Overview of Physical and Chemical, Operational Properties of Nitrous Oxide Used as a Propellant for Low-thrust Rocket Engines
2019-03-04Boryaev
A. A. Boryaev
(Saint Petersburg State University of Architecture and Civil Engineering, 4 Vtoraya Krasnoarmeyskaya Str., Saint Petersburg, Russia, 190005)
Abstract: The results of a system analysis of the efficiency of nitrous oxide (N2O) as a propellant component for small space vehicles (SSV) were presented. A criterion for mass efficiency of the SSV propulsion system (PS) is determined. The current global state-of-the-art of SSV PSs is shown. The application field of nitrous oxide in SSV PSs is calculated and mass efficiency of N2O application is quantitatively determined. An overview of physical and chemical as well as operational properties of nitrous oxide as a promising, non-toxic component of rocket propellant is provided. Main physical and chemical constants of gaseous and liquid nitrous oxide; chemical properties of N2O, thermal stability of N2O, catalytic decomposition of N2O, a mechanism of decomposition of N2O, catalysts for decomposition of N2O, ballast additives to N2O, application of nitrous oxide, nitrous oxide as a rocket propellant, production of nitrous oxide, toxicity of nitrous oxide, fire hazard of N2O, requirements to equipment when handling N2O; storage and transportation of N2O are considered. It is demonstrated that nitrous oxide is a chemical compound meeting the requirements to rocket propellants, including those related to the environmental friendliness of propellants. With 75 references.
Keywords: rocket propellant; nitrous oxide; physical and chemical properties
Introduction
Nitrous oxide was first synthesized in 1793. Despite its well-studied physical and chemical properties, it was not made use of for a long time. Only 100 years after the discovery, nitrous oxide was gradually put into practice in medicine due to its anesthetic properties.
During the rapid development of rocket and space technology in the second half of the past century, the attention of experts of the field was drawn to highly energetic properties of nitrous oxide, its ability to show strong oxidizing properties at high temperatures and decompose into nitrogen and oxygen at a ratio of 2∶1 which is close to the air composition. Those properties made it possible to recommend nitrous oxide as a monopropellant which could be used as a basis for designing monopropellant and bipropellant low-thrust rocket engines for the system of control factors in space vehicles (SVs), as well as gas generators ensuring life support on board an SV[1-5].
Due to toughening requirements to the environment friendliness of rocket propellants, nitrous oxide gains attention as an environmentally-friendly ("green") propellant.
1 A system analysis of nitrous oxide efficiency
The state-of-the-art of space technology imposes significant restrictions on mass and size, as well as dynamic and specific characteristics of propulsion systems (PSs) in small space vehicles (SSVs)[6-7]. Some of those requirements are presented in Table 1.

Table 1 General requirements to SSVs
Currently, SSVs of the Micro class are the most popular. In order to increase mass efficiency, reduce the cost of designing and testing PSs of such SSVs, a number of foreign companies performed researches of promising propellant components and new designs[8].
One of the stated focus areas[7, 9]is design of PSs using nitrous oxide (N2O). Nitrous oxide as a propellant component is widely used by XCOR Aerospace[10]. Since 2000, at the request of NASA and DARPA, XCOR Aerospace together with ATK GASL have developed and tested more than 10 designs of liquid rocket engines (LREs) with the thrust range of 10-2000N using N2O as an oxidant.
According to the statistical data shown in Figure 1, the proportion of PS dry mass (PS mass without fuel) increases with a decrease in sizes of SSV[9]. The data shown in Figure 1 demonstrate that for vehicles weighing less than 100kg this effect results in a significant reduction in mass energy efficiency. The generalized criterion of SSV PS mass efficiency (energy output) is a ratio of the total momentum generated by the PS to the sum of the masses—the dry mass of the PS plus the mass of the fuel.

Fig.1 Proportion of dry mass regarding SVs of various purpose
One of the main reasons for increase in PS dry mass with a decrease in SSV linear dimensions is the fact that the thickness of structural elements and dimensions of units remain constant and are not subject to further scaling when reaching the minimum, technologically possible limit value.
Some increase in the mass efficiency parameter can be achieved using high-energy monopropellants or bipropellants of high density. However, in most cases, their application requires the availability of mass-consuming propellant pressurization and storage systems, introduction of devices for operation initiation. Moreover, designing a rocket engine (RE) with an efficient engine cycle at a rated thrust less than 0.5N requires solving many technical issues which results in an increase in the structure mass.
According to the literature analysis, starting from 1970[8, 11], a quite definite trend (Figure 2) characterizing changes in mass energy efficiency of PSs in SSV for various purpose can be revealed. The analysis included SSVs weighing up to 500kg with a reference speed of up to 100m/s. The reference speed of a rocket (ideal speed) is a speed that can be reached in vacuum by a rocket moving rectilinearly under the action of engine thrust only.
According to several works[8, 11], for an SSV weighing 1-100kg with combined PSs including LREs or SREs (solid rocket engine), it is possible to plot an average diagram (Figure 3) characterizing the achieved global state-of-the-art mass efficiency indicator at various SSV reference speeds.
Figure 2 shows a significant decrease in the growth rate of mass energy efficiency of SSV PSs starting from the 1990s. It should be noted that the PS mass efficiency value in modern SSVs does not exceed 250m/s (Figure 3).

Fig.2 Values of mass energy efficiency for SSVs for the period of 1970-2010

Fig.3 Mass energy efficiency of PSs of SSVs weighing 10-20kg
For further increase of the mass efficiency value, it is necessary not only to introduce promising propellant components but to implement designs allowing significantly reducing dry mass of the SSV PS.
As mentioned above, one of the ways to increase mass energy efficiency of the SSV PS is the use of nitrous oxide. The most important advantages of N2O are its ability to decompose into free oxygen and nitrogen with a release of thermal energy in the amount of 82kJ/mol, the ability to be stored in the liquefied state, simplification of the feeding system due to the effect of self-pressurization by own saturated vapors with a pressure more than 4MPa at 290K, as well as non-toxicity.
The above properties of nitrous oxide allow designing an SSV PS using this propellant component, combining maintainability and operability, reliability and variety of operating conditions. Some options of SSV PS designs are shown in Figure 4.
Such PSs consist of sustainer engines with thrustFs=5-500N and control engines with thrustFc<1N. The considered designs have cold gas thrusters as control engines, while a monopropellant RE with catalytic decomposition of nitrous oxide, a hybrid RE (HRE) or a liquid RE (LRE) may serve as sustainer engines. Several works[9, 12-13]showed the possibility of designing monopropellant and bipropellant LREs satisfying the requirements imposed on SSV PS components with regard to thrust, switching frequency and duty ratio (pulse mode), durability, afteraction impulse and environmental friendliness.

