Role of pancreatoscopy in management of pancreatic disease: A systematic review
2019-02-27TarunKauraFieldWillinghamSaurabhChawla
Tarun Kaura, Field F Willingham, Saurabh Chawla
Abstract BACKGROUND Per-oral pancreatoscopy (POP) plays a role in the diagnosis and therapy of pancreatic diseases. With recent technological advances, there has been renewed interest in this modality.AIM To evaluate the efficacy and safety of POP in management of pancreatic stone disease and pancreatic ductal neoplasia.METHODS To determine the safety and efficacy of POP in the management of pancreatic diseases, a systematic search was conducted in MEDLINE, EMBASE and Ovid.Articles in languages other than English and case reports were excluded. All published case series were eligible. Data specific to POP were extracted from studies, which combined cholangiopancreatoscopy. Ten studies were included in the analysis of POP therapy for pancreatic stone disease, and 15 case series satisfied the criteria for inclusion for the role of POP in the management of pancreatic ductal neoplasia. The examined data were subcategorized according to adjunctive modalities, such as direct tissue sampling, cytology, the role of intraoperative POP, intraductal ultrasound (IDUS) and POP combined with image-enhancing technology.RESULTS The success rate for complete ductal stone clearance ranged from 37.5%-100%.Factors associated with failure included the presence of strictures, multiple stones and the inability to visualize the target area. Although direct visualization can identify malignant and premalignant conditions, there is significant overlap with benign diseases. Visually-directed biopsies provide a high degree of accuracy,and represent a unique approach for tissue acquisition in patients with ductal abnormalities. Addition of pancreatic fluid cytology increases diagnostic yield for indeterminate lesions. Protrusions larger than 3 mm noted on IDUS are significantly more likely to be associated with malignancy. The rate of adverse events associated with POP ranged from 0%-35%.CONCLUSION Current evidence supports wider adoption of pancreatoscopy, as it is safe and effective. Improved patient selection and utilization of novel technologies may further enhance its role in managing pancreatic disease.
Key words: Pancreatoscopy; Cholangiopancreatoscopy; Chronic pancreatitis; Pancreatic duct stones; Intraductal papillary mucinous neoplasm; Pancreatic cancer; Pancreatic duct stricture
INTRODUCTION
Evaluating the pancreatic duct (PD) is challenging due to its anatomy, which occasionally limits visualization by cross-sectional imaging, relative inaccessibility to available endoscopic devices, and certain unique obstructive disease entities. These may limit diagnostic and therapeutic endeavors under fluoroscopic guidance.Evaluation of these entities has relied heavily on various radiologic modalities including computed tomography (CT) scans, magnetic resonance imagings (MRIs),endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasonography (EUS)[1]. ERCP-guided brushings of pancreatobiliary strictures for cytological examination has a diagnostic yield ranging from 30%-57%[2-4]. Even with the addition of endobiliary biopsy forceps and endoscopic needle aspiration, the diagnostic yield and negative predictive value remains low[5]. Stone extraction from the PD may be limited by stone impaction at side branch take-offs, or a narrow proximal PD, which may limit balloon extraction. Furthermore, non-endoscopic interventions of the pancreas are associated with significant morbidity and mortality.
For these reasons, direct visualization of the pancreatic ductal system is helpful in evaluating and managing certain pancreatic diseases. Attempts at direct visualization of the PD with per-oral pancreatoscopy (POP) were initially described in the 1970s using a mother-baby system[6]. However, there were drawbacks, including the need for two endoscopists, scope fragility and poor image resolution, which limited its adoption for mainstream use.
The recent development of catheter-based systems, primarily developed for bile duct use (single operator cholangioscopy), has addressed some of these limitations,thus promoting widespread application of this modality for both biliary and pancreatic ductal use. Features, such as four-way tip deflection, dedicated irrigation,accessory channels, and digital image acquisition with significant improvement in image quality, field-of-view and ability to add image-enhancing technology, have made these systems more user-friendly. They have also resulted in diagnostic and therapeutic advances in the management of complex pancreatic diseases.
We present an updated review of the current literature on POP for the management of pancreatic diseases.
MATERIALS AND METHODS
To determine the safety and efficacy of POP in the management of pancreatic diseases, a systematic search was conducted in MEDLINE, EMBASE and Ovid. We used the key words “pancreatoscopy”, “cholangiopancreatoscopy”, “IPMN”, “chronic pancreatitis” and “pancreatic stone disease” to identify relevant articles. Articles in languages other than English and case reports were excluded. All published case series were eligible. Data specific to POP was extracted from studies that combined cholangiopancreatoscopy. The subject population was heterogeneous among the studies reviewed. Ten studies were included in the analysis of POP therapy for pancreatic stone disease (Table 1). Fifteen case series satisfied the inclusion criteria for the role of POP in the management of pancreatic ductal neoplasia (Table 2). The examined data were subcategorized according to the adjunctive modality, such as direct tissue sampling, cytology, role of intraoperative POP, intraductal ultrasound(IDUS) and POP combined with image-enhancing technology.
