuringiensis.For the toxicity of dosage, previous reports indicated that B. thuringiensis did not cause the pathopoiesis under control of specific strains and given doses. A new formulation of B. thuringiensis SH-14 exhibited no acute toxicity via dermal route, low eye irritation, no symptom of dermal irritation or hypersensitivity to tested animals[23]. The dosages of 2 500, 5 000 and 7 500 mg/kg mbof B. thuringiensis Vip3Aa16 toxin did not cause the health risk to animal. The concentrations of 106and 108spores/mL of B. thuringiensis did not result in the lethality of mice[21]. The present study also showed the similar results in lethality and effects on the mouse bone marrow by B. thuringiensis. The high dosage intake of the B. thuringiensis with a concentration of 4 000 mg/kg mbled to clinical adverse symptoms. At a low dosage of 2 000 mg/kg mbor less, this strain isolated from Chouguiyu did not result in a distinct harm to the tested mice.The dosage of intake should be a key factor inf l uencing the health of mice. In practice, the B. thuringiensis dosages of intake by mammals are far below the dose used in this study.Therefore, the B. thuringiensis 29.118 from Chouguiyu was regarded as safety to the consumer.
About toxicological crystal proteins, more investigations focused on the mechanisms of its action. The toxicity of B. thuringiensis mainly depends on the insecticidal function of spore-crystal proteins[24-25], and these proteins highly restrain the cellular and humoral components of immune systems.The humoral defense system is blocked by both inhibitor A and inhibitor B. Furthermore, the response and defense of crystal protein of B. thuringiensis are regulated by p38 MAP kinase pathway. Nevertheless, not all the crystal proteins for insecticides are toxic to mammals. Cry1C protein expressed by B. thuringiensis is considered to be one of main toxic components as an insecticide, nevertheless, Cry1C protein is not a source as a potential allergen or toxin in vitro[1].The present study showed that all the proteins expressed by B. thuringiensis 29.118 did not cause distinct symptoms of pathopoiesia. Therefore, the expression of sporecrystal protein should be one of key directions in further investigating B. thuringiensis 29.118.
B. thuringiens is regarded as a pathogen with an appropriate condition to produce enterotoxins, emetic toxins,and other biological toxins. In addition, this strain could lead to diarrhea, vomiting and other diseases[26]. Nevertheless,no vomiting, diarrhea, high body temperature, or death were observed in any of the mice in the present study. This may be attributed to less expression of biological toxins by the B. thuringiensis. The production of biological toxins by this organism is severely inhibited under adverse conditions. The process of Chouguiyu product is fermented in the condition of high humidity, no starch, no sugar[27]and low dissolved oxygen[28]. Therefore, the expression of toxin was inhibited under the condition of anaerobic fermentation.
Generally, the assessment indexes of food safety at least include acute oral toxicity, genetic toxicity, thirty-day feed test, etc. The assessments of food safety for B. thuringiensis lack the detailed and systemic investigations[29]. Most reports focused on the specif i c issue of B. thuringiensis toxics, such as the cytotoxicity, hematotoxicity and genotoxicity[30],acute toxicity and cytotoxicity[21], the genotoxicity of B. thuringiensis spore-crystal Cry1Ac[31]. In the present study, all the acute oral toxicity, genetic toxicity, thirty-day feed test and the microstructure of colon were investigated to in total assess the toxicology of B. thuringiensis 29.118.Therefore, the comprehensive assessments in this study help to the scientif i c evaluation of food safety.
4 Conclusions
A dose of 4 000 mg/kg mbcauses distinct poisoned symptoms, acute oral toxicity and the damage to colonic intestinal epithelial cells, however, a dose of 2 000 mg/kg mbdid not cause the symptom of acute oral toxicity. With a dose of 2 000 mg/kg mb, the studies of genetic toxicity showed that the B. thuringiensis 29.118 had no genetic toxicity to the mice, all the mice grew and developed well after 30 days feed, and no distinct differences were found among the seven serum items and three liver homogenate items, no distinct pathological symptoms existed in the liver tissues and colon tissues. The given doses of 2 000 mg/kg mbfor B. thuringiensis 29.118 are much higher than those of the surrounding environment. Therefore, the investigated results indicated that the isolated strain B. thuringiensis 29.118 was regarded to be safe.
