Effect of silicon to aluminum ratio on the selectivity to propene in methanol conversion over H-ZSM-5 zeolites
2019-01-22HUANGHuiwenZHUHuiZHANGShanheZHANGQiangLIChunyi
HUANG Hui-wen, ZHU Hui, ZHANG Shan-he, ZHANG Qiang, LI Chun-yi
(State Key Laboratory of Heavy Oil Processing, China University of Petroleum (East China), Qingdao 266580, China)
Abstract: A series of H-ZSM-5 zeolites with a silicon to aluminum ratio of 50-4000 but similar crystal size were synthesized and characterized by XRD, N2 sorption, NH3-TPD and Py-FTIR; the intrinsic effect of silicon to aluminum ratio on the selectivity to propene in the conversion of methanol to propene (MTP) was investigated. The results show that a complete conversion of methanol can be initially achieved over H-ZSM-5 with a silicon to aluminum ratio from 50 to 1600 and then the initial conversion of methanol decreases progressively with further increasing the silicon to aluminum ratio. Meanwhile, the selectivity to propene increases monotonically with an increase in the silicon to aluminum ratio of H-ZSM-5 for MTP with a complete methanol conversion, suggesting that a high Si/Al ratio for H-ZSM-5 may enhance the propagation of the alkene-based methylation/cracking cycle relative to the arene-based methylation/dealkylation cycle in MTP. A critical value of acid density, viz., [AS]S, is required to achieve the maximum propene selectivity for MTP with a complete methanol conversion; this critical [AS]S value is 0.175 μmol/m2 for the H-ZSM-5 zeolite under current reaction conditions.
Key words: methanol; propene; H-ZSM-5; silicon to aluminum ratio; catalytic acidity
As a basic feedstock of petrochemical industry, propene is used in the production of various polymers and chemical intermediates, such as polypropene, acrylonitrile, acrolein, and propene oxide[1-3]. Nowadays, propene is mainly produced as a by-product of naphtha steam cracking and catalytic cracking processes, which are both dependent on the crude oil resources[4]. Due to the increasing demand for propene and the shortage of crude oil resources in recent years, finding an alternative process of non-oil route for propene production has become more and more important[5,6]. Since methanol can be expediently produced via synthesis gas from multifarious sources like coal, natural gas and biomass with lower prices and greater availability than crude oil, methanol to propene (MTP) process has attracted significant attention[7-12]. Over the past decades, several zeolite catalysts have been investigated in MTP, such as ZSM-5[13,14], ZSM-11[15], and EU-1[16]. At present, ZSM-5 zeolite is regarded as the most promising archetype catalyst for this process because of its high surface area, adjustable acid properties and well-defined pore network.
MTP is an acid-catalyzed reaction, in which methanol conversion and product distribution depend strongly on the amount and strength of acid sites on H-ZSM-5 zeolites under optimized reaction conditions[14,17-19]. Hence, most of the studies are focused on the effect of framework silicon to aluminum ratio of ZSM-5 zeolites on their catalytic performance in MTP, which is an intrinsic factor to determine the acidic properties of zeolites[20,21]. Although certain significant achievements have been made, there are still no accordant acknowledgements about the relationship between the acidic properties and performance of zeolite catalyst for MTP. For example, Wan et al[22]studied the effect of silicon to aluminum ratio (SiO2/Al2O3= 23, 47, 107, 217 and 411) on the performance of nanocrystal ZSM-5 zeolite catalysts in methanol to gasoline (MTG) and found that nanocrystal catalyst with a moderate silicon to aluminum ratio of 217 showed the highest gasoline yield with a complete methanol conversion. However, Michels et al[23]reported that a suitable range of silicon to aluminum ratio was 30-70 for the micron-sized zeolitic catalysts in MTG. Liu et al[2]discovered that the aromatization reaction was significantly suppressed by ZSM-5 zeolites (SiO2/Al2O3= 24, 200, 300, 440 and 720) with a higher silicon to aluminum ratio, over which propene became the predominant product in MTP. In contrast, Zhang et al[24]found that compared with H-ZSM-5 with a silicon to aluminum ratio of 150, more and less aluminum species in the H-ZSM-5 catalysts (SiO2/Al2O3= 68, 154 and 410) were not favorable for increasing propene selectivity. Wei et al[25]investigated the product distribution of methanol conversion over ZSM-5 zeolites with different silicon to aluminum ratios (SiO2/Al2O3= 60, 120, 180 and 220) and concluded that a silicon to aluminum ratio of 220 afforded the highest propene selectivity in MTP. In short, the silicon to aluminum ratio of ZSM-5 zeolites plays an important role in methanol conversion and it is necessary to deeply understand the relationship between the acidic properties and catalytic performance so as to develop an efficient catalyst in methanol conversion.