Fig.4 PS designs using nitrous oxide with a monopropellant rocket engine (RE) (a), hybrid rocket engine (HRE) (b) or liquid rocket engine (LRE) (c) as a sustainer engine
The reference[12]determines the areas of the efficient application of nitrous oxide systems in SSV PSs (with regard to the SSV Micro class) and includes a comparison of the above PS designs using nitrous oxide (Figure 4) with analogues using hydrazine (Figure 5(a)), gaseous O2/H2(Figure 5(b)), unsymmetrical dimethylhydrazine (UDMH)/nitrogen tetroxide (N2O4) (Figure 5(c)) and cold gas thrusters (Figure 5(d)) by the value of mass efficiency. The analysis was carried out at given values of thrust of sustainer REs (Fs=50N) and control REs (Fc=0.5N). The SSV mass rangeMSV=10-100kg with total PS impulse of It=10-5000N·s was accepted as a parametric area under study. It is understood that 60% of total impulse is accounted for by sustainer REs and 40%— by control REs.
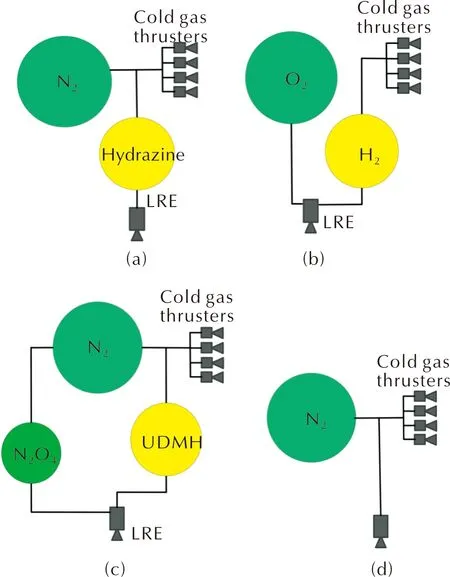
Fig.5 PS designs using hydrazine (a), gaseous oxygen and hydrogen (b), unsymmetrical dimethylhydrazine (UDMH) and nitrogen tetroxide (N2O4) (c) "cold gas" (d)
Mass efficiency was estimated in accordance with reference[14]. The propellant mass was determined through the value of the specific impulse of the sustainer RE and specific impulse of the control RE. The PS mass was estimated as the sum of PS component masses. The PS design units were selected with account for the existing and prospective designs[14]. The mass of each PS unit complies with the nomenclature of PS components manufactured in the Russian Federation[15-17]. The RE specific impulse was selected in accordance with Table 2[6].

Table 2 RE specific impulse
The calculations allowed to define the values of mass energy efficiency depending on the SSV PS total impulse (Figure 6).
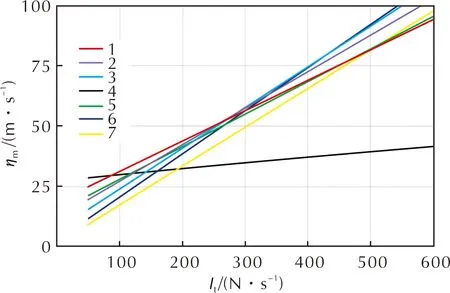
Fig.6 Dependence of the mass efficiency value on the total PS impulse for options using: monopropellant RE using N2O (1), HRE using butadiene propellant and N2O (2), LRE using ethanol and N2O (3), cold gas thruster (4), monopropellant RE using hydrazine (5), RE using gaseous H2 and O2 (6), N2O4/UDMH (7)
The calculation data shown in Figure 6 demonstrate that the application of a PS using nitrous oxide allows reducing the SSV mass if the range of values of the total PS impulse is 120-470N·s. AtIt<260m/s, the application of a PS with a monopropellant sustainer RE using N2O, and, atIt>260m/s, the application of a PS with a bipropellant LRE using N2O as an oxidant become more expedient. According to the calculation methods, the use of nitrous oxide makes it possible to reduce the SSV PS mass by 10%-15% relative to PSs of currently applied conventional designs.
It should be recognized that the above calculation is estimative. However, it allows concluding that in some cases the application of a RE using nitrous oxide provides a considerable positive effect. An analysis and evaluation of physical and chemical as well as operational properties of nitrous oxide, presented in the overview, will allow determining its advantages to the fullest extent possible when designing rocket engines and gas generators of various purposes for space technology.
2 Physical and chemical properties of nitrous oxide as a propellant for low-thrust rocket engines
In normal conditions, nitrous oxide is a colorless gas with a specific pleasant slight odor and sweet taste. It can be liquefied under pressure and stored in cylinders as a liquid.
Gross formula, N2O; molecular mass, 44.01;Molecular volume at 0℃ and 0.1MPa, 22.25L.
Nitrous oxide molecules are low-polar (dipole moment μ=0.17) and have a linear, asymmetric structure: N≡NO.
Bond lengths in a molecule of nitrous oxide:N—N , 0.1125nm; N—O,0.1186nm.
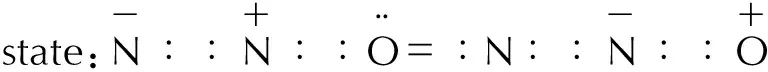
The ionization potential of N2O is 12.6eV.
The force constants of valence bond are: 18.2(N≡N) and 12.0(NO).
In a free state, nitrous oxide is a constant component of air (0.00005 volume %).
Physical and chemical properties of nitrous oxide have been studied fairly well; its physical and chemical constants are published in various literature sources[18-25]. According to their analysis, a spread in the same values is observed, especially in the temperature range that is of greatest interest for space technology. The possible reason for such spread in values is related to different measurement and calculation methods. Some physical and chemical constants of nitrous oxide are shown below.
2.1 Basic physical constants of liquid and gaseous nitrous oxide
Thermophysical properties:boiling point,184.7K (-88.7℃);melting point (freezing point) 182.3K (-90.7℃).
Critical parameters:temperature,309.6K (36.6℃); pressure,7.25MPa (7.17MPa); density,454kg/m3; volume,0.0973dm3/mol.
Triple point parameters:pressure,87.9kPa (658.9mm Hg); temperature,182.4K (-90.6℃).
Heat of:
gas-phase formation at standard conditions (enthalpy):at 0K(-273℃),85.029kJ/mol;at 289.18K(16.18℃),81.6kJ/mol;decomposition (standard) at 298℃,81.5kJ/mol; melting,6.54kJ/mol; evaporation,16.55kJ/mol.
evaporation of liquid nitrous oxide at saturation line at various temperatures:
atT=260K(-13℃),11.58kJ/mol;
atT=295K (+22℃),7.30kJ/mol;
atT=305K (+32℃),4.75kJ/mol.
Entropy at 298K(25℃) at standard conditions,219.90J/(mol·K)
Thermal capacity
atT=273K (0℃) andP=0.1MPa (1kgf/cm2):liquid,111.3J/(mol·K); gaseous,37.4J/(mol·K);
at 298K (25℃) at standard conditions ,8.63J/(mol·K).
In reference[26], enthalpy and entropy were estimated using the values of thermal capacity of solid nitrous oxide.
Thermal conductivity coefficient of liquid (λl, W/m·K) and gaseous (λg, W/m·K) nitrous oxide at saturation line at various temperatures:
atT=260K (-13℃),λl=0.1200,λg=0.0176;
atT=295K (+22℃),λl=0.1276,λg=0.0230;
atT=305K (+32℃),λl=0.2341,λg=0.0290.
