Design and Synthesis of Phenothiazine Function-alized Spiro 〔fluorene-9,9′-xanthene〕 for Understanding Structure-property Relationships in Photoelectrical Properties
2019-01-18ZHAOXianghuaWANGLiminMAXiaoXUWenjuanCHENMingYUANShundongXUZhiJie
ZHAO Xiang-hua, WANG Li-min, MA Xiao, XU Wen-juan,CHEN Ming, YUAN Shun-dong, XU Zhi-Jie
(1. College of Chemistry and Chemical Engineering, Xinyang Normal University, Xinyang 464000, China; 2. Key Laboratory for Organic Electronics and Information Displays & Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts & Telecommunications, Nanjing 210023, China;3. College of Science, China University of Petroleum, Qingdao 266580, China)*Corresponding Authors, E-mail: 4773zxh@163.com; yuansd@upc.edu.cn
Abstract: A novel 10-(spiro[fluorene-9,9′-xanthen]-2-yl)-phenothiazine(SFXPz) with deep HOMO(the highest occupied molecular orbital)(-6.15 eV) and high triplet energy(T1, 2.82 eV) has been synthesized, which is expected to fabricate high-efficient blue phosphorescent organic light-emitting diodes(PhOLEDs) due to the importance of a wide energy gap(Eg, 4.33 eV) and deep enough HOMO in blue PhOLEDs. The good thermal stability(Td, 259 ℃) and high stable morphology(Tg, 206 ℃) of SFXPz were demonstrated by thermo gravimetric analysis(TGA) and differential scanning calorimetry(DSC) curves. The complete separation of HOMO and the lowest unoccupied molecular orbital(LUMO) of the compound is preferable to prevent the back of energy transfer. The UV-Vis spectrum peaks of SFXPz exhibit at around 230, 260, 292, 310 nm, respectively, and the corresponding fluorescence emission spectrum peaks show at about 311, 324 nm, respectively. In addition, the molecular structure is characterized detailedly by LC-MS, 1H NMR and 13C NMR, respectively.
Key words: spiro[fluorene-9,9′-xanthene]; phenothiazine; deep HOMO; structure-property relationships; steric hindrance
1 Introduction
Since the pioneering work of electrophosphorescence reported by Forrest and his group, PhOLEDs have drawn great attention due to their theoretical 100% internal quantum efficiency through harvesting singlet and triplet excitons caused by spin-orbit coupling[1], which is considered as one of the most promising candidates as the next-generation illumination technology to replace liquid crystal displays (LCDs) and plasma display panels (PDP) due to its advantage characteristics such as wider viewing angles, thinner and lighter,etal[2-3]. However, phosphorescent emitters often encounter concentration quenching and/or triplet-triplet annihilation at high concentration that usually leads to dramatical efficiency decrease of PhOLEDs[3]. In order to obtain high performance of PhOLEDs, phosphor emitters are often doped into a proper host to resolve this problem[4]. Although, variuos excellent host materials have been designed and synthesized for high perfromance PhOLEDs[3,5], high efficiency, long lifetime and low cost are still crucial factors for the commercialization of PhOLEDs in full-color display and white solid lighting. Thus, it is urgent to set the state-of-the-art models of host materials by ingenious build to discover the deep relationships among each molecular srtucture modification in PhOLEDs for purposely cumulative enhancement of device performance to accelerate their commercialization. To resolve the inherent conflict between optical and electrical properties, it is increasing an urgent issue to adjust a single optoelectronic property selectively for rational design organic host to realize high-efficient device. However, the modulation of a single property is a bigger challenge than that of multiple properties because the uncontrollable and unfavorable change of device properties could be avoided effectively resluted from multiple properties alteration simultaneously. Xu and his co-wokers developed dibenzothiophene-based phosphine oxide hosts with short-axis substitution approach which just modulated 0.09 eV of the LUMO energy level without any undulation of the HOMO energy level and corresponding T1[6]. They also reported a series of phosphine oxide based dibenzofuran with short-axis linkages and ternary ambipolar phosphine oxide based fluorene-carbazoly blue hosts by indirect linkage to controllably reduce S1but retain T1energy level, which is expected to be helpful in hole transportation (HT)/hole injection (HI) and electron transportation (ET)/electron injection (EI)[7-9]. All of these works demonstrate that selectively regulate single electrical properties with unchanged T1would be beneficial to realize high-efficient PhOLEDs.