RESULTS
Endoscopic pancreatic ductal stone therapy
Chronic pancreatitis is characterized by ongoing inflammation that leads to fibrotic changes in the pancreas, resulting in diminished exocrine and endocrine function.Chronic abdominal pain is the main symptom, which may be severe enough to limit quality of life. Several mechanisms, such as outflow obstruction leading to ductal hypertension from strictures/stones and perineural inflammation, have been implicated in the pain pathogenesis of chronic pancreatitis. Continued ductal obstruction may eventually lead to parenchymal atrophy and loss of exocrine and endocrine function, which may cause other symptoms including anorexia,malabsorption and weight loss. Therefore, relief of pancreatic ductal obstruction is a cornerstone in the management of this disease.
Options for therapy depend on ductal morphology and the presence of PD stones and/or strictures. Pancreatic ductal stones, which can occur in up to 90% of patients,represent a significant target for therapeutic intervention[7]. Stone predominant disease, associated with a uniformly dilated PD, is often seen in patients with idiopathic or genetic etiologies, as compared to the complex ductal morphology with strictures seen in patients with chronic alcoholic pancreatitis[8].
Traditional ERCP techniques using extraction balloons and stone extraction baskets have a limited success rate of around 50%, even in expert hands[9]. The complication rate of pancreatic mechanical lithotripsy is three-fold higher than biliary lithotripsy,including trapped and broken baskets that occur in up to 10%[10]. Extra corporeal shockwave lithotripsy (ESWL) is an important adjunct to managing pancreatic ductal stones, with a success rate of 60% for pain relief[11]. However, the limited availability,cost, need for multiple sessions, along with concomitant ERCP to remove stone fragments and treat downstream strictures, have limited widespread use[9].Furthermore, ESWL also requires a radiopaque target such as a calcified stone or the tip of a stent, thus limiting applicability with radiolucent stones. The management of radiolucent stones is more demanding, as it may require ultrasound guidance or contrast injection through a nasopancreatic catheter[12]. In addition, ESWL is less effective in patients with dense or multiple stones[13].
POP-guided intraductal lithotripsy has the potential to combine the advantages of endoscopy and ESWL. POP-guided intraductal lithotripsy was initially described by Howell et al[14], and significant advances have been achieved since then. Intraductal lithotripsy under direct visualization can be achieved by either electrohydraulic therapy (EHL) or laser lithotripsy (LL). The EHL probe consists of two coaxially insulated electrodes attached to a generator producing high voltage electrical impulses at a frequency of 1 to 20 Hz, with power settings between 50%-100%[15].Sparks at this site produce high amplitude hydraulic pressure waves during water immersion, which help in stone fragmentation[16]. Neodymium: yttrium-aluminumgarnet lasers have been used for pancreatobiliary stone fragmentation by transforming optical energy into mechanical energy in the form of shockwaves vialocal plasma formation[17].
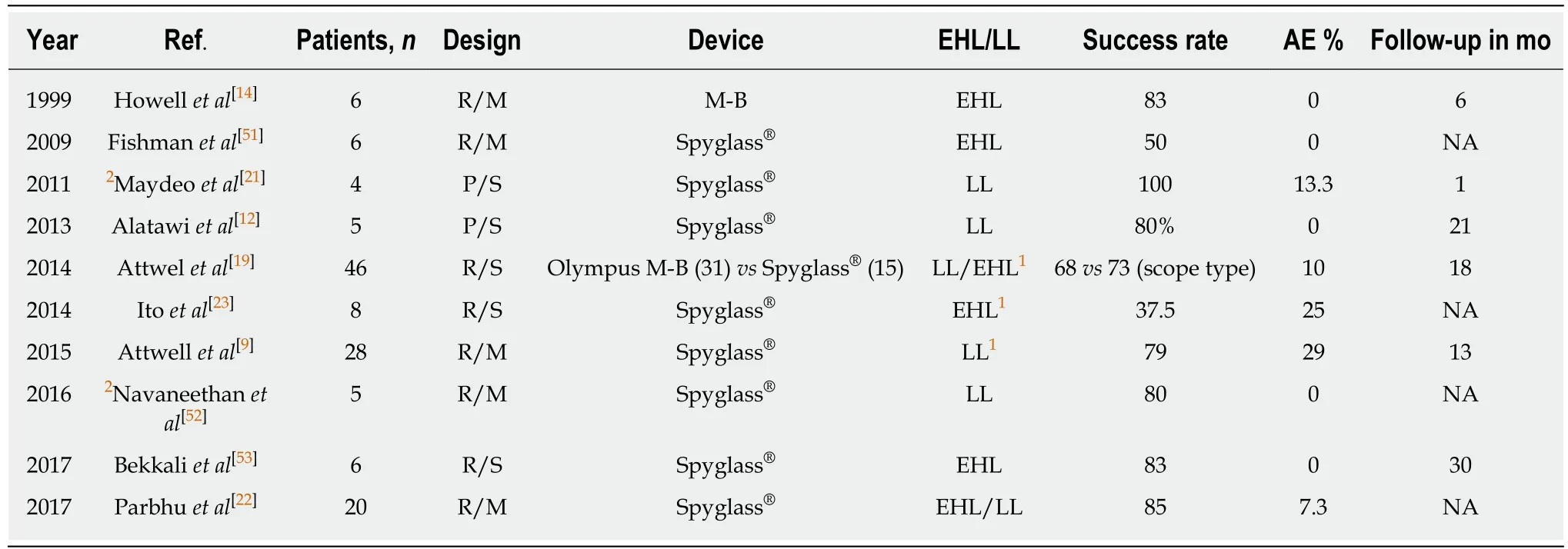
Table 1 Per oral pancreatoscopy-guided pancreatic ductal stone therapy
Pancreatoscopy-guided lithotripsy
Ten published studies were selected for review based on the inclusion criteria. Only two prospective studies with a total of 9/134 patients were identified. There were no prospective randomized studies. Only three published studies had more than ten patients, however, they are all retrospective in nature. A majority of the included patients had chronic pancreatitis due to excessive alcohol use.