Density and viscosity of nitrous oxide:
Density
gaseous: atT=273K (0℃) andP=0.1MPa (1kgf/cm2) -1977.8kg/m3;
liquid:atT=253K (-20℃),1000kg/m3;
at T=293K (20℃),784kg/m3;
at gas-liquid solubility line at 25℃:gas, 0.18g/cm3;liquid, 0.75g/cm3.
Dynamic viscosity coefficient of the liquid(ηl×10-4Pa·s) and gaseous (ηg×10-4Pa·s) phases and kinematic viscosity coefficient of the liquid (λl×10-6m2/s) phase at saturation line at various temperatures:
atT=260K (-13℃),ηl—0.8565;ηg—0.1472;λl—0.0875;
atT=295K (+22℃),ηl—0.4985;ηg—0.2043;λl—0.0639;
atT=305K (+32℃),ηl—0.3812;ηg—0.2554;λl—0.0569.
Vapor pressure atT=290K (17℃),4.71MPa (48.02kgf/cm2).
Vapor pressure over liquid N2O:
at 0℃,30.3×105Pa;
at 18℃,49.5×105Pa;
at 35.4℃,75.0×105Pa.
A dependence of saturated vapor pressure (PS, MPa) and density of the liquid (ρl, kg/m3) and gaseous (ρg, kg/m3) phases at saturation line on temperature (T, K) at various pressures is shown in Tables 3 and 4.

Table 3 A dependence of saturated vapor pressure (PS),density of the liquid (ρl) and gaseous (ρg) phases of nitrous oxide at saturation line on temperature
According to the presented data, with an increase in temperature even in such narrow range (2-32℃), saturated vapor pressure and density of the gaseous phase increase sharply, and density of liquid nitrous oxide at saturation line decreases sharply.
Density of nitrous oxide decreases with an increase in temperature from 27 to 727℃ in the pressure range from 0.1 to 20 MPa; and with an increase in pressure this manifests more intensely.
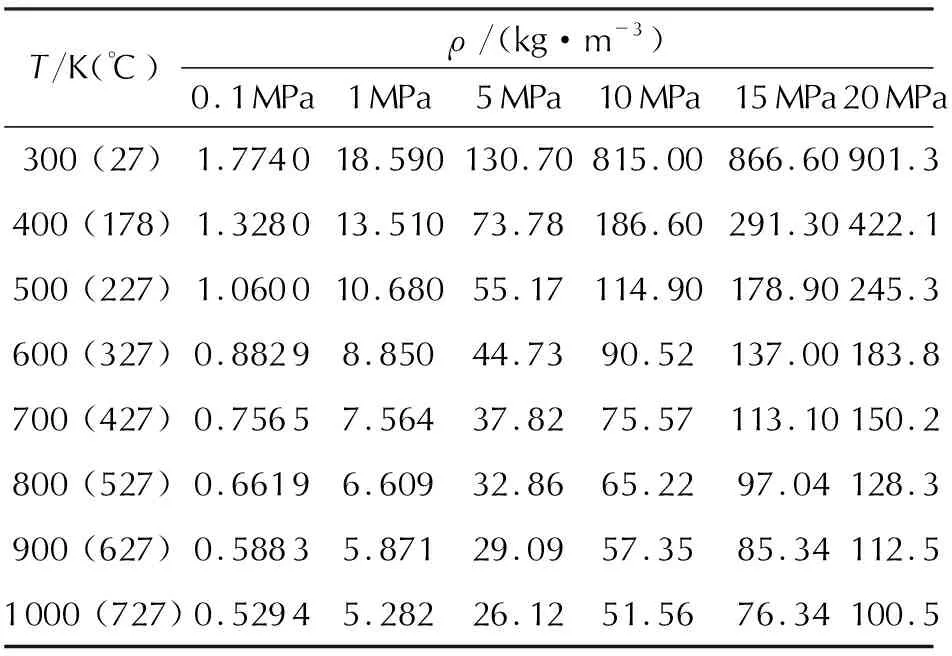
Table 4 A dependence of nitrous oxide density (ρ, kg/m3)in a single-phase area on temperature at various pressures
Refractive index of nitrous oxide:
liquid:atT=183K(-90℃),1.330;
gaseous: atT=273K (0℃) andP=0.1MPa,558.
Coefficient of isobaric volumetric expansion:
atT=273K (0℃) andP=0.1MPa,α=3.761×10-3K-1;
atT=473K (200℃) andP=0.1MPa,α=3.669×10-3K-1.
Isothermal compressibility coefficient
atT=273K (0℃) andP=0.1MPa,βT=1.33×10-3Pa-1.
Speed of sound in the vapor phase of nitrous oxide atT=273K (0℃),a=263m/s.
Electrical properties:
Specific volumetric electrical resistance of liquid nitrous oxide in the temperature range from -40 to +25℃,(2.5-3.5)×1014Ω×m (dielectric).
Dielectric permeability of gaseous nitrous oxide at 298K (25℃), 1MPa and 106Hz,1.00103.
2.2 Chemical properties of N2O
Under normal conditions (P=0.1MPa,T=293K (20℃)), nitrous oxide is an inert chemical substance and in the temperature range from -50 to +50℃ it is stable and relatively non-reactive. It does not interact with water, solutions of acids and alkalis. At the same time, N2O can dissolve in water, organic solvents (acetone, acetic acid, methyl and ethyl alcohols, aniline, amyl acetate, chloroform, benzene, etc.) and in solutions of inorganic salts (chlorides, sulphides, nitrites) under pressure. Its solubility in water at 5℃ is 1.048, and at 20℃ is 0.629 volumes of gas in one volume of water[27].
Formally, the solution of nitrous oxide in water corresponds to the unstable hyponitrous acid H2N2O which easily decomposes into water and nitrous oxide:
2H2N2O=2N2O+H2O
(1)
Nitrous oxide does not interact with oxygen under normal conditions (it could not be oxidized). It also could not be oxidized by such strong oxidants as KMnO4, Cl2O, etc.
At higher temperatures (upon heating) nitrous oxide ignites in oxygen:
2N2O+3O2=4NO2+Q
(2)
Upon heating, charcoal may burn in nitrous oxide. Besides, nitrous oxide is able to react with alkali metals and a number of transition metals (Fe, Co, Cr, etc.)[9- 10]:
2N2O+C=CO2+2N2+Q
(3)
N2O+Mg=MgO+N2+Q
(4)
N2O+Fe=FeO+N2+Q
(5)
Nitrous oxide reacts with hydrogen, ammonia, carbon monoxide and hydrocarbons, producing a significant amount of heat[11-12]:
N2O+H2=N2+H2O-329.5kJ
(6)
3N2O+2NH3=4N2+3H2O-879.2kJ
(7)
4N2O+CH4=CO2+4N2+2H2O-Q
(8)
N2O+CO=CO2+N2-Q
(9)
However, it is difficult to initiate combustion (reactions 6-9). At temperatures above 600℃, nitrous oxide acts as a strong oxidant due to the release of active oxygen and additional heat during its decomposition. At the same time, combustible substances, which have electron affinity, burn more intensely in nitrous oxide than in oxygen.