According to the aforementioned basis, it is very important to recognize the selective tuning of various single electrical prioperties without affecting other properties in the same series of hosts, which would be helpful to uncover which factors are positive in enhancing device performance and which ones are negative elements in impairing device properties. Therefore, excellent hosts with further optimized structures would be designed and synthesized and high performance devices would be fabricated accumulatively through purposive and feasible modifications. Then, the relationship between molecular structure and device performance would provide guidance about how to modify molecular structure is prior to achieve better device performance. Subsequently, highly efficient devices would be fabricated using excellent host materials with optimized molecular structure after uncovering the relationship between molecular structure and device performance.
Spirocyclic bifluorene cores have attracted widespread interest in the field of molecular tectonics and organic electronic due to their three-dimensional orthogonal framework and two independent π-conjugated arms lingked by sp3carbon atom at the 9-position of fluorene, which could endow them with good thermal and morphological stability, binary channels injecting/transporting characteristics, as well as bukly steric hindrance[10-11]. Hence, spirocyclic cores are employed widely to construct high-efficient host materials because of their versatile properties[12-13]. Nevertheless, the multiple synthesis steps of spirobifluorenes(SBF) are relative tedious and time consuming. Since Xie and Huang first discovered the convenient one-pot method to synthesize 3D bulky spiro[fluorene-9,9′-xanthene](SFX) in mild synthesis condition with high yield(over 90%), spiro[fluorene-9,9′-xanthene] and its derivatives have been drawn increasing interesting not only due to their fascinating properties but also because of their concise and efficient synthesis method compared to that of SBF[14]. In the application of OLEDs, SFX-based materials have been extensively used as hosts for PhOLEDs, ligands for Pt(Ⅱ) or Ir(Ⅲ) emitters, and thermally activated delayed fluorescence (TADF) OLEDs[12-17]. In our previous works, carbazole-endcapped SFX(SFX-Cz) has been explored as hole-transporting materials for heavily-doped PhOLEDs with good performance[17]. Then, Huang and Sun also introduced carbazole and diphenylamine into SFX core (SFX-Cz, SFX-DPA) as hosts for PhOLEDs[18]. However, the device performances based on SFX-Cz or SFX-DPA are not high enough compared to other SFX-based derivatives as hosts[17-18]. To achieve highly efficient PhOLEDs, a wideEg(energy gap) with deep enough HOMO is more important than electron mobility to hold up holes, while the high T1confines triplet excitions into the emitting layer to hinbit energy reverse from guest to host for high performance devices, which has been proved by Kido and Xiao’s group work using tetraphenylsilane/pyridine hybrid as host achieving 100% internal quantum efficiency (IQE) under weak electron transporting ability[19-20]. Therefore, it is quite important to research the relationship between molecular structure and properties of SFX-based materials for realizing high perfroamance devices. In this work, phenothiazine (Pz) as an electron donor was incorporated into SFX, then, a novel SFX/Pz hybrid (SFXPz) with a wideEgand deep HOMO level was synthesized (Scheme 1).
Thus, the understanding structure-property relationships among SFXPz, SFX-Cz and SFX-DPA in photoelectrical and physical properties were discussed detailedly, which would be helpful to fabricate high-efficient device. Subsequently, state-of-the-art models of SFX-based materials would be further set to uncover the relationship between molecular structure and properties, which would provide useful experimental data for realizing the application of commercialized devices in full-color display and white solid-state lighting after being further explored and researched.