In this contribution, H-ZSM-5 zeolites with a silicon to aluminum ratio from 50 to 4000 but similar crystal size were prepared, characterized, and evaluated as catalysts for MTP. Based on the catalyst characterization and reaction results, the relationship between methanol conversion and propene selectivity and relevant acid sites was elucidated in detail. It was revealed that for MTP over high-silica HZSM-5 zeolites, the alkene-based methylation/dealkylation cycle dominates the reaction and contributes primarily to propene formation. Furthermore, a critical value of acid density required for the maximum propene selectivity in MTP with a complete methanol conversion was addressed.
1 Experimental
1.1 Catalyst preparation
A series of hierarchical ZSM-5 zeolites with a silicon to aluminum molar (SiO2/Al2O3) ratio of 50-4000 were synthesized through the traditional hydrothermal crystallization method. In a typical procedure for the synthesis of HZ-5(50) with a SiO2/Al2O3ratio of 50, 0.28 g sodium aluminate (NaAlO2, 26.3%) was dissolved thoroughly in 10 mL tetrapropylammonium hydroxide (TPAOH, 25%) aqueous solution under stirring, followed by adding 35 g tetraethylorthosilicate (TEOS, A.R.). The final pH value was adjusted to be about 10 by drop-wise addition of sulfuric acid (H2SO4, 95%-98%). After vigorously stirring under room temperature for 5 h, the gel was transferred into a static Teflon-lined stainless-steel autoclave and crystallized at 443 K under autogeneous pressure for 48 h. The synthesized product was filtered, washed, dried, and calcined at 823 K in air for 6 h to remove the template. ZSM-5 zeolites with different silicon to aluminum (SiO2/Al2O3) ratios were then synthesized by varying the amount of NaAlO2in the synthetic gel.
The synthesized ZSM-5 zeolites were turned into H-form (H-ZSM-5) by three consecutive ion-exchange cycles with 1 mol/L NH4NO3solution at 353 K for 2 h under continuous agitation. After that, the sample powders were filtered and washed with deionized water until the pH value was about 7. The filtered powders were then dried at 393 K in an oven and calcined again at 823 K for 2 h. The obtained samples were denoted as HZ-5(x), wherexrefers to the SiO2/Al2O3molar ratio. Prior to reaction tests, all H-ZSM-5 powder samples were pressed into pellets and subsequently crushed and sieved into 40-60 mesh.
1.2 Catalyst characterization
X-ray diffraction (XRD) patterns were recorded on an X’Pert PRO MPD diffractometer (PANalytical Co., Netherlands,λ= 0.15406 nm) at 40 kV and 40 mA with CuKαradiation, in the 2θrange from 5° to 65° with a step size of 2°.
Scanning electron microscope (SEM) images were taken on an S-4800 field emission scanning electron microscope without deposition of any conducting materials or layers.
Nitrogen adsorption-desorption isotherms were obtained on a Quadrasorb SI instrument (Quantachrome, USA) at liquid nitrogen temperature. Prior to measurements, the samples were degassed at 573 K under vacuum for 12 h to remove the adsorbed moisture. The total surface area (ABET) was calculated by the Brunauer-Emmett-Teller (BET) equation and the total pore volume (vtotal) was estimated from the nitrogen adsorption at a relative pressure of 0.99. Both the micropore surface area (Amicro) and micropore volume (vmicro) were determined by thet-plot method. The external surface area (Aext) and the mesopore volume (vmeso) were the difference between the total values and the corresponding micropore data.