When handling nitrous oxide, it should be borne in mind that under certain conditions and proportions of the above substances, their mixtures with nitrous oxide may explode when heated. For example, a mixture of equal volumes of N2O and H2, when contacting with flame, generates an energetic flash; a mixture of N2O and NH3may explode with a great force upon ignition. When studying the ignition of N2O mixtures with CO depending on the concentration of initial components in the temperature range of 627-1627℃, it was established that the addition of CH4, C2H4, H2, H2O, NO could both accelerate the reaction of interaction between N2O and CO (H2and H2O) and inhibit the reactions between hydrocarbons and NO)[28].
The process of hydrogen, ammonia and hydrocarbon oxidation by nitrous oxide considerably accelerates when mixtures are diffused over the catalyst surface. For example, the oxidation of hydrogen on a copper catalyst starts at 150℃ with a noticeable speed and occurs in an interval of 200-300℃[29].
Studies of the reduction reaction of nitrous oxide with hydrogen, carbon monoxide, ammonia and methane on supported cobalt catalysts made it possible to establish a reductant activity series:
H2>CO>NH3>CH4>C2H6
It has been established[28, 30-31]that the replacement of molecular oxygen with nitrous oxide leads to an increase in the selective character of the reaction during conversion of methane and other hydrocarbons into various chemical products. This is also facilitated by the use of catalysts in the reaction of hydrocarbon oxidation by nitrous oxide. For example, iron ions represent an active catalyst for the gas-phase reaction of ethane oxidation[32]:
C2H6+2N2O→CH3CHO+H2O+2N2
(10)
Period 4 metals direct the CH4reaction of oxidation by nitrous oxide towards the formation of methanol[33- 34].
The oxidizing properties of nitrous oxide manifest most strongly in such specific reaction as oxidation of benzene into phenol which can be performed with 100% conversion compared to the use of oxygen as an oxidant[35].
The above reactions are of fundamental importance for the arrangement of industrial production of a number of chemical substances that could not be obtained with a sufficient conversion without the use of nitrous oxide.
2.3 Thermal stability of N2O
Pure nitrous oxide at atmospheric pressure is thermally stable at a temperature of up to 500℃. However, being an endothermic compound, it is able to decompose. Nitrous oxide can decompose in several ways: thermal, catalytic, photochemical and in electrical discharge. It was established in reference[35]that a visible homogeneous thermal decomposition of N2O starts at 590℃ which matches the data of reference[36]. In general terms, decomposition of N2O can be represented by the following equation which suggests that the amount of heat released (as well as the temperature of decomposition products) depends heavily on the degree of its decomposition:
N2O→(1-x)N2O+0.5xO2+xN2-xQ
(11)
wherexis the degree of nitrous oxide decomposition.
At a decomposition degree of 0.2, the estimated temperature of decomposition products is about 300℃, and about 1600℃ to reach 100% decomposition degree, i.e. it varies within wide limits. On the basis of the characteristics of this relation, the specific impulse and thrust of rocket engine will also vary within wide limits.
When heated over 520℃, nitrous oxide dissociates noticeably, and at a temperature of 900℃ it completely decomposes releasing a large amount of heat according to the reaction:
2N2O=2N2+O2-163.3kJ
(12)
Nitrous oxide also dissociates under the influence of electric field.
Thermal decomposition activation energy of nitrous oxide is quite high and amounts to 246.8kJ/mol. This means that a lot of heat is required for its thermal decomposition.
The standard decomposition heat of nitrous oxide at 25℃ is 91.5kJ/mol. A considerable number of researches are devoted to studying thermal decomposition of nitrous oxide. The influence of various factors on the decomposition rate constants is explored[26-27, 37-38]. The relative efficiency rates of various dilution gases (including water vapors) influencing the rate of decomposition at low and high pressures in a wide temperature range are provided[Allen M. T. et al.; Loffler G. et al.]. A considerable number of papers are devoted to studying thermal decomposition of nitrous oxide in shock waves in the temperature range of 1700-2500K in the atmosphere of Ar, He, N2, and CO[26, 41, 42]. Estimations, decomposition schemes, constants, and activation energies are provided in paper[26]. For example, the decomposition rate constant for decomposition of N2O in argon can be calculated using the following equation:
KAr= 4.4×1011exp (-56000/RT)L/mol.
A ratio of the decomposition rate constants for decomposition of N2O in various dilution gases is provided below, demonstrating their effectiveness as dilutants:
KHe/KN2/KCO/KAr=4∶2∶3∶1.
The authors of the researches propose to represent the scheme of thermal decomposition of N2O in shock tubes in the following form:
(1) N2O+N→N2+O+N
(2) O+N2O→NO+NO
(3) O+N2O→O2+N2
Based on the analysis of the available literature data, it may be deduced that decomposition of gaseous nitrous oxide occurs sequentially in two reactions: a bimolecular activation reaction of a nitrous oxide molecule and a monomolecular decomposition reaction of an activated molecule. At low pressures, the first reaction becomes the limiting one, and at high pressures (over 10MPa) —the second reaction becomes the limiting one.
2.4 Catalytic decomposition of N2O
The reaction of catalytic decomposition of nitrous oxide has been studied in detail. A rather large number of publications are devoted to its results. However, those papers mainly contain data on the mechanism and kinetics of the process, as this reaction was used as a model one when developing the theory of catalysis.
Both pure metals and oxides, mixed catalysts and zeolites were used as catalysts. Let us review the published literature data.
2.4.1 Mechanism of N2O decomposition
The kinetics of catalytic decomposition of nitrous oxide was studied most extensively using catalysts of various composition[43-66]. The authors of those papers believed that the reaction rate is proportional to pressure of nitrous oxide and the process is inhibited by adsorption of oxygen. Most papers propose the following reaction mechanism with the adsorption equilibrium of oxygen over the catalyst ((·) is the surface of the catalyst):

(13)
(2) (N2O)→(O)+N2
(14)
(15)
2N2O=2N2+O2
(16)
The following equation is proposed to describe the process occurring according to this mechanism where the decomposition rate is unrestrictedly inhibited by oxygen upon increase in its pressure:
(17)
where:Kis reaction rate constant;Pis partial pressure of O2and N2O;bO2is mole fraction of O2in reaction products.
However, the results of the reference[32]do not match the equation; the experimental data provided in the paper suggest the existence of a limit of reaction inhibition by oxygen which occurs when a certain value ofPo2is reached and the reaction rate does not depend on it. Based on the provision that adsorption of oxygen is not reversible, an adjusted mechanism including two directions of the reaction is proposed:
for the first direction:
(1) N2O+(·)→N2+(O)
(18)
(2) N2O+(O)→N2+O2+(·)
(19)
2N2O=2N2+O2
(20)
for the second direction:
(1)N2O+(O)→N2+O2+(·)
(21)

(23)
As we can see, despite the formation of identical reaction products when N2O interacts with adsorbed oxygen in both directions, sources of adsorbed oxygen are different. For example, in the first direction, particles (O) form during decomposition of N2O, and in the second direction — during adsorption of O2.
Since the necessary adsorbed oxygen for the reaction in the second direction forms from O2, then, at lowPo2values, the first direction has the advantage which can be described by the following first-order equation:
(24)
whereK1andK2— rate constants of reaction (18) and (19).