Based on the available data, the success of POP-guided PD stone therapy ranges between 37.5%-100% (Table 1) as compared to the success rate of ESWL, which ranges between 59%-76%[18]. Only one study retrospectively compared single-operator pancreatoscopy with traditional mother daughter technique. This study showed no significant differences in success rate, although there was a trend towards better success with the catheter-based system, with a complete clearance rate of 68%-73%[19].Dorsal duct POP-guided endotherapy via minor papilla access was successfully attempted in cases in which the duct immediately upstream of the major papilla was inaccessible[9,19]. This can be performed in patients with pancreatic divisum or acquired obstruction of the ventral duct (pseudo-divisum) from strictures or stones.Brauer et al[20]reported 80% clinical success via minor papilla in five patients with painful pancreatolithiasis.
Most studies included patients who had failed conventional ERCP techniques[12,21,22]or ERCP with ESWL[9,14,19,23]. Median reported PD stone size ranged from 5 mm[22]-15 mm[9]. Some studies[9,19]reported 23 h observation after index POP procedure or pancreatic sphincterotomy. Most studies reported the placement of plastic PD stents for drainage after POP-guided therapy, necessitating multiple procedures. Shin et al[24]placed a self-expanding fully covered metal stent for downstream PD stricture prior to successful POP-guided EHL lithotripsy of a 1.1 cm large PD stone.
Parbhu et al[22]reported a 50% success rate in 20 patients using only balloon or basket sweeps due to better visualization with POP. Complete clearance in a single procedure was reported in 100% patients by Maydeo et al[21]and 61% by Attwell et al[9].The majority of patients required multiple procedures to achieve clinical success.
Attwell et al[9]attained better technical success of complete clearance in patients who had stones in the head/neck (92%) as compared to the body/tail (67%). The same study demonstrated better success for patients with single stone (87%) vs patients with multiple stones (69%). Factors predicting the failure of therapy include multiple strictures, multiple stones and direct visualization failure.
POP was also reported to have an adjunctive intraoperative role with POP-guided EHL during lateral pancreatojejunostomy, having shown reduced rates of subsequent hospitalization and surgeries[25].
The risk of side effects ranges between 0%-29% (Table 1), without any reported mortalities. Broad-spectrum antibiotic prophylaxis was used before POP[9,19], although no clear study to date has evaluated its benefit. Side effects include post-procedure pain and pancreatitis, which was mild in most of the patients using the Cotton criteria. A single study reported perforation with guidewire[13], which was managedconservatively. There is a risk of ductal wall injury if the high energy produced is directed towards it[26], although none were reported in the evaluated studies. Two studies with more than 25% risk of side effects[9,23]had combined use of ESWL and LL/EHL, likely related to patients having more complex stone disease. In the study by Ito et al[23], POP-guided EHL was used as a rescue therapy in patients who failed ESWL.
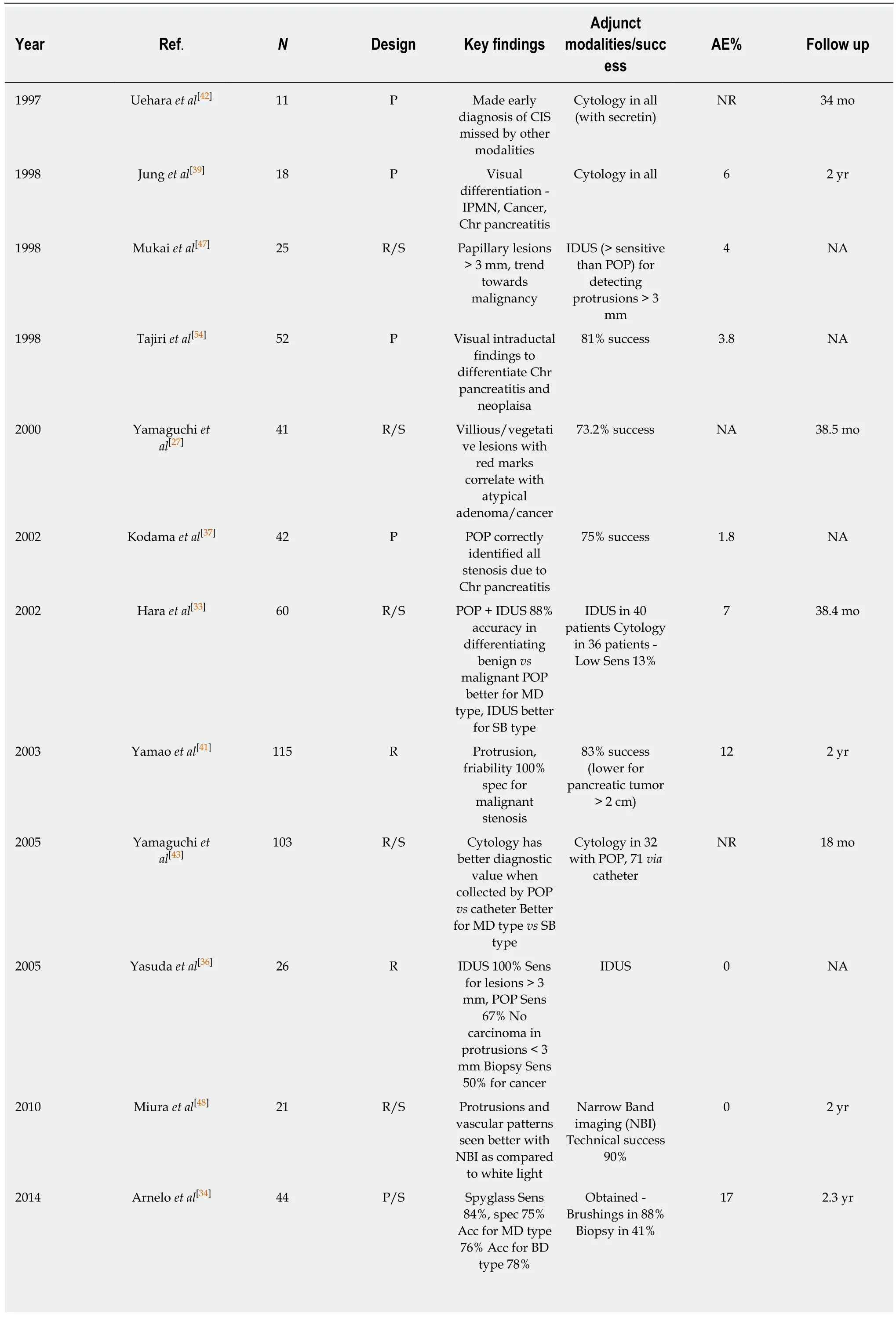
Table 2 Role of per oral pancreatoscopy in pancreatic ductal neoplasia
POP: Per oral pancreatoscopy; IDUS: Intraductal ultrasound; P: Prospective; R: Retrospective; S: Single-center; M: Multicenter; AE: Adverse events; CIS:Carcinoma in situ; MD: Main duct; SB: Side branch; Sens: Sensitivity; Spec: Specificity; Acc: Accuracy.
The overall safety profile is similar as compared to ESWL, which so far has only one reported mortality, along with a few rare complications that include splenic rupture, bowel perforation and liver trauma[18].
