Energy-Transfer Processes of Xe(6p[1/2]0,6p[3/2]2,and 6p[5/2]2)Atoms under the Condition of Ultrahigh Pumped Power
2019-01-10ShnHeJunzhiChuDongLiuXueyngLiJingweiGuoJinboLiuShuHuHuiLiPengyunWngYingChenFengtingSngYuqiJin
Shn He,Jun-zhi Chu,Dong Liu,Xue-yng Li,Jing-wei Guo∗,Jin-bo Liu,Shu Hu,Hui Li,Peng-yun Wng,Ying Chen,Feng-ting Sng,Yu-qi Jin
a.Key Laboratory of Chemical Lasers,Dalian Institute of Chemical Physics,Chinese Academy of Sciences,Dalian 116023,China
b.University of Chinese Academy of Sciences,Beijing 100049,China
The kinetic processes of Xe(6p[1/2]0,6p[3/2]2,and 6p[5/2]2)atoms under the focused condition were investigated.The atomic density of the laser prepared state significantly increases.Therefore,the probability of the energy-pooling between two high-lying atoms increases.There are three major types of the energy-pooling collisions.The first type is the energy-pooling ionization.Once the excitation laser is focused,the obvious ionization can be observed from the side window whenever the laser prepared state is 6p[1/2]0,6p[3/2]2,or 6p[5/2]2state.Ionization of Xe is attributed to the energy-pooling ionization or a Xe∗atom reabsorbing another excitation photon.The second type is energy-pooling with big energy diflerence.When the 6p[1/2]0state is the laser prepared state,the energy-pooling collision between two 6p[1/2]0atoms can produce one 5d[3/2]1atom and one 6s′[1/2]0atom.The third type is energy-pooling with small energy diflerence.The intensities of fluorescence lines are much stronger that five secondary 6p states act as the upper states,and the rising edges of these fluorescence lines are much steeper.The primary mechanism of generating the secondary 6p atoms is energy-pooling collision instead of collision relaxation.Based on the collision probability,the rate of energy-pooling between two 6p[1/2]0atoms is deduced(6.39×108s−1).In addition,the 6s atoms also increase under the focused condition.Therefore,all the fluorescence lines are serious trailing by radiation trapping.
Key words:Energy-pooling,Kinetics,Xe,Ultrahigh pumped power
I.INTRODUCTION
Recently,diode-pumped metastable rare gas laser is a promising subject for its unique advantages including mild working conditions and inert chemical property etc.So many groups pay their attentions to this subject[1−9].Heaven and co-workers have demonstrated a CW diode-pumped Ar∗laser providing 4 W[9].It is a great progress in the regime of diode-pumped rare gas laser.
The study about diode-pumped metastable Xe laser is sparse.In comparison with the lighter rare gas atoms,the metastable Xe atoms are easier to produce.However,the kinetics between the high-lying Xe states is very complex because the energy diflerences between the high-lying Xe states are relatively low.The energytransfer processes between the high-lying Xe states have been studied[10,11],but the power of the excitation laser is relatively low.However,the laser system usually requires high power pumped sources.Consequently,it is important to study the energy-transfer processes between the high-lying Xe states under the high power pumped condition.
Energy-pooling collisions can be produced in diodepumped alkali lasers(DPAL)under the strong pumping condition[12].Energy pooling is a kinetic process in which two excited atoms collide to produce one atom in a higher state and the other one in a lower state.This process has been widely studied in alkali metals[13,14]and alkaline earth metals[15,16].However,studies about energy-pooling collision between the high-lying Xe states are sparse.The high-lying states of the rare gas atoms are more abundant.The type of energypooling collisions between the rare gas atoms probably is more abundant.
Previously,we have studied kinetics of the 6p[1/2]0state under the condition of strong excitation laser,and found that the high power of the excitation laser can trigger the ASE of 3408 nm(6p[1/2]0-6s′[1/2]1)[17].We have also systematically studied the kinetics of 6p[1/2]0atoms in bufler gases and found that the Kr,Ar,and Ne bufler gases can accelerate the transfers of 6p[1/2]0→5d[1/2]1[18].However,the energy-pooling collision has never been observed.Although the power of the excitation laser we used was relatively high,the energy-pooling collision may need even higher power.