At high values ofPo2, the reaction mainly occurs in the second direction and can also be described by a first-order equation, however, of a different form, and the rate constant has the physical meaning differing from that in equation (24):
(25)
whereKis rate constant of reaction (21);PN2Ois partial pressure of N2O vapors in the mixture.
Despite the simplicity of the nitrous oxide decomposition reaction using oxide catalysts, at the present time, no clear representation of the decomposition reaction mechanism exists.
According to the mechanism suggested by most authors, which is represented by the equations below, the limiting stage is desorption of oxygen, which depends on the interaction of O-with free positive holes:
(26)

(27)
where (p) is a hole symbol.
At the same time, according to the data of the reference[34], the limiting stage of the reaction is somewhat different but it is also related to the participation of free holes in a semiconductor:

(28)
However, more recent studies have shown that the relation between catalytic and chemisorption properties and a semiconductor with its electron properties is more complex than it was previously assumed. For example, according to isotope exchange data, at high temperatures, sorbed oxygen is indistinguishable from oxygen of the surface layer. This has made it possible to assume that, at high temperatures (above 400℃), the reaction of N2O decomposition can occur without the participation of electrons and holes, i.e. according to another mechanism, e.g. by oxygen exchange between the surface and the gas phase. In insulators, stable defects —Fcenters — contribute to this mechanism:
N2O+F-center→N2+O2-
(29)
2O2-→O2+2F-center
(30)
whereFis center of a single oxygen vacancy on the catalyst surface.
Forst W.[38]has a similar point of view and suggest a hypothesis of the geometrical structure of the N2O adsorption complex on oxides. If we assume that decomposition occurs according to equations 11 and 12, at the first stage, nitrous oxide binds to the F-center, attaching the electron and forming a surface compound of the following structure:
(31)
In this case, it is assumed that the N2O molecule in the complex is not linear (in contrast to the molecule in the gas phase).
Based on the literature data on the kinetics and mechanism of N2O decomposition, we can draw the following general conclusion:
(1) The reaction rate is proportional to partial pressurePof N2O.
(2) In many cases, the decomposition process is inhibited by O2.
(3) Nitrous oxide can be adsorbed (the degree of filling is very low — up to 1%), the heat of adsorption activation is low — no more than 42kJ/mol.
2.4.2 Catalysts for decomposition of N2O
An analysis of the literature data shows that studies of the kinetics and mechanism of N2O decomposition were carried out using a wide range of chemicals including metals and their alloys, oxides, spinels, carbides, zeolites, etc.[43-66].
Metal catalysts were tested both in a compact form (foil, powder, wire) and in the form of supported catalysts.
As for compact metal catalysts, the following metals were most commonly used: Pt, Pd, Ag, less often Cr; Ru, Au, and Ge.
As for supported catalysts, the studies were carried out using the catalysts where the following metals or their oxides were used as an active component:
Cobalt Co/Al2O3,ZSM-5;CoO;CoO/Al2O3,TiO2,ZrO2,SiO2;CoO/MgO;Co,CuO/ZnO;
Nickel Ni;Ni/ZnO,Cr2O3,SiO2;NiO;NiO/MgO;TiO2,ZrO2,Al2O3,SiO2;NiCr2O4
Iron Fe2O3/Al2O3;Al2O3·Li2O;/ZSM-5;SiO2;/TiO2,ZrO2,Al2O3,SiO2;/ZrO2
Copper Cu/Al2O3,SiO2;complexes of Cu;/ZSM-5
Palladium Pd/Al2O3,SiO2,TiO2;PdO/Al2O3-SiO2
Rhodium Rh/Al2O3;/ZSM-5;ZnO,CeO2,ZSM-5;CeO2,ZSM-5;
Ruthenium Ru/Al2O3;ZSM-5
Iridium Ir/Al2O3
Silver Ag/Al2O3
Molybdenum Mo/Al2O3;SiO2
Al2O3
ZrO2ZrO2/Al2O3
However, taking into account numerous studies of metal catalysts, it is difficult to compile a series of specific catalytic activities of metals in the reaction of N2O decomposition. This is due to the fact that in the published works there is no information about the specific surface of catalysts and the active component, conditions of preparation and phase composition, which makes it impossible to compare the presented data under the same conditions. Nevertheless, it can be stated that, in the reaction of N2O decomposition, catalysts based on rhodium, supported on carriers with a large specific surface, such as γ-Al2O3, zeolite of the mordenite type or ZnO, exhibit the greatest activity. Moreover, in its oxidized form, Rh shows more activity than in the reduced form.
In the reaction of N2O decomposition, the activity of complexes containing cobalt, manganese, and copper, based on non-hydrocarbon high-molecular compounds, where metal ions are in the chelating environment, has also been studied. It has been found that in these complexes the onset temperature of N2O decomposition and the activation energy are 250-300℃ and 3-4 times lower than in the polycoordination compounds of the corresponding metals, respectively. However, their loss of activity at temperatures above 300℃ indicates that they will agglomerate under the conditions of operation of a monopropellant rocket engine with catalytic decomposition of N2O, where the temperature is above 1000℃.
Far more works are devoted to studies of N2O decomposition on oxide catalysts which were mainly represented by transition metal oxides. The studies were carried out both on pure oxides and on those supported on various carriers. For example, in the reference[67], using the static method at a pressure of 0.13-0.20atm, the activity of 19 metal oxides was defined, which, according to the specific catalytic activity, can be ranged (decreasingly) as follows:
Rh2O3>IrO2>CaO>CuO>SrO>HfO2>Fe2O3>NiO>ThO2>MnO2>SnO2>CeO2>MgO>>Cr2O3>ZnO>Ga2O3>BeO>Al2O3>TiO2.
In the same paper, the author attempted to correlate the catalytic properties with adsorption properties in relation to oxygen and N2O.
The reference[68]presents the activity of 21 oxides using the flow method at atmospheric pressure and the onset temperature of decomposition. According to their activity, the oxides are arranged as follows:
Co3O4>NiO>CuO>ThO2>Al2O3>CdO>CeO2>ZrO2>SnO2>Fe2O3>Cr2O3>ZnO>Nd2O3>>MgO>CаO>Sb2O4>WO3>U3O8>BeO>SiO2>GeO2
Oxides of cobalt and nickel have the highest activity; their minimum onset temperature of decomposition is 280 and 294℃, respectively. The activities of oxides following them in the series of activity are much lower: as for copper oxide, the onset temperature of decomposition is 400℃.
Comparison of both series of activity shows that they basically coincide (except for Al2O3and CaO), although they were obtained by different methods.
It should be noted that the reference[68]did not take into account possible differences in the surface of catalysts (the oxides were heat-treated at different temperatures and during different periods) during compilation of the series of activity. Apparently, this can explain the observed discrepancies in activity.
More than 50 different catalysts in the reaction of N2O decomposition were tested in reference[69]). It was found that catalysts based on nickel oxide supported on zirconium oxide, iridium oxide or rhodium oxide supported on γ-Al2O3were the most promising. Zakirov V A.[69]made those conclusions on the basis of laboratory and first-time conducted bench tests of catalysts in a model reactor, which makes them very useful. Unfortunately, the paper did not present the main physical and chemical characteristics of the catalyst (general and specific surface, porous structure) and the rationale for the choice of the active component and carriers for the development of N2O decomposition catalysts.