Even though there are many published case series evaluating the efficacy of POP-guided therapy for pancreatolithiasis, there is lack of robust randomized prospective data. In addition, most of these studies are from tertiary care centers, and therefore may not be generalizable to the community. PD stone therapy remains challenging,and new prospective data will be needed to better define indications of POP-guided therapy for pancreatic stones. We feel a multidisciplinary consensus meeting between pancreatic endoscopists, pancreatic surgeons and radiologists may help determine the best approach for these patients.
DISCUSSION
Role of POP in pancreatic ducal neoplasia
Ohashi et al first described mucin-producing tumors of the pancreas (MPTP) in 1982[27]. Mucin-producing tumors are comprised of two separate entities: Intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN). IPMN is characterized by papillary proliferation of mucin-producing neoplastic epithelium,which causes cystic dilation of the PD[28]. The entity is comprised of a spectrum of epithelial changes ranging from hyperplasia to carcinoma[29]. IPMN accounts for up to 7% of clinically-diagnosed pancreatic neoplasms, and up to 50% of incidentallydiagnosed pancreatic cysts[30].
Diagnosis of IPMN has increased in recent decades, mainly due to the widespread use of high-resolution cross-sectional abdominal imaging[31]. Since IPMN has malignant potential in 65%-70% of patients[29], the differentiation between benign and malignant tumors is crucial to plan the appropriate therapy, along with timing and extent of surgery if needed.
Various modalities have been employed to assess these lesions. A number of factors, such as main duct diameter, cyst diameter, and the presence or absence of septa and nodules, have been useful in identifying lesions with a higher risk of malignant transformation. However, these features are less prominent in uncharacteristic or early lesions. The multicentric nature of IPMN poses an additional challenge, and may lead to recurrence even after surgical resection with negative margins. Sauvanet et al[32]reported the limitation of using frozen sections by the existence of discontinuous (“skip”) lesions that range from 6%-19% of IPMN in surgical series, and can lead to reoperation in up to 8% of cases. Direct pancreatoscopy has been shown to be useful in differentiating benign mucinproducing tumors of the pancreas from more dysplastic lesions[27].
Role of POP visual impression and POP-guided biopsy
In 2000, Yamaguchi et al[27]investigated the efficacy of POP in differentiating between benign and malignant MPTP by comparing findings in 41 patients with surgical pathology, and characterized them according to the shape of the intraductal elevations and the color features on the lesions. They reported a technical success rate of 73.2%, where failure of examination was associated with branched ductal-type lesions. They classified elevated lesions as sessile, semi-pedunculated, villous and vegetative, and color markings were reported as white or red (spotty/linear). Red color markings were noted only over semi-pedunculated or villous-type lesions. The correlation of POP findings with surgical pathology indicated that villous and vegetative tumors were observed only in patients with severely atypical adenoma and adenocarcinoma. Red color markings were also characteristic of this group, with a sensitivity of 87.5% compared with 16.7% for the group, including hyperplasia and mild/moderately atypical adenoma. In this series, 23% of the patients underwent segmental pancreatic resection with favorable outcomes. Pancreatoscopy also helped identify synchronous lesions at different sites, which were missed by other modalities in three patients, helping to determine the location of surgical resection.