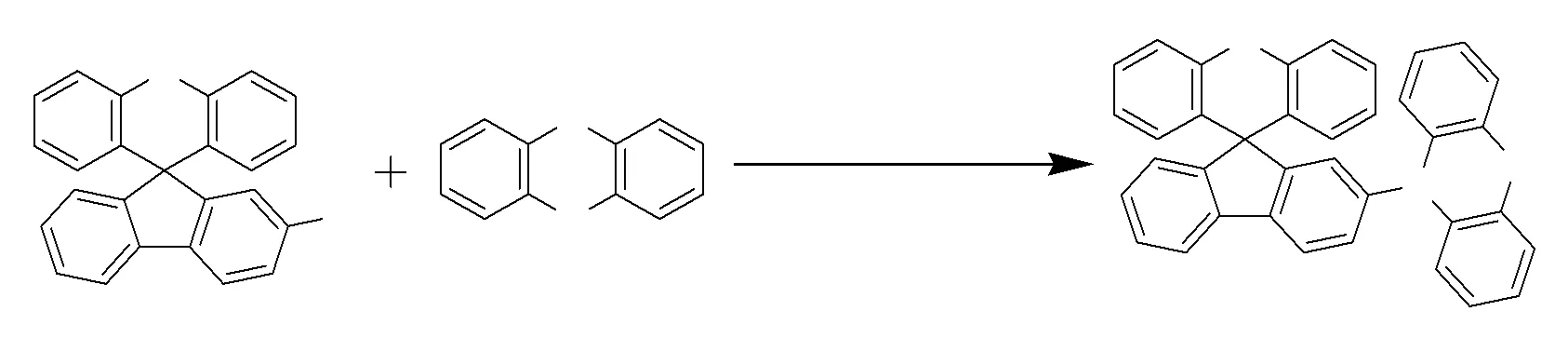
Scheme 1 Synthesis of SFXPz
2 Experiments
2.1 Materials and Instruments
2-bromospiro[fluorene-9,9′-xanthene],phenothiazine and other reactants were purchased from Alfa Aesar and used without further purification. All reactions were carried out under nitrogen unless otherwise stated. Molecular mass was perfromed by an ultra performance liquid chromatography(Waters)/gas chromatography(Agilent) mass spectrometry coupled with quadrupole/time-of-flight mass spectrometry(LC/GC-QTOF-MS).1H and13C NMR were recorded on a Bruker 400 MHz and JNM-ECZ600R/S 3 600 MHz spectrometer in CDCl3using etramethylsilane(TMS) as the internal reference. Absorption and fluorescence spectra were carried out by a Shimadzu UV-3600 spectrometer and Shimadzu RF-5301(PC) luminescence spectrometer at room temperature, respectively. The triplet energy was estimated by the phosphorescence spectra of the host that was performed on Edinburgh FPLS 920 fluorescence spectro-photometer in degassed-DCM frozen glass at 77 K cooled under liquid nitrogen atmosphere. To research electrochemical properties of the host, cyclic voltammetry studies were measured in acetonitrile solution with doping film on a platinum sheet working electrode at 0.1 mol·L-1of Bu4NPF6at room temperature. HOMO and LUMO spatial distributions of the compound were obtained from the optimized ground state structures under the assistance of density functional theory(DFT) calculations. The HOMO, LUMO, and HOMO-LUMO energy gap(Eg) of the compound were estimated from the equationsEHOMO=-[Eox-E(Fc/Fc+)+4.8]eV,ELUMO=-[Ered-E(Fc/Fc+)+4.8] eV, whereE(Fc/Fc+) is about 0.012 3 V. The geometry and electronic cloud density of the host were further studied by DFT calculations.