It was found that the activity of individual oxides can be changed by introducing additives of other oxides. For example, the introduction of Li2O and Ga2O3additives to ZnO and additives of Al2O3and WO3in TiO2decreases the catalytic activity of ZnO and TiO2. At the same time, the introduction of Li2O in NiO increases, and the introduction of In2O3reduces its catalytic activity, although Li2O accelerates the chemisorption of O2(electron acceptor) on ZnO and NiO.
A significant number of studies on N2O decomposition were carried out on catalysts represented by complex oxide systems such as solid solutions and spinels. The activity of the following solid solutions was studied in that manner:
Based on double oxides of CoO, Fe2O3-MgO; NiO-MgO; Fe2O3-MgO; CuO-ZnO, CoO,- CuO, ZnO; CaO-MgO; ZnO-Fe2O3with addition of transition metal oxides; based on aluminates of Co, Ni, Cu, Mg, Zn and Li;
based on chromites CuCr2O4, NiCr2O4.
In the reaction of N2O decomposition, the activity of more complex oxide systems was determined as well:
mixed oxides with the structure of delafossite—CuMO2, where M—Al, Cr or Fe (CuFeO2>CuCrO2);
mixed oxides—manganites with the structure of perovskite MMnO3, where M—La, Nd, Sn or Gd;
oxide compounds of Ni, Mn, and Cu with rare-earth elements (such compounds as LnNiO4and LnCuO4, where Ln—La, Nd, or Pr or LaCo3O4compound);
multi-component systems based on lanthanides and transition metals such as La(1-x)Srx, MO3, where M—Mn, Mo or Cu; La2TiMO6, where M—Cu, Ni or Zn; La2Cu1-xNixO4; La2CuxZn(1-x)O4.
Comparison of the activities of the above-mentioned oxide systems has shown that cobalt aluminates have higher activity (the onset temperature of decomposition is 470℃) than Cu and Ni aluminates, Ni and Zn chromites, Na, Ca, Ba stannates. At the same time, aluminates of non-transition metals (Mg, Zn and Li), Mg, Co, Ca chromites and Mg ferrites are of low activity. It should be noted that the data on activity significantly differ in different sources. For example, MgAl2O4is noticeably more active than MgCr2O4, while the specific rate of N2O decomposition on MgCr2O4is massively more than that on MgAl2O4. Apparently, this is due to the different surface values of catalysts, which are in fewer reported than all papers.
The data obtained on the samples of double oxides with their ratio varied—in the CoO-Al2O3system, changes in the ratio of components have virtually no effect on the activity—are of great interest.
At the same time, during studies of the activity of solid solutions of the MgO-Cr2O3system, it has been found that dilute Cr3+solutions are characterized by the increased catalytic activity in the range of 400-500℃ referred to one chromium atom. A similar increase in atomic activity during dilution is observed when N2O decomposes at a temperature of 300-400℃ on NiO-MgO solid solutions.
Despite the fact that catalytic activity of those complex systems, characterized by the minimum onset temperature of the reaction ranges from 350 to 600℃, and some of them, such as lanthanides, have the activity comparable with the activity of palladium catalysts, they are of no practical interest for the development of catalysts. This is due to the fact that, on the one hand, the studies were carried out on catalysts of complex chemical composition and, therefore, are difficult to reproduce. On the other hand, they were basically carried out to establish a mechanism of decomposition of nitrous oxide and clarify the role of oxygen in this process, therefore, they are of interest for the theory of catalysis.
The data obtained during the determination of activity and studies of the kinetics of nitrous oxide decomposition, where zeolites of the following type are used as catalysts, have similar values:
natural and synthetic mordenite, in the cavities of which there were multiply charged metal cations Be, La, Mg, Ba, Sr —Tdecomposition=(250-600)℃, cations of alkaline earth metals and ion-exchange iron, as well as synthetic mordenite containing cations of transition metals;
Y-zeolites containing F3+,Tdecomposition=(350-650)℃;
ZSM-5 zeolite used as a catalyst and as a carrier for Rh and Cu (Tdecomposition=300℃); for Rh and Ru,Tdecomposition=(250-300)℃; for Cu,Tdecomposition=(375-450)℃.
Carbides of Ti, Zr, Hf, W, Mo, Gr, Si, B, Ca were also used as catalysts in the decomposition reaction. It was found that N2O decomposition on titanium, zirconium, and tungsten carbides starts at 310-370℃; and the temperature can be lowered by carburizing (increasing the proportion of carbon in the sample through metal removal). We should note the catalytic activity of calcium carbide: the onset temperature of decomposition on the carbonized catalyst is 170℃. This catalyst is of some interest for further researches.
An analysis of the data shows that, in the reaction of nitrous oxide decomposition, a quite large number of chemical compounds of individual metals and their oxides, complex systems based on those oxides, as well as zeolites, both natural and synthetic, were studied. However, those studies were mainly aimed at elucidating the mechanism and role of O2in the reaction of N2O decomposition and establishing the relation between the catalytic and adsorption properties of the chemical compounds under study. Therefore, the published data can serve only as a basis for further researches aimed at creating a highly efficient catalyst meeting the requirements to products of rocket and space technology.
2.5 Ballast additives
It is known during decomposition of nitrous oxide a significant amount of heat releases, which contributes to high temperatures of reaction products. For example, at a decomposition degree of 0.8, the temperature of decomposition products reaches 1300℃, which greatly complicates the choice of structural materials necessary for making combustion chambers. One of the ways to simplify the technology of creating low-thrust engines and their operation is to lower the temperature of N2O decomposition products by diluting the initial component with other chemical compounds—so-called ballast additives.
Taking into account chemical properties and good diffusibility of N2O into other gases, inert gases He, Ar, N2, and CO2can be used as ballast additives since they do not interact with nitrous oxide, and, therefore, will not produce additional heat in the decomposition chamber.
At the same time, when stored under pressure, N2O will liquefy, therefore, its mixture with He, N2and Ar can be stratified, and when throttled from a cylinder, the composition of such gas mixture will be undefined.
Carbon dioxide, which is liquefied to a colorless liquid at 20℃ and 5.7MPa, is of great interest as a ballast component. When the liquid mixture of those gases is throttled, we can obtain a gas mixture which is more homogeneous in composition than that with inert gases.
Physical and chemical properties of possible ballast mixtures are presented in a number of publications. For example, Kestin S.and Ro S. T.[23]considered the viscosity and diffusion coefficient of binary nitrous oxide systems with N2, Ar, and CO2in the temperature range of 25—200℃. It was found that the calculated values of the binary diffusion coefficients of mixtures were consistent with the law of the corresponding states. According to Prajapati S. R.[70], values of thermal conductivity of two binary systems of nonpolar gases, N2-N2O and NO-N2O, in the temperature range of 50—1800℃ were calculated.
Preliminary calculations have shown that the temperature of nitrous oxide decomposition products depends greatly on the amount of ballast gas introduced. For example, when 20% vol. He is introduced, the temperature of N2O decomposition products can decrease almost twice. Therefore, this gas mixture deserves attention of researchers due to good dissolution of helium in nitrous oxide. Stratification of this gaseous mixture is out of question. However, the composition of the ballast gas mixture should be determined for each input component individually.