Similar conclusions were noted in a retrospective study of 60 patients who underwent POP (IDUS performed in 40) by Hara et al[33]. They found protruding lesions by POP in 67% of the patients, with better yield in main ductal-type lesions as compared to branching ductal-types. A fish egg appearance with vascular patterning and villous and vegetative lesions were significantly more likely to be malignant as compared to granular appearance or fish eggs without vascular markings.
Arnelo et al[34]prospectively studied the utility of POP in evaluating IPMN in 44 patients with a technical success rate of 93%. They reported additional diagnostic information provided by POP-affected clinical decision-making in 76% of the patient cohort. With operated cases as a reference, the sensitivity of POP was 84% and specificity was 75% in identifying malignant lesions. A classic fish eye papilla was noted in only 35% of the patients with a final diagnosis of MD-IPMN. POP-guided biopsy was diagnostic in 13 of the 17 patients, with inadequate tissue acquisition in four. Nagayoshi et al[35]evaluated 17 patients with radiological diagnosis of IPMN.They used the Spyglass®optical probe inserted into a regular ERCP catheter to inspect lesions in patients with non-dilated MPD or severe angulation, with success in 4/5 patients. Ten patients with protruding lesions were identified, but biopsies could only be obtained in seven due to insufficient angulation of the probe. Targeted biopsies had a sensitivity of 25% and a specificity of 100%. Yasuda et al[36]reported that targeted biopsies had 50% sensitivity and 100% specificity for detecting malignant IPMN in 11 patients. Targeted biopsies may be more challenging in pancreatoscopy as compared to cholangioscopy due to smaller MPD diameter, more tortuous course and the inability to adequately visualize side branch lesions. The diagnostic accuracy could also be affected by the quality of images obtained.
Pancreatoscopy findings in pancreatic cancer may include findings similar to the above, along with erythema, friability, erosions, infiltrative strictures (with near occlusions of the lumen) with irregular margins, or signs of extrinsic compression with normal mucosa. In a series by Kodama et al[37], 5/8 cases of pancreatic cancer were seen adequately, and all had stenosis with a ductal cut-off of MPD.
Parbhu et al[22]studied the impact of POP in 16 patients who had EUS suggestive of IPMN, but definitive diagnosis could not be achieved. They achieved 100% success in obtaining biopsies with a diagnostic accuracy of 75%. Four patients in this cohort had negative biopsies, but strong visual impression led the authors to recommend surgery, with a postoperative diagnosis of IPMN.
El Hajj et al[38]investigated the role of POP in 79 patients with suspected pancreatic ductal neoplasia, with a technical success of 97%. In the subset of patients with confirmed neoplasia (n = 33), POP-guided tissue sampling with the index procedure could confirm diagnosis in 88%. The sensitivity, specificity and accuracy of POP was 87%, 86%, 87%, respectively, whereas it was 91%, 95% and 94%, respectively, for POP plus targeted tissue sampling. The diagnostic yield reported here may be higher due to more extensive methods employed - a minimum of three passes with either POP-guided direct biopsy, POP-assisted fluoroscopic-guided biopsy or POP-guided brushings; a combination of the above was employed in eight patients.
POP-directed tissue acquisition has been shown to be very useful in distinguishing benign from malignant PD strictures. Jung et al[39]prospectively evaluated 18 patients who had indeterminate ductal abnormalities using POP with brush cytology and biopsy (EUS used in three patients only). They confirmed neoplasia in seven and chronic pancreatitis in eight. Macroscopic features of strictures in chronic pancreatitis include white-gray smooth narrowing without superficial vessels. These visual impressions may be critical in distinguishing various etiologies of unexplained pancreatic ductal abnormalities (Table 3). Other findings may include turbid pancreatic juice, protein plugs, indistinct vascular markings, erythema or rough surfaces[40]. Similar findings were noted by Yamao et al[41], where benign stenotic lesions in the PD demonstrated smooth mucosa without protrusions, friability or tumor vessels.
Parbhu et al[22]were successful in dilating 100% strictures in five patients in their study, and were able to obtain targeted biopsies in 80%. Dorsal ductal pancreatoscopy(DDP) via minor papilla can be considered in patients with true or pseudo-divisum presenting with indeterminate strictures, which may be inaccessible via major papilla.Brauer et al[20]attempted DDP in five patients, with technical success of 80%. One failure reported was the inability to obtain biopsies due to acute angulation. These studies suggest the possible role of POP in patients with indeterminate PD strictures.