In this work,the time-resolved fluorescence and ASE spectra were detected under the focused condition.Only when the excitation laser is resonant and focused,can ionization phenomenon be observed.The ionization should be produced by energy-pooling collision or the high-lying atoms reabsorbing excitation photons.When the laser prepared state is 6p[1/2]0state,two new ASE lines at 1732 nm(5d[3/2]1-6p[5/2]2)and 2026 nm(5d[3/2]1-6p[3/2]1)appear.The substantial 5d[3/2]1atoms are produced by energy-pooling collision between two 6p[1/2]0atoms.By virtue of the unique arrangements near the 5d[3/2]1and 6s′[1/2]0states,the probability of self-pooling can be pretty high.Besides,all the intensities of fluorescence lines with the higher states being secondary 6p states become stronger,and the rising edges of those lines are much steeper under the focused condition.Therefore,the primary mechanism of producing the secondary 6p atoms should be the energypooling collision instead of collision relaxation.
II.EXPERIMENTS
The experimental apparatus has been described in detail previously[17,18].Only a brief description was given here. The excitation laser was obtained from the second harmonic of dye laser(Sirah CBST-LG-18-EG).The Xe(6p[1/2]0,6p[3/2]2,and 6p[5/2]2)atoms were prepared by two-photon excitation at wavelengths about 249.5,252.4,and 255.9 nm,respectively.The dye laser was pumped by the third harmonic of a Nd:YAG laser(Beamtech SGR-10).A quartz lens(f=200 mm)was used to focus the excitation laser.A stainless-steel sample cell was used to contain the gases.It has four windows.One window is made of sapphire to ensure the MIR pass through.The rest three windows are made of fused quartz.An uncoated Si plate was placed between the sapphire window of the cell and the slit of the MIR monochromator(HORIBA micro HR MHRA-2A-MS).It can absorb the excitation laser and transmit the MIR ASE.
A series of lenses were placed along the axis perpendicular to the axis of excitation laser to collect thefluorescence. The focal point of the excitation laser and that of the fluorescence collection lens systems nearly overlapped.The fluorescence was separated by a monochromator(Princeton Instrument SpectraPro 2500i)with a 1200 g/mm grating.A single spontaneous emission line was measured by an APD and recorded by a 2 GHz oscilloscope(LeCroy waverunner 620zi).The schematic diagram is shown in FIG.1.
All the gases used in this experiment were ultrahigh purity:Xe(99.999%),Kr(99.999%),Ar(99.999%).

FIG.1 Schematic diagram of the experimental apparatus.

FIG.2 The phenomena directly observed from the side window. The experimental conditions from top to bottom are resonant and unfocused,non-resonant and focused,and resonant and focused,respectively. The excitation state is 6p[1/2]0,6p[3/2]2,or 6p[5/2]2. The pressure of Xe is 6.0 Torr.The energy of excitation laser is 2.30 mJ(3.54×1010W/cm2).
III.RESULTS AND DISCUSSION
FIG.2 shows the phenomena directly observed from the side window.Evidently,strong visible emissions are produced under the resonant and focused condition. The wavelengths of emissions with the upper states being six 6p states are all longer than 800 nm.So there is no visible emission under the resonant and unfocused condition.As a result,the upper states of these visible emissions are not the six 6p states.There must be some new processes happening under the resonant and focused condition.To probe the new process,the fluorescence spectrum is observed under the resonant and focused condition,as shown in FIG.3.Firstly,the typical fluorescence lines of 6p-6s such as 828 nm(6p[1/2]0-6s[3/2]1),823 nm(6p[3/2]2-6s[3/2]2),and 882 nm(6p[5/2]3-6s[3/2]2)are observed.Secondly,the continuous spectrum from 400−700 nm is obviously produced by ionization.The Xe atoms are ionized under the resonant and focused condition and these two factors are both indispensable.The ionization processes should occur as follows(Xe∗is the laser prepared state including the 6p[1/2]0,6p[3/2]2,and 6p[5/2]2states):

FIG.3 Fluorescence spectrum under the resonant and focused condition.The laser prepared state is the 6p[1/2]0 state.The pressure of Xe is 6.0 Torr.The energy of excitation laser is 2.30 mJ(3.54×1010W/cm2).