Temperature-programmed desorption of ammonia (NH3-TPD) was conducted on a TP-5078 chemisorption analyzer (Xianquan Co., China). About 200 mg of sample (40-60 mesh) was pretreated at 873 K for 0.5 h under flowing helium. The sample was then cooled down to 373 K and saturated with NH3. After that, the sample was purged with flowing helium to remove the weakly and physically adsorbed NH3until a stable TPD signal was attained; the sample was then heated from 373 to 923 K at a ramp of 10 K/min. The acidity of the zeolite samples was determined on the basis of the quantity of NH3desorbed at the corresponding temperature range.
Fourier transform infrared spectra of adsorbed pyridine (Py-FTIR) were measured on a Tensor 27 FT-IR instrument (Bruker, USA) equipped with a MCT detector. Prior to measurements, self-supporting wafers of the zeolite samples were preheated at 423 K for 12 h in an evacuated Pyrex glass cell equipped with CaF2windows, and then cooled down to room temperature. The adsorption of pyridine was performed by exposing the zeolite wafers to the pyridine vapor at room temperature for 2 h. After that, the samples were evacuated at 423 K and 1.0 Pa for 4 h and the FT-IR spectra were then recorded at room temperature in the range of 4000-600 cm-1with a resolution of 4 cm-1and 64 scans. The integrated molar absorption coefficients used to determine the quantity of Brønsted and Lewis acid sites were 1.67 and 2.22 cm/μmol, respectively[26].
1.3 Catalytic activity tests
MTP reaction was carried out at 723 K in a stainless steel fixed-bed reactor (12 mm i.d.) under atmospheric pressure. The reactor was heated by a resistive furnace, which was regulated by a Watlow 96 series temperature controller, and the bed temperature was monitored by using a K-type thermocouple positioned in the center of the catalyst bed. For each test, 1.0 g catalyst powder of 40-60 mesh was loaded into the center of the reactor, and the volume up- and down-flow to the catalyst bed was filled with 200 mg inert quartz sands to sustain the temperature profile along the catalyst bed. Prior to each reaction test, the catalyst was pretreated in dry air at reaction temperature for 2 h. The liquid methanol at a weight hourly space velocity (WHSV) of 9.72 h-1was then fed through a HPLC infusion pump together with nitrogen at a constant flow of 20 mL/min into the reactor. The effluent stream was separated into gaseous and aqueous phases by a cold trap. The gaseous phases were analyzed by a Bruker 450 gas chromatography (GC) equipped with a flame ionization detector (FID) and two thermal conductivity detectors (TCDs). Likewise, the aqueous phases were analyzed on an Agilent 6820-GC fitted with HP-INNOWAX capillary column (30 m × 0.32 mm × 0.25 μm) and a FID, using ethanol as the internal standard for calibration.
The catalyst activity is expressed in terms of methanol conversion, which is calculated from the difference between the inlet and outlet concentrations of methanol and dimethyl ether (DME). Product selectivity on carbon basis (CH2) is defined as the mole ratio of each product referred to the converted methanol and DME. The mass balance was above 95%.
2 Results and discussion
2.1 Physicochemical properties of H-ZSM-5 zeolites
Figure 1 shows the XRD patterns of H-ZSM-5 zeolites; all the synthesized samples display the typical diffraction peaks of intrinsic MFI structure. Taking HZ-5(4000) zeolite as a reference, they all have a relative crystallinity of 90%-100%, demonstrating their highly crystalline MFI structure. The textural properties determined from the nitrogen adsorption-desorption isotherms are summarized in Table 1; all the synthesized H-ZSM-5 samples have a similar BET surface area (415-430 m2/g) and micro surface area (306-341 m2/g), as well as almost the same pore volume (0.20 cm3/g) and micropore volume (0.15 cm3/g). In addition, as estimated by Scherrer’s method from the diffraction peak of (011) plane at 7.9°, all the as-synthesized samples have a similar crystal size of 66-73 nm (Table 1).

Figure 1 XRD patterns of the H-ZSM-5 zeolites with different SiO2/Al2O3 ratios
The acidic properties of the synthesized H-ZSM-5 zeolites were first determined by NH3-TPD; the ammonia quantities desorbed in the temperature ranges of 373-500, 500-600 and 600-923 K are assigned to the weak, medium and strong acid sites, respectively.