POP with cytology
Uehara et al[42]reported the early diagnosis of pancreatic carcinoma in situ (CIS) in their study of 72 patients using POP with cytology. Of these, 11 patients had presented with minimal symptoms and abnormal imaging, showing dilated PD without any localizing signs seen by other modalities such as EUS/ERP/CT. A combination of POP with pancreatoscopic cytology was useful in diagnosing and locating CIS, with 100% recurrence-free post-operative survival up to a median of 34 mo. Cytology with POP assistance had a better diagnostic yield compared to catheterassisted collection (100% vs 60%). Hara et al[33]assessed the value of pancreatic juice cytology in 36 out of 60 patients, with low sensitivity of 13% and accuracy of 44% in identifying malignant lesions. K-Ras point mutations were noted in 31 out of 36 patients with high conversion regardless of histologic grade, which manifests as low specificity. Similar results were elicited from a retrospective study of 103 patients by Yamaguchi[43], who found a suboptimal impact of pancreatic juice cytology in differentiating between benign and malignant IPMN. The sensitivity was higher for main PD tumors as compared to branch type (57.9% vs 47.4%) with better results when the pancreatic juice was collected by POP as compared to catheter. In this study,there was a small additional benefit of cytology, even when no high-risk lesions were seen on POP, as 4/7 patients with no malignant stigmata on POP exams had positive cytology. Nagayoshi et al[35]also compared regular pancreatic cytology with irrigation cytology, with reported sensitivity and specificity of 67% and 100%, respectively.
The exact cytological discrimination between benign and malignant lesions is difficult, and results from different studies are variable due to diverse reasons that include observational bias and location of tumors. For this reason, the use of pancreatic juice cytology remains controversial, although supplementary benefits with other modalities can be appreciated. EUS-FNA has the advantage of sampling mural nodules and a superior ability to assess branch-type lesions, which is clearly advantageous in certain settings.
Intraoperative POP
The specific utility of POP to guide surgical therapy in patients with MPTP has been studied prospectively by Kaneko et al[44]in 24 patients. Using surgical pathology as the standard, they reported that the sensitivity, specificity and overall accuracy of intraoperative pancreatoscopies were 100% as compared to 43.8%, 100%, and 60.9%for endoscopic retrograde pancreatography, and 47%, 100%, and 62.5% for endoscopic ultrasonography, respectively. Ten patients were noted to have intraductal MPT that were missed by ERCP and EUS. Five out of these ten patients had multicentric lesions,with three requiring an extension of the planned surgical margin. The overall accuracy to identify lesions was 100% for intraoperative POP vs 60.9% for ERCP and 62.5% for EUS. Similar findings were demonstrated by Navez et al[45]from a retrospective review of 21 patients with suspected IPMN who underwent intraoperative POP, revealing eight occult lesions. Five of these eight patients underwent modified surgery, with 90.5% disease-free survival at a mean of 93 mo.Tyberg et al[46]outlined the role of POP in guiding surgical therapy for lesions in the PD. Out of 13 patients who underwent POP, the initial surgical plan was altered in eight (62%), with an overall correlation of 88% between pancreatoscopy and final surgical histology.
This confirms that intraoperative pancreatoscopy is safe and effective in evaluating main ductal IPMN, with the specific advantage of diagnosing multicentric lesions.These may be missed on ERCP or EUS, thus highlighting its complimentary nature to these modalities. Preoperative thorough direct examination of the PD may be limited due to the acute angle noted at the junction of the duct of Wirsung and Santorini, and intraoperative POP helps in overcoming this problem.
IDUS with POP
Mukai et al[47]evaluated mucin-producing tumors in 25 patients with POP and IDUS.They concluded that papillary tumor height of more than 3 mm implied moreadvanced dysplastic lesions. The sensitivity of detecting lesions more than 3 mm was 29% for US, 21% for CT, 86% for EUS, 100% for IDUS and 83% for POP. Adequate examination of papillary lesions using POP was technically successful in 60% of the total patients. The sensitivity for detecting protrusions more than 3 mm was 100% for IDUS and 67% for POP in a study of 26 patients by Yasuda et al[36]. In this study, out of the six patients with adenocarcinoma, none had protrusions less than 3 mm on the resected pathological specimen. The same study demonstrated the suboptimal diagnostic capability of cross-sectional imaging for protruding lesions, with 16 % for CT scan and 20% for MRI.