2.2 Synthesis of The Product
Synthesis of 10-(spiro[fluorene-9,9′-xanthen]-2-yl)-phenothiazine(SFXPz): a mixture of 2-bromo-spiro[fluorene-9,9′-xanthene] (0.88 g, 2 mmol), phenothiazine (0.44 g, 2.2 mmol), P(t-Bu)3(0.56 mL, 2.4 mmol), Pd(OAc)2(0.04 g, 0.2 mmol) and KOtBu (0.27 g, 2.4 mmol) were added in 30 mL toluene refluxed at about 110 ℃ for 72 h under nitrogen atmosphere. Then, 30 mL water was poured into the mixture after the reaction cooling to room temperature, and extracted with dichloromethane for several times. The organic phase was combined and dried over sodium sulfate. The solution was filtered under vacuo and solid residue was washed with CH2Cl2. The solvent was removed by rotary evaporators and the product was purified by column chromatography on silica gel using the eluents of ethyl acetate and petroleum ether, yield 47.3% of white crystals. LC-MSm/z529.149 7[M+];1H NMR(400 MHz, CDCl3):δ(ppm) 7.80(d,J=7.6, 3H), 7.37(t,J=7.2, 3H), 7.24(d,J=8.0, 2H), 7.19(t,J=6.2, 8H), 7.16(s, 1H), 6.65(t,J=7.2, 3H), 6.40(d,J=7.2, 3H).13C NMR(120 MHz, CDCl3):δ(ppm) 155.11, 151.50, 139.73, 128.47, 128.12, 127.95, 127.86, 125.66, 124.94, 123.35, 119.98, 116.87. Anal. calcd for C37H23NOS: C, 83.90; H, 4.38; N, 2.64; found: C, 83.66; H, 4.40; N, 2.62%.
3 Results and Discussion
3.1 Synthesis and Thermal Stability
Scheme 1 shows the synthetic route of SFXPz that was synthesized by combing SFX with phenothiazine using palladium acetate as catalyst under nitrogen atmosphere refluxed for 72 h. The initial material 2-bromo-spiro[fluorene- 9,9′-xanthene] was prepared using the one-pot method reported by Xie and Huang[15]. LC-MS and1H NMR spectroscopy were employed to characterize the molecular structure of SFXPz detaily, which has good solubility in common solvents such as dichloromethane, acetic ether, chloroform, and tetrahydrofuran,etal. The bulky cruciform spiro-annulation structure endows SFXPz with good thermal stability and morphological stabity showed in Fig.1, which shows SFXPz loses 5% weight at 259 ℃ and has glass transition temperature of 206 ℃ and no melting phenomenon in the range from 40 to 250 ℃. The TGA and DSC curves demonstrate SFXPz(Td, 259 ℃;Tg, 206 ℃) has lower thermal ability than that of SFXCz(Td, 345 ℃) and SFXDPA(Td, 338 ℃) but owns better morphological stability than that of SFXCz(Tg, 97 ℃), which manifests the introduction of rigid plane group and flexible unit into SFX could enhance the thermal and morphological stability of SFX-based compounds, respectively[17-18].