3 Nitrous oxide application. N2O as a propellant
Nitrous oxide is a well-studied chemical compound. It is largely used in medicine during surgical operations because of its narcotic properties. Currently, nitrous oxide is recommended for use in the food and cosmetic industry as a dispersant for the preparation of various creams. It has been found that the introduction of N2O in the combustion chamber of an internal combustion engine leads to an increase in its power. As an excellent refrigerant, it could be used in the refrigeration industry[70], and as a strong oxidant — in the chemical industry. However, until recently, nitrous oxide was not used in industry. Thanks to recent works, N2O, as a strong oxidant allowing more efficient catalytic synthesis than oxygen, is used in liquid-phase catalytic oxidation processes.
Besides, nitrous oxide decomposes into nitrogen and oxygen (at a ratio of 2∶1), which is an excellent respiratory mixture with the composition close to the air composition. That means that N2O can be a source of oxygen ensuring life support in a confined space. This property allows recommending N2O as a source of oxygen for life support systems in emergency situations (under water, under the ground or in outer space). This requires the development of a gas generator for its catalytic decomposition.
Since a high-temperature gas mixture (more than 1600℃) is generated during N2O decomposition, it can serve as a source for obtaining high-energy nitrogen in laser installations.
And finally, nitrous oxide can be used as a high-energy propellant[1-5]. For a long time, it was of no interest for specialists in rocket technology. Gradual improvement of space vehicles, including the creation of low-thrust engines, and the requirements for the use of environmentally-friendly propellants (so-called "green propellants") made the specialists in this industry pay attention to nitrous oxide as a promising "green" propellant. In the process of its decomposition, high-energy products are generated, which, on the one hand, allow developing engines with the gram level of thrust. On the other hand, due to the unique property of forming a mixture of oxygen and nitrogen during decomposition, life support in a confined space can be ensured with a clean respiratory mixture.
In this case, nitrous oxide can be stored on-board a spacecraft in a liquefied state and gasified when introduced into the combustion chamber. This property of N2O allows using it as a source of "cold gas".
Below there are some design energy characteristics of generator gas that were obtained by the author during decomposition of liquid and gaseous nitrous oxide at a pressure of 40-60MPa:

Table 5 Energy characteristics of generator gas
As it can be seen, during decomposition of gaseous N2O, the temperature of decomposition products (T) can reach 1900K (1627℃) and the reference speed (v) can reach 1105m/s. For comparison, when using hydrazine, the theoretical values of the temperature of decomposition products and reference speed (ifX=0.25) reach 1450 and 1340, respectively.
The theoretical specific impulse for N2O is also quite high -206s; for comparison, in case of hydrazine and hydrogen peroxide — monopropellants which are currently successfully used in rocketry — it amounts to 245 and 179s, respectively.
Nitrous oxide can also be used as an oxidant in a bipropellant engine. High specific propellant parameters can manifest upon N2O combustion with ammonia or hydrazine: the design specific impulse and temperature of decomposition products (atα=1.0), are 3000m/s and 2990K for ammonia, and 3190m/s and 3290K for hydrazine, respectively.
Based on the foregoing, taking into account non-toxicity, high stability, corrosion resistance, and compatibility with various storage tank materials, we can conclude that nitrous oxide is one of the most promising propellants.
4 Preparation of nitrous oxide
Nitrous oxide can be obtained by the interaction of nitrogen acid or nitrous acid with various reducing agents (hydrogen sulphide, sulfurous acid, etc.). In industry, one of the most common and oldest methods of nitrous oxide preparation is thermal decomposition of nitrates, in particular, ammonium nitrate, at a temperature of 170-260℃. The process occurs according to the following equation and is accompanied by a release of a large amount of heat:
NH4NO3→N2O+2H2O-36.8kJ
(31)
The process of decomposition proceeds with acceleration, therefore, a special caution is required since rapid heating can lead to an explosion.
According to this technology, N2O is obtained for pharmacology. The obtained nitrous oxide shall have the following composition (in volume fractions, %):
basic substance, not less than 96.0;
noncondensable substance, not more than 4.0
carbon monoxide, not more than 0.005
Due to the development of a number of new chemical industrial processes where N2O is used as an oxidant and spent in large quantities, the method of obtaining N2O by thermal decomposition of nitrates is unacceptable.
A number of methods are proposed for those purposes. Among them, the method of catalytic reduction of NO by carbon monoxide or hydrogen in the presence of homogeneous or heterogeneous catalysts and the thermocatalytic method of direct oxidation of ammonia by oxygen shall be noted:
2NH3+2O2→N2O+3H2O
(32)
Based on the latter, a new technology was developed and a pilot plant for the preparation of N2O, which meets the requirement of chemical industry, was created[71].
5 Toxicity
Nitrous oxide is a low-toxic compound. The maximum allowable concentration in the air of the working area is 500mg/m3[72- 73].
Gaseous N2O has sweet taste when inhaled. When inhaled in a mixture (with a volume fraction of up to 20% N2O) with air, it affects the nervous system, causes intoxication, weakening of pain, a bout of laughter. That is why N2O is called "laughing gas". In small concentrations with oxygen, it is used as a narcotic anesthetic in medicine; when inhaled in high concentrations, it can cause suffocation due to the expulsion of oxygen from the lungs. Therefore, indoor works with nitrous oxide can be carried out only if a supply and exhaust ventilation is available. Besides, both self-contained and hose gas masks must be available at the workplace.
Liquid nitrous oxide can cause frostbite of skin and conjunctiva, since when a lot of nitrous oxide flows out of a vessel, it turns into a snow-like mass with a temperature of -90℃. Therefore, workers using N2O should be provided with special protective clothes, footwear, masks and wool gloves.
In case of intoxication with nitrous oxide, the affected person should be immediately removed from the gassy area to fresh air, laid in a horizontal position, and handed over to medical professionals.
6 Fire hazard
Nitrous oxide falls under the class of oxidants, therefore, it is a fire-hazardous liquefied gas. It can react intensively with some flammables. The minimum energy of gas ignition at a pressure of 0.5-3.0MPa and an initial temperature of 18.3℃ is (10ρ-2.27) J[74- 75].
Gaseous nitrous oxide forms explosive mixtures with hydrocarbon vapors (methanol, ethylene, acetylene, oil, kerosene, etc.), ammonia, hydrogen, carbon monoxide and carbon disulfide. Concentration explosion limits of some gas mixtures (volume fraction of N2O in a mixture of vapors of some substances) at a pressure of 0.1MPa are presented in Table 6.