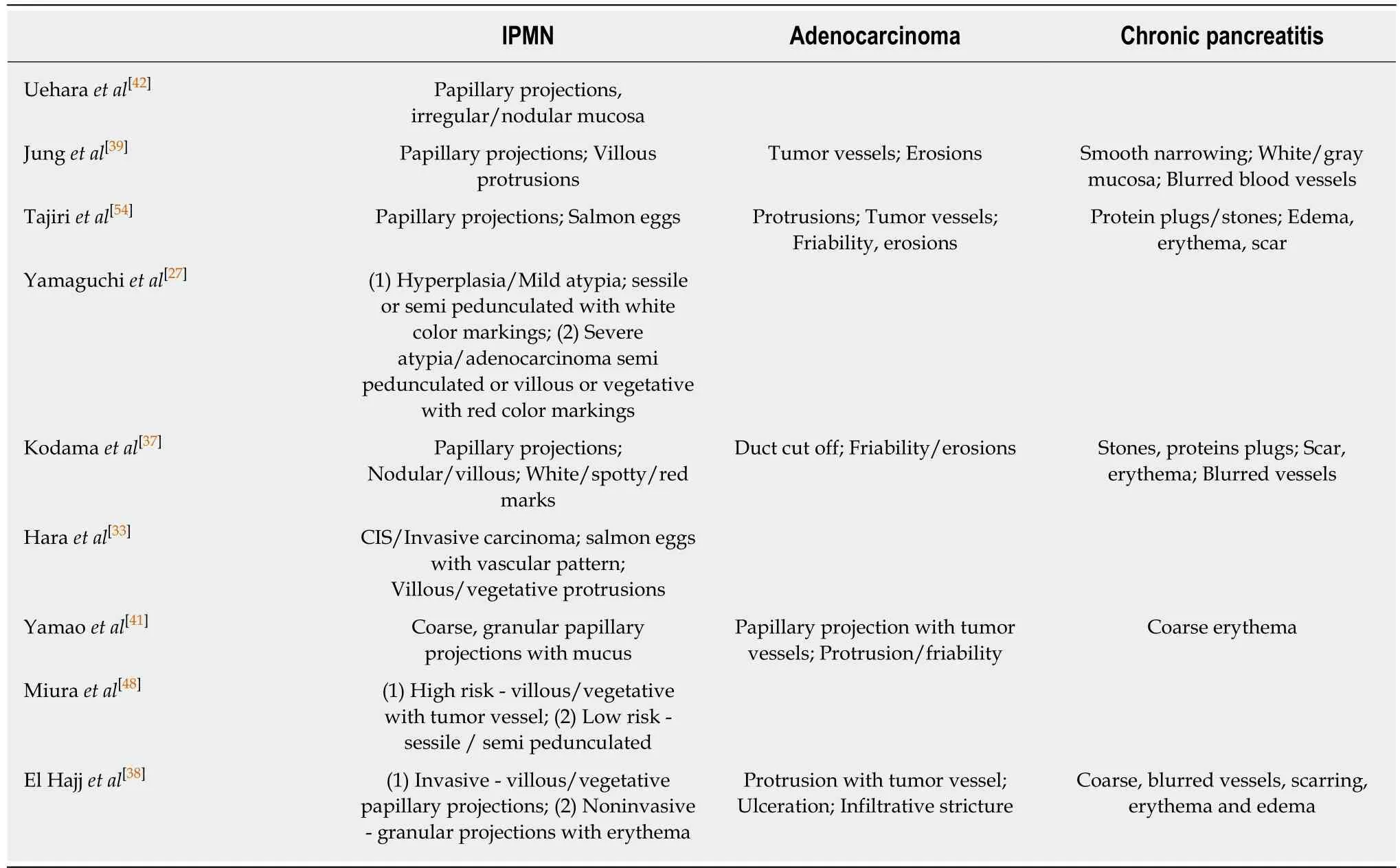
Table 3 Per oral pancreatoscopy visual findings for pancreatic ductal abnormalities
In the study performed by Hara et al[33], 88% of the lesions with villous projections more than 4 mm on IDUS were malignant. The diagnostic accuracy of POP alone in differentiating benign/malignant was 88% and 67% for main duct and branch duct,respectively, as compared to IDUS with an accuracy of 63% and 88%. Their study confirmed that adding IDUS to POP improves the evaluation of branch ductal-type lesions. The combined accuracy rate for different modalities such as CT, EUS, POP and IDUS was 55%, 65%, 75% and 78%, respectively, with the highest rate of 88% for POP plus IDUS combined. Surgical pathology served as the gold standard in this study. Most malignant tumors had POP visual morphology types III, IV or V (as per the Yamaguchi classification). The benefit of using this combined modality was evident in the fact that reduced operations were performed in 33 out of 60 patients,with only one positive resection margin that was due to infiltrative parenchymal changes. Critically, management based on these criteria culminated in an extraordinary 95% 3-year cumulative survival rate and a 93% disease-free survival rate.
IDUS is particularly useful to visualize branches distant from the probe and the parenchyma, and plays a crucial complementary role to POP. IDUS also has better efficacy for early lesions like CIS, due to higher resolution and probe location as compared to EUS.
POP with image-enhancing technology
Miura et al[48]assessed POP-guided NBI (narrow band imaging) in 21 patients with IPMN. They used a small diameter videoscope CHF-BP260 (Olympus medical systems) with an outer diameter of 2.9 mm, and achieved technical success of 90%.Vascular patterns and protrusions were detected more clearly in NBI images as compared to examination under white light. Similar findings were observed by Ito et al[49]. NBI identified skip tumor lesions in the tail of the pancreas, which were not detected by conventional POP.
Other adjuvant imaging modalities utilizing POP are also being evaluated. Meining et al[50]prospectively studied the role of probe-based confocal laser endomicroscopy(pCLE) in assessing indeterminate pancreatobiliary strictures. The accuracy of the combination of ERCP and pCLE was significantly higher compared with ERCP, with tissue acquisition (90% vs 73%, P = 0.001) having higher specificity in the exam when the probe was delivered via cholangiopancreatoscopy as compared to a standard catheter.
The risk of pancreatitis in these series, which ranged between 0%-35%, seemed to be higher in patients without dilated MPD, and also depended on the level of experience of the operator[34,35]. Arnelo et al[34]recorded one fatal case of post-POP pancreatitis. They postulated that reducing the flow rate could help in minimizing the risk of it, however this needs further evaluation.