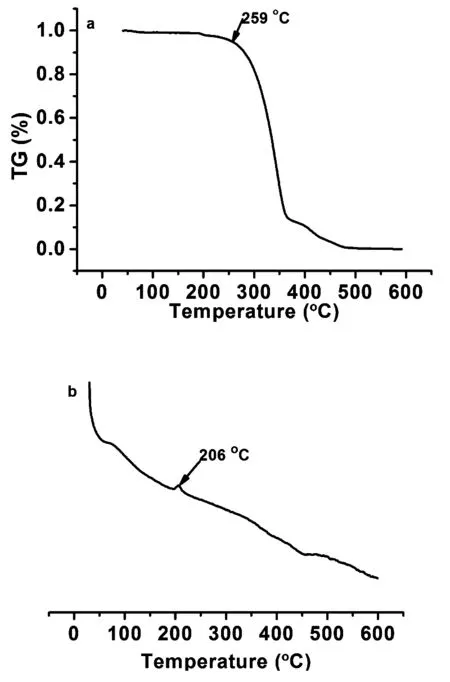
Fig.1 TGA(a) and DSC(b) curves of SFXPz
3.2 Optical Properties

Fig. 2 UV-Vis and flurescent emission spectra of SFXPz in dichloromethane solvent
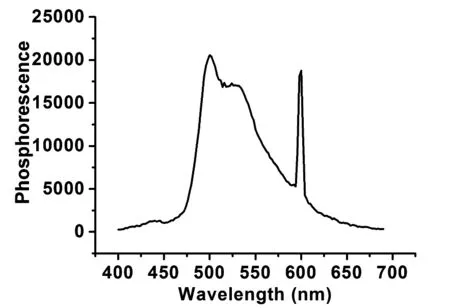
Fig.3 Phosphorescence spectrum of SFXPz measured at 77 K in CH2Cl2
3.3 Electrochemical Properties and DFT Calculations
The influence of carrier transporting groups on the frontier molecular orbitals(FMOs) was further researched by density functional theory(DFT) calculations, and the relevant data were listed in Tab.1. The HOMO and LUMO energy levels are -4.89 eV and -1.21 eV, respectively, which are mainly localized on phenothiazine and fluorene, respectively. The complete separation of HOMO and LUMO between phenothiazine and fluorene is helpful to efficient hole- and electron-transporting characteries, and prevent the reverse energy transfer[23]. In addition, the electrochemical properties of SFXPz were further studied by cyclic voltammetry measurements (Tab.2 and Fig.4). The HOMO(-6.15 eV) and LUMO(-1.82 eV) were estimated from the oxidation and reduction threshold of the compound. The energy gap(Eg, 4.33 eV) was calculated from the difference of HOMO and LUMO. Compared to the corresponding data of SFX-Cz based our previous work[17], HOMO and LUMO of the compound are decreased and rised, respectively. Consequently, an enlargedEgis realized, which is necessary to construct high-efficient blue PhOLEDs[19].
Tab.1HOMOandLUMOsurfacesfromDFTcalculations

CompoundHOMOLUMOSFXPz-4.89 eV-1.21 eV
Tab.2ElectrochemicalpropertiesandtripletenergyofSFXPz

CompoundHOMO/eVLUMO/eVEg/eVT1/eVSFXPz-6.15-1.824.332.82