Figure 2 NH3-TPD profiles of the H-ZSM-5 zeolites with different SiO2/Al2O3 ratios
As shown in Figure 2, all NH3-TPD profiles have a similar shape; however, the peak position and intensity of the H-ZSM-5 zeolites are distinctly dependent on the silicon to aluminum ratio. As expected, the amounts of weak, medium and strong acid sites all decrease dramatically with an increase in the silicon to aluminum ratio (Table 2), that is, the number of the acid sites on H-ZSM-5 is related to the aluminum content[27]. Meanwhile, a maximum ratio of strong to weak acid sites (S/W) is observed for the H-ZSM-5 zeolite with a silicon to aluminum ratio of 1600 (Table 2).

Table 1 Textural properties and relative crystallinity of the H-ZSM-5 zeolites with different SiO2/Al2O3 ratios
a: surface area was obtained by the BET method; the micropore surface area and volume were determined by thet-plot method; total pore volume was estimated from the nitrogen adsorption atp/p0= 0.99; the external surface area and mesopore volume were the difference between the total calculated value and the corresponding micropore data;
b: crystal size was estimated by Scherrer’s method from the XRD patterns using the diffraction peak at about 7.9°;
c: relative crystallinity was estimated by comparing the XRD peak intensities at 2θof 8.0°-9.0° and 22°-25° with those of the reference HZ-5 (4000) sample
The Py-FTIR spectra of H-ZSM-5 zeolites were further measured in the region of 1700-1400 cm-1; two bands at around 1540 and 1450 cm-1are assigned to the Brønsted and Lewis acid sites, respectively[28]. Likewise, both the Brønsted and Lewis acid sites decrease sharply with the increase of the silicon to aluminum ratio. In contrast, the ratio of Brønsted to Lewis acid sites (B/L) decreases first with an increase in the silicon to aluminum ratio from 50 to 1600, and then increases with further increasing the silicon to aluminum ratio from 1600 to 4000, as given in Table 2; that is, the H-ZSM-5(1600) exhibits the maximum S/W ratio and the minimal B/L ratio.
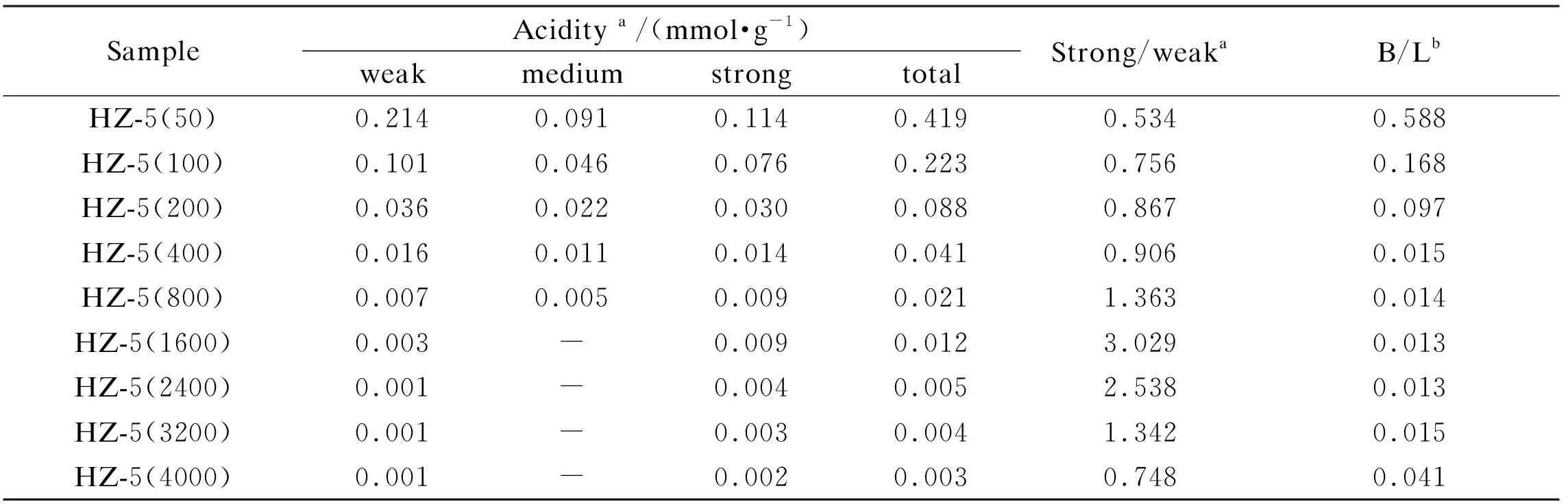
Table 2 Acidity of H-ZSM-5 zeolites with different SiO2/Al2O3 ratios
a: the amounts of weak, medium, and strong acid sites were calculated from the NH3-TPD profiles by integrating the ammonia desorption lines in the range of 373-500, 500-600, and 600-923 K, respectively;
b: the ratio of Brønsted to Lewis (B/L) acidity is obtained by comparing the band at 1550 cm-1to that at 1455 cm-1in the Py-FTIR spectra
2.2 Catalytic performance of H-ZSM-5 zeolites in MTP