The role of POP for intraductal pancreatic neoplasia has evolved over time with the availability of longitudinal data and rapid technological improvements. Prospective multicenter studies of POP with selected adjunct modalities may eventually address the true value of POP in the evaluation and management of pancreatic ductal neoplasia. POP will continue to serve a crucial complementary role for such patients,in addition to cross-sectional imaging and EUS. Appropriate application will likely be restricted to high volume tertiary care centers where multidisciplinary approaches will guide the treatment of such rare diseases.
In conclusion, this review illustrates the crucial role POP may play in the management of pancreatic disease by providing direct macroscopic assessment,targeted tissue acquisition and the opportunity for guided endotherapy. The application of this technology has been largely limited to high volume expert centers due to the procedural complexity, the morbidity of the conditions being treated,technical challenges, and cost. There is significant heterogeneity in the available data,with variable patient follow-up, lack of control arms and retrospective designs.Innovations like larger fields-of-view, higher image resolution, integrated image enhancements, and larger working channels may augment the capability of the procedure. Well-designed and powered prospective trials would refine the role of POP in the management of pancreatic disease.
ARTICLE HIGHLIGHTS
Research background
Pancreatoscopy has been used for over 30 years in the diagnosis and management of pancreatic diseases; however, its use remains limited to large volume referral centers. Data regarding its efficacy and safety are limited and have been available mainly from single or multicenter retrospective case series. Well-designed large randomized controlled trials are lacking and may be difficult to conduct due to a heterogeneous patient population. With this study, we have compiled a systematic review of available data, thus highlighting the valuable role of per oral pancreatoscopy in managing pancreatic diseases.
Research motivation
The main aim of our study was to systematically analyze available data regarding the therapeutic potential of pancreatoscopy in managing difficult pancreatic stone disease and pancreatic ductal neoplasia. It appears to be safe, with rare serious side effects, and serves a crucial complementary role to other pancreatic endoscopic modalities.
Research objectives
The main objective of the study was to gather data related to the safety and efficacy of pancreatoscopy. We wanted to identify the success rates and factors associated with treatment failure for pancreatoscopic management of stone disease. We also aimed to analyze the pancreatoscopic visual findings associated with pancreatic ductal neoplasia, and how it can be differentiated from benign pancreatic duct strictures. The diagnostic potential of adjunctive techniques like POP guided/assisted biopsy, pancreatic juice cytology and intraductal ultrasound (IDUS) was evaluated separately.
Research methods
This is a systematic review of available studies published in English. We performed an extensive medical database search to identify relevant publications. Case reports and stand-alone abstract publications were excluded from the final analysis. Data regarding safety and efficacy were extracted and presented. Studies addressing the role of POP in management of pancreatic ductal neoplasia with adjunctive modalities were examined separately.
Research results
Pancreatoscopy is overall safe, with rare reported serious side effects. The success rate ranges between 37.5%-100% for treating pancreatic stone disease. Factors associated with failure include the presence of multiple stones, stones in side branches causing failure of visualization, and the presence of stricture. Visual impression during pancreatoscopy provides important information in patients with indeterminate pancreatic ductal strictures. The key finding in our study was the association between villous projections with red color markings, which is associated with highrisk advanced neoplastic lesions across multiple studies. Smooth narrowing with the presence of coarse mucosa, protein plugs or stones, and blurred mucosal vessels are seen in patients with strictures caused by chronic pancreatitis. POP-assisted tissue acquisition, as well as adjunctive techniques such as cytology, narrow band imaging and IDUS, greatly enhance the diagnostic potential and help in treatment planning.
Research conclusions
Pancreatoscopy is an overall safe and effective diagnostic and therapeutic modality. It serves as an important bridge for patients with pancreatolithiasis who fail conventional Endoscopic retrograde cholangiopancreatography or ESWL. Patients with multiple stones in body/tail, or those with pancreatic strictures, may have risk of decreased success with POP-guided therapy;the recognition of these factors may help in treatment planning. POP visual impression provides a plethora of information regarding etiology in patients with indeterminate pancreatic ductal strictures, although there is an overlap between benign and malignant conditions. POP-guided tissue acquisition has been shown to greatly enhance the diagnostic yield, but limitations persist due to technical challenges. The addition of newer imaging technology may further augment the potential of POP in managing such scenarios.
Research perspectives
Appropriate future action may involve multicenter prospective studies to identify patient characteristics, which may make them amenable to POP-guided endotherapy for pancreatic diseases. Continued improvement in imaging technology, such as narrow band imaging and probe-based confocal laser endomicroscopy, need to be evaluated extensively before mainstream use is implemented.
杂志排行
World Journal of Gastrointestinal Endoscopy的其它文章
- Safety and efficacy of over-the-scope clip-assisted full thickness resection of duodenal subepithelial tumors: A case report
- Narrow band imaging evaluation of duodenal villi in patients with and without celiac disease: A prospective study
- Age, socioeconomic features, and clinical factors predict receipt of endoscopic retrograde cholangiopancreatography in pancreatic cancer
- No significant difference in clinically relevant findings between Pillcam® SB3 and Pillcam® SB2 capsules in a United States veteran population
- Spectrum of gastrointestinal involvement in Stevens - Johnson syndrome
- Endoscopic ultrasound-guided drainage of the biliary system:Techniques, indications and future perspectives