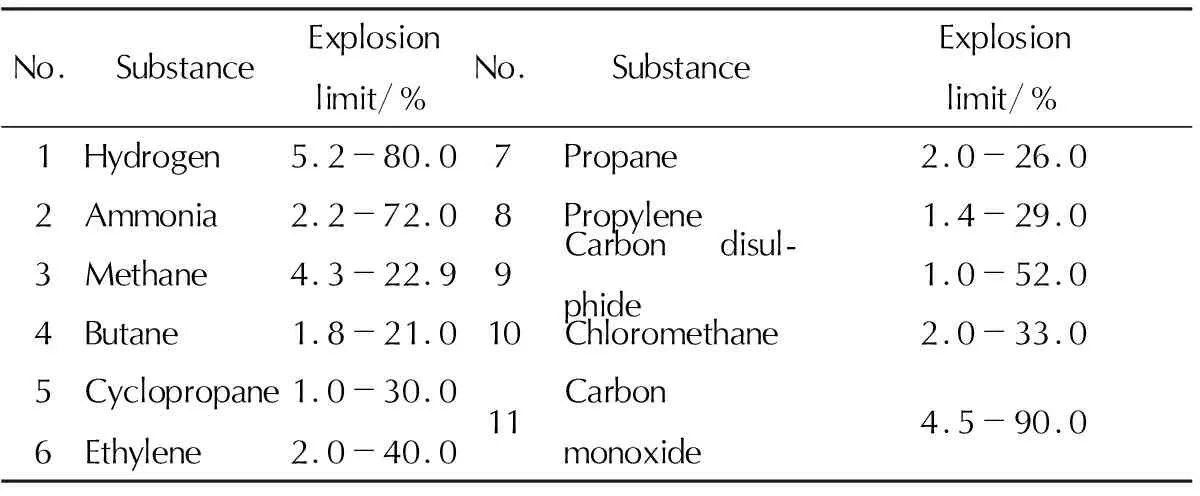
Table 6 Explosion limits of vapors of nitrous oxide mixtures with various chemical substances
Under normal conditions, liquid and gaseous nitrous oxide does not detonate. At the same time, at high pressures (more than 0.5MPa), gaseous nitrous oxide is explosive. This can be attributed to the fact that it is an endothermic compound capable of spontaneous decomposition upon initiation of ignition from an external source, which is accompanied by a release of a large amount of heat (-81.5kJ/mol) and a sudden increase in pressure in an enclosed volume. This can lead to the destruction of a container for N2O storage.
Electric discharge, shock waves, hot wires, and, at pressures above 6.5MPa, static electric charges and sparks from friction can be potential initiators of explosive decomposition of nitrous oxide.
At normal temperature and pressure up to 0.5MPa and absence of organic impurities and other combustible additives, nitrous oxide is a thermostable and non-explosive compound. Therefore, in working areas, the use of N2O does not create an explosive environment. However, in the presence of vapors of the above mentioned chemical compounds, its sensitivity to explosion increases. The introduction of phlegmatizing gases (nitrogen, air, carbon dioxide) reduces nitrous oxide explosivity. Reducing of the probability of explosive decomposition is facilitated by a decrease in the temperature and, correspondingly, the partial pressure of saturated vapors.
In the event of a fire initiated by nitrous oxide in a working area, standard means of fire extinguishing are used: water, dry sand, asbestos cloth, carbon dioxide fire extinguishers.
7 Requirements to equipment when working with N2O
Under normal conditions, nitrous oxide is a chemically stable compound, therefore, it does not have corrosiveness in relation to many structural metal materials in a temperature range from -50 to +50℃. This means that almost all materials applied in the chemical industry can be used to manufacture equipment for works with nitrous oxide. Chromium, chromium-nickel, and carbon steels, as well as a number of copper, aluminum, and nickel alloys are allowed for works with nitrous oxide. It is recommended to use fluoropolymers and pyrographite as sealing materials. Rubber materials used in the chemical industry lose their strength properties when they come into contact with liquid or gaseous nitrous oxide. It should be taken into account that the lower limit of the use of structural materials is determined by the boiling point of nitrous oxide (-88℃).
For safe and stable operation of plants using nitrous oxide, it is important to ensure clean internal surfaces of the equipment (cylinders, pipelines, reactors, instrumentation and control, etc.) that are in contact with nitrous oxide. This especially applies to vessels and pipelines made of carbon steel, which, in the presence of moisture, can form scale that catalyzes its decomposition. Therefore, all equipment shall be duly prepared prior to operation according to the following stages:preparation of equipment for degreasing;degreasing;water washing;drying.
When using the equipment made of carbon steel, it is recommended to clean it by etching before degreasing. It is recommended to carry out quality control of degreasing and drying of equipment by the fluorescent or nephelometric method.
It is recommended to use materials based on fluorine and perfluorinated polymers as lubricants.
8 Storage and transportation
Since nitrous oxide under pressure liquefies easily, it is usually stored and transported in the liquefied form in cylinders with a volume of up to 10L which is the most convenient in operation. The cylinders that are subject to inspection every 5 years are painted in blue in Russia. Refueling of cylinders with liquefied gas shall be carried out by the weight method. Cylinder filling factor -0.62kg/L.
Nitrous oxide in a cylinder is simultaneously in the liquid (at the bottom) and gaseous (at the top) form, which allows (if necessary) obtaining both gas and liquid from a cylinder (by means of turning the cylinder valve downwards). The pressure in a filled cylinder will be about 57 atm at 20℃ and it will remain constant until the liquid part is completely consumed, and then the pressure in the cylinder will begin to drop. Recommended residual pressure — no less than 5×105Pa.
In laboratories, cylinders are installed in a vertical position at a distance of at least 1m from heating elements and fixed.
Cylinders with nitrous oxide can be transported by rail and road transport, but as it is a dangerous cargo, they shall be transported in accordance with the current regulations for this category of products.
Cylinders with nitrous oxide shall be stored in ventilated warehouse facilities or in open air under roof at a temperature of no more than 30℃ and separately from combustible gases.
9 Conclusion
The presented overview of physical and chemical, as well as operational and thermodynamic properties of nitrous oxide, chemical stability of various materials in N2O, its decomposition products, as well as the data on the mechanism and catalysts for its decomposition, the corresponding requirements applied to them as catalysts for the decomposition of propellants in model rocket engines showed the following:
(1) Nitrous oxide is a chemical compound meeting the requirements to monopropellants, including those applied to the environmental friendliness of propellants.
(2) The variety of data available in the literature is mainly devoted to studies of physical and chemical properties, the mechanism and kinetics of decomposition of nitrous oxide on various chemical substances as a model reaction and does not address the issues of creating a catalyst for its decomposition.
(3) The conditions of catalytic decomposition of nitrous oxide presented in the overview showed the complexity of its operation in model rocket engines and high requirements for the expected active components of the catalyst:
the catalyst shall operate in extremely harsh conditions: high temperature (more than 1000℃), oxidizing environment at high temperatures (nitrous oxide and oxygen), multiple launches (several thousand impulses) and the impact of various vibration, linear, and shock loads;
currently, there is no unified theory for the selection of an active component meeting the requirements;
there is no consensus on the mechanism and kinetics of nitrous oxide decomposition, despite the apparent simplicity of the reaction;
it is difficult to perform an experiment for the determination of the catalyst activity due to the high temperature of reaction products and their chemical aggressiveness.
Only after the recognition of nitrous oxide as a promising monopropellant, works related to the selection of catalysts and a method for assessing their performance under conditions simulating their operation under real conditions (in low-thrust rocket engines) began.
The results of works on the nitrous oxide decomposition catalyst development will be published later.