Figure 3 illustrates the initial conversion of methanol over the H-ZSM-5 zeolites with different silicon to aluminum ratios. In general, the conversion of methanol decreases constantly with an increase in the silicon to aluminum ratio. All the H-ZSM-5 catalysts with a silicon to aluminum ratio below 1600 demonstrate almost a complete conversion of methanol at the beginning; however, a further increase in the silicon to aluminum ratio to 2400 leads to an incomplete methanol conversion (93.21%) even at the very beginning of the MTP reaction. It is generally accepted that the initial conversion of methanol depends heavily on the structural integrity of the catalyst and the number of acid sites on the catalyst surface[29,30].

Figure 3 Methanol conversion (●/■), catalyst weight (1/2/3) and total acidity (0.003-0.419) of the H-ZSM-5 zeolites as a function of SiO2/Al2O3 ratio (reaction conditions: 723 K, 0.1 MPa, WHSV= 9.72 h-1)
Since all the synthesized H-ZSM-5 zeolites exhibit the MFI structure (Figure 1) with high crystallinity (Table 1), the incomplete initial methanol conversion over HZ-5(x) samples ofx> 1600 should be ascribed to their insufficient acid sites on the catalyst surface. The conversion of methanol over HZ-5(x) ofx≤ 200 remains steady over the reaction time studied (130 min), suggesting a sustained activity of H-ZSM-5 zeolites with a low silicon to aluminum ratio.
The methanol conversion is closely related to the total acidity and a certain number of acid sites are then required for a complete transformation of a definite quantity of methanol. Figure 3 also shows the initial conversion of methanol over 2 g HZ-5(2400) and 3 g HZ-5(3200), which indicates that if the total acidity reaches a critical value by adding more catalyst, almost 100% of initial methanol conversion can be attained. That is to say, a complete conversion of methanol cannot be achieved until sufficient active centers or acid sites are provided; therefore, a critical quantity of acid sites ([AS]C) is required for the complete transformation of a certain quantity of methanol, which is about 18.93 μmol here for MTP over H-ZSM-5 under current reaction conditions.
The initial methanol conversions over the HZ-5(x) catalysts (x= 50-1600) are similar, although these zeolites are different in the nature, amount and strength of available acid sites on the catalyst surface. It suggests that the amount of acid sites on the HZ-5(x) catalysts (x= 50-1600) is enough for a complete conversion of methanol during the initial reaction period. As reported by Campbell et al[31], only a small amount of active/acid sites is necessary for H-ZSM-5 to be an effective catalyst in MTG. Furthermore, it seems that the conversion of methanol depends mainly on the strong acid sites rather than on the weak acid sites, consistent with the early observation that the number of strong acid sites controlled the initial reactivity, though the weak acid sites might also contribute to reaction for methanol conversion[18,32].
The propene selectivity, ethene selectivity and the selectivity ratio of ethene to 2-methylbutane and 2-methyl-2-butene (2MBu) as a function of silicon to aluminum ratio are shown in Figure 4.