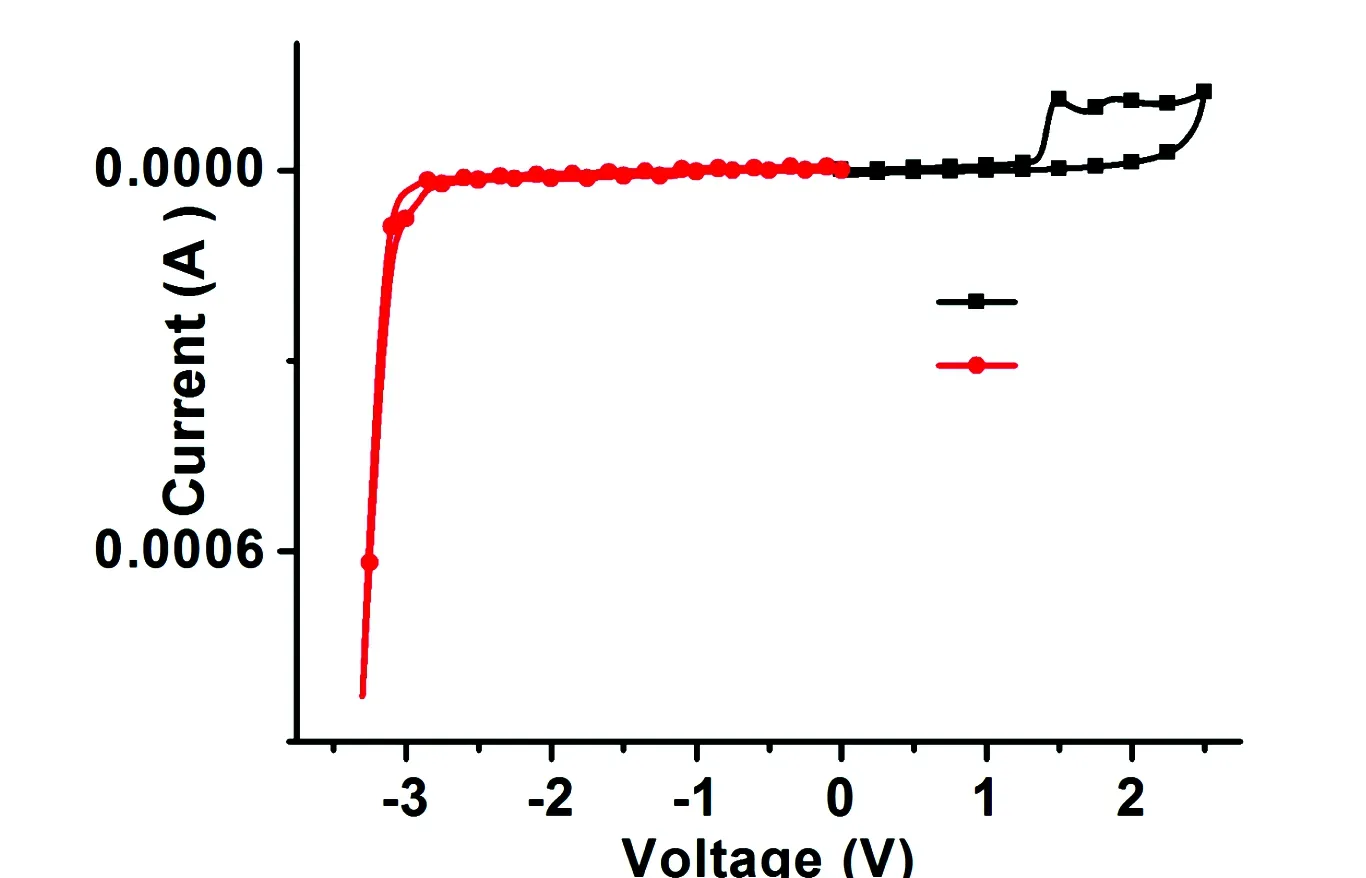
Fig. 4 CVs of SFXPz measured at a scan rate of 100 mV·s-1in acetonitrile solution
4 Conclusion
In conclusion, a phenothiazine funcationalized SFX molecule SFXPz is synthesized using PdAc2as a catalyst. The completely separated HOMO and LUMO
of the compound are preferable for inhibiting intramolecular energy reverse and enhance hole- and electron-transporting properties. The HOMO, LUMO, andEgare -6.15, -1.93, 4.22 eV, respectively, estimated by cyclic voltammetry moments. The deep HOMO indicates this material could block hole injecting/transporting, which is benecifical to balance holes and electrons. The high T1(2.82 eV) and largeEg(4.22 eV) demonstrate the method of using interruption π-conjugation link between phenothiazine and SFX could realize blue host with high T1successfully. The UV-Vis spectrum shows four aborption peaks around 230, 260, 292, 310 nm, respectively. SFXPz exhibits two emission peaks at about 311 and 324 nm, respectively, which are shorter than that of SFXCz and SFXDPA caused by sulphur atom of phenothiazine. The TGA and DSC curves demonstrate SFXPz has good thermal ability(Td, 259 ℃) and high stable morphology(Tg, 206 ℃), which would be preferable to constructing stable device with long liftime. Moreover, the further investigations of the relationship among molecular structure, material properties and device performance based on SFXPz researched in our following work are also undergoing.
Acknowledge
We thank the Key Laboratory of Functional Inorganic Material Chemistry (Heilongjiang University), Ministry of Education, P. R. China for financial support.