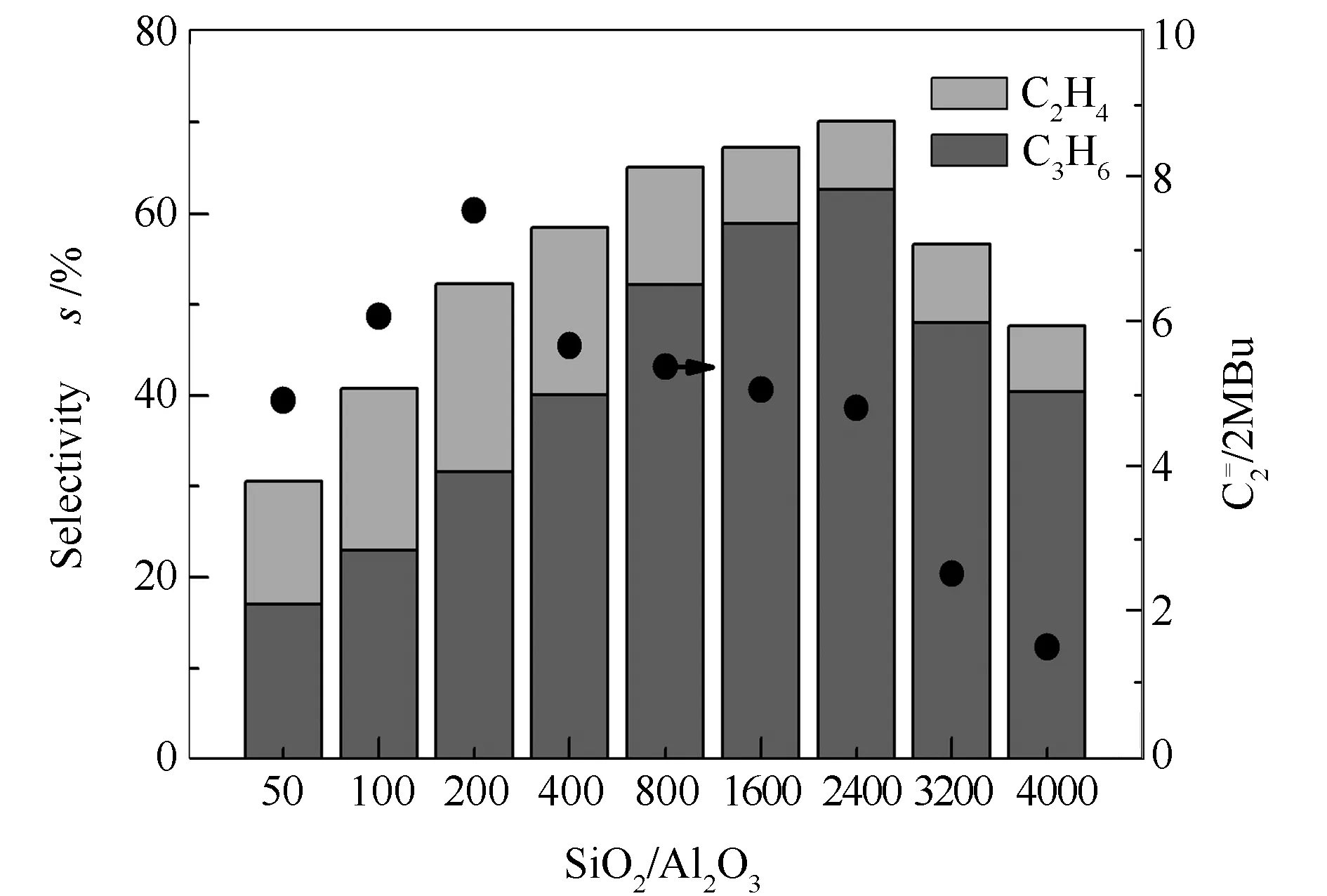
Figure 4 Selectivity to propene and ethene and the ratio for MTP over the H-ZSM-5 zeolites with different SiO2/Al2O3 ratios
Both the selectivities to propene and ethene increase first and then decrease with the increase of silicon to aluminum ratio; there is a proper silicon to aluminum ratio to achieve the maximum selectivity to propene. In particular, for MTP with a complete methanol conversion, the selectivity to propene increases from 17.06% to 58.91% over the H-ZSM-5 zeolites with an increase of the silicon to aluminum ratio from 50 to 1600; the selectivity to propene reaches its maximum value of 62.62% on HZ-5(2400), although the conversion of methanol is no more complete . With a further increase in the silicon to aluminum ratio (>2400), the selectivity to propene decreases perpetually. However, when the complete transformation of methanol can be achieved by loading more catalyst, the selectivity to propene and butene reaches its maximum value for HZ-5(2400). That is, the product selectivity for MTP is also strongly related to the silicon to aluminum ratio of the H-ZSM-5 zeolites. Similarly, a maximum selectivity to ethene is observed for H-ZSM-5 with a silicon to aluminum ratio of 200.


Table 3 Methanol conversion, product distribution and C4 hydrogen transfer index of for MTP over H-ZSM-5 zeolites with different SiO2/Al2O3 ratios
The conversion of methanol to hydrocarbons (MTH) involves a series of reactions, in which methanol is first dehydrated to DME and water, and then the mixture of the oxygenates is converted to light olefins[11,32,33]. Later, the light olefins react to form higher olefins, alkanes, aromatics, and even coke through hydrogen transfer, cyclization and oligomerization reactions. The complex network of reactions for methanol conversion over acidic zeolites has been summarized to many mechanisms and recent studies have demonstrated that the dual-cycle mechanism is dominant in the steady state period of MTH reaction[34-36]. In this mechanism, the alkene-based cycle leads predominantly to propene and higher alkenes through rapid methylation and cracking reactions, whereas the arene-based cycle produces mainly ethene from methylbenzenes followed by re-methylation. Furthermore, two catalytic cycles are not independent of each other as higher alkenes can participate in the arene-based cycle via hydrogen transfer and cyclization reactions, and light olefins dealkylated from methylbenzenes can also be recycled into the alkene-based cycle by methylation and oligomerization reactions, as illustrated in Figure 5.
It has been previously mentioned that both the strong and weak acid sites are essential for the initial reactivity of methanol. Likewise, they are crucial to the production of hydrocarbons from methanol. In particular, the strong acid sites facilitate the hydrogen transfer, cyclization and oligomerization reactions for the formation of alkanes, aromatics, and even coke, whereas the weak acid sites promote the production of light olefins through alkylation and methylation reactions[33,35-37]. Accordingly, the hydrogen transfer and cyclization reactions are significantly suppressed by increasing the silicon to aluminum ratio (50-1600) and meanwhile, propene becomes the predominant product accompanied with a limited generation of alkanes and aromatics (Table 3). In addition, according to the dual mechanism, strong acid sites are probably more favorable to the propagation of the arene-based cycle, whereas weak acid sites are more conductive to the alkene-based cycle. That is, the relative propagation of the arene-based and alkene-based catalytic cycle can be viewed as a consequence of the relative number of strong and weak acid sites on the H-ZSM-5 zeolites. The alkene-based cycle propagates to a greater extent due to an increase in silicon to aluminum ratio with relatively less strong acid sites and more weak acid sites, and consequently results in higher selectivity to propene, with lower selectivity to alkanes and aromatics.

Figure 5 Proposed reaction pathways for methanol conversion over the H-ZSM-5 zeolites[33]

2.3 A single-value descriptor for methanol conversion and propene selectivity in MTP
As discussed above, the silicon to aluminum ratio of H-ZSM-5 zeolites has an important effect on methanol conversion and propene selectivity in MTP. Methanol conversion decreases constantly with an increase in the silicon to aluminum ratio; however, a complete initial conversion of methanol can be attained by increasing the total acidity over a critical value through loading more catalyst. Likewise, the selectivity to propene increases with an increase in the silicon to aluminum (50-1600) for MTP with a complete methanol conversion; if the amount of acid sites of loaded catalyst reaches its critical value, the maximum propene selectivity can be achieved. That is, only a small amount of acid sites is necessary for H-ZSM-5 to achieve a complete methanol conversion and the H-ZSM-5 zeolites with different silicon to aluminum ratios (HZ-5(x),x= 50-4000) exhibit similar methanol conversion in MTP if they are provided with the same amount of acid sites, especially the strong acid sites.
Since MTP is an acid-catalyzed reaction, both the conversion of methanol and the selectivity to propene are strongly related to the number of acid sites on the catalyst surface[24,29,30]. A higher amount of acid sites provide sufficient active centers for methanol molecules and then benefit the conversion of methanol; besides, a higher surface area accelerate the desorption of light olefins and thereby lower the probability of subsequent reactions of light olefins to alkanes and aromatics. Meanwhile, MTP is very sensitive to the amount of acid sites on catalyst surfaces, only when the amount of acid sites on catalyst surfaces reaches a critical value, good catalytic capability in terms of methanol conversion and propene selectivity can be attained. Namely, the underlying mechanistic basis for the effect of silicon to aluminum ratio is the appropriate concentration of acid sites on the catalyst surface, which can be combined into a single-value descriptor ([AS]S, mmol/m2, the average number of acid sites on the catalyst surface).
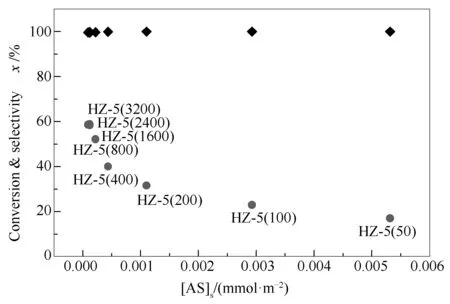
Figure 6 Methanol conversion () and propene selectivity () as a function of acid density
As shown in Figure 6, the selectivity to propene increases progressively with a decrease in the value of [AS]S, implying that a lower concentration of acid sites on the catalyst surface can enhance the propagation of the alkene-based cycle relative to the arene-based cycle. An increase in the silicon to aluminum ratio may reduce the interaction between methylbenzenes and acid sites and thereby decrease the probability of sequential chain growth-ring closure-hydride transfer reactions required to form aromatics, leading to an increase in the selectivity to propene[44]. As a result, there exists an optimal silicon to aluminum ratio in terms of both methanol conversion and propene selectivity. In this work, the critical value of acid density ([AS]S) required to achieve a maximum selectivity to propene for MTP with a complete methanol conversion is estimated to be 0.175 μmol/m2under current reaction conditions when 0.162 g/min methanol is fed; HZ-5(1600) is propoably provided with the suitable acidity distribution with intrinsic MFI structure and then exhibits a high yield of propene (58.60%) with a initial complete conversion of methanol.
3 Conclusions
A series of H-ZSM-5 zeolites with a silicon to aluminum ratio of 50-4000 but similar crystal size were synthesized and the intrinsic effect of silicon to aluminum ratio on the catalytic activity of H-ZSM-5 and selectivity to propene in MTP was investigated.
The results indicate that the silicon to aluminum ratio of H-ZSM-5 zeolite has a great influence on methanol conversion and product distribution and high-silica H-ZSM-5 zeolite can be an effective catalyst for MTP. A complete conversion of methanol can be initially achieved over H-ZSM-5 with a silicon to aluminum ratio from 50 to 1600 and then the initial conversion of methanol decreases progressively with a further increase in the silicon to aluminum ratio. Meanwhile, the selectivity to propene increases monotonically with an increase in the silicon to aluminum ratio of H-ZSM-5 for MTP with a complete methanol conversion, suggesting that a high silicon to aluminum ratio for H-ZSM-5 may enhance the propagation of the alkene-based methylation/cracking cycle relative to the arene-based methylation/dealkylation cycle in MTP.
HZ-5(1600) with a silicon to aluminum ratio of 1600 exhibits both a high selectivity to propene and a complete initial conversion of methanol, which can be attributed to its appropriate acidity distribution with intrinsic MFI structure. A critical value of acid density, viz., [AS]S, is required to achieve the maximum propene selectivity for MTP with a complete methanol conversion; this critical [AS]Svalue is 0.175 μmol/m2for the H-ZSM-5 zeolite under current reaction conditions.