The first mechanism(reaction(1))is the“energypooling ionization”.Substantial Xe∗atoms are generated near the focal point under the resonant and focused condition.It increases the probability of the eflective collision between the two Xe∗atoms.Since the potential energy of Xe2+is∼90000 cm−1[19],the potential energies of two Xe∗atoms(higher than 154000 cm−1)are high enough to trigger the reaction(1).
The second mechanism(reaction(2))is that a Xe∗atom absorbs another excitation photon.Not only the Xe∗density but also the photon density of excitation laser is very high in the area near the focal point.Thus the probability of reaction(2)can also increase.In addition,the potential energy of Xe∗is bigger than 78000 cm−1. The energy of a ∼250 nm photon is∼40000 cm−1.The energy sum of a Xe∗and an excitation photon is high enough to trigger the ionization.Because no ionization phenomenon appears under the non-resonant and focused condition,the mechanism of a ground state Xe atom absorbing three or more photons is excluded.
Thirdly,the atomic lines in 450−500 nm can be owed to the lines of 6p′-6s and 7p-6s,such as 450 nm (6p′[1/2]0-6s[3/2]2),467 nm (7p[5/2]3-6s[3/2]2),482 nm(7p[3/2]1-6s[3/2]1).The energy differences between the 6p[1/2]0state and 6p′,7p states are∼9000 cm−1.Relaxation normally cannot produce exothermic transfer,let alone the exothermic transfer with such a big energy diflerence.The following two mechanisms may explain the production of 6p′and 7p atoms.The first mechanism is a Xe+combining an electron to populate a 6p′or 7p atom.The second mechanism is energy-pooling between two 6p[1/2]0atoms.
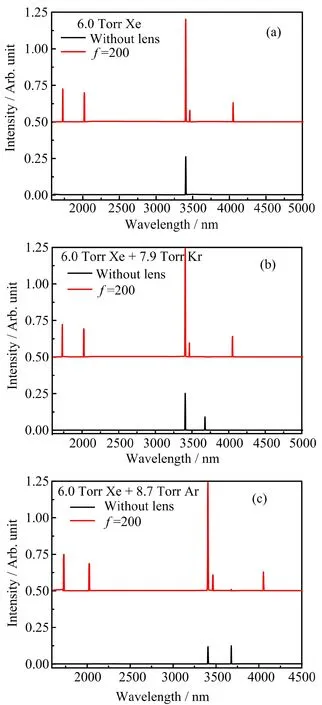
FIG.4 The mid-infrared ASE spectra in the forward direction along the excitation laser.The laser prepared state is the 6p[1/2]0state.The energy of excitation laser is 2.30 mJ(3.54×1010W/cm2).For clear comparison,the spectrum under the focused condition is moved upward 0.50 Arb.unit.
FIG.4 is the mid-infrared ASE spectra measured in the forward direction along the excitation laser.The laser prepared state is the 6p[1/2]0state.As shown in FIG.4(a),the intensity of ASE at 3408 nm(6p[1/2]0-6s′[1/2]1)in pure Xe under the focused condition is much stronger than that under the unfocused condition.The density of the 6p[1/2]0atoms significantly increases in the region near the focal point.It results in the high gain coefficient of the ASE at 3408 nm(6p[1/2]0-6s′[1/2]1).So the intensity of ASE at 3408 nm(6p[1/2]0-6s′[1/2]1)becomes stronger.Unexpect-edly,new peaks at 1732,2026,3464,and 4052 nm emerge.The peaks at 1732 nm and 2026 nm should be attributed to the transfers of 5d[3/2]1-6p[5/2]2and 5d[3/2]1-6p[3/2]1,respectively.And the peaks at 3464 and 4052 nm are the second order diflraction of the peaks at 1732 and 2026 nm,respectively.This phenomenon indicates substantial 5d[3/2]1atoms are produced. The kinetic process for the generation of 5d[3/2]1atoms should be energy-pooling collision illustrated as reaction(3).The probability of reaction(3)must be pretty high,because the population inversions can be formed between the 5d[3/2]1state and the 6p[5/2]2,6p[3/2]1states. This can be owed to the following two aspects.Firstly,the energy diflerence between the 6p[1/2]0state and the 5d[3/2]1state is very close to that between the 6p[1/2]0state and the 6s′[1/2]0state.To some extent,this energy-pooling collision is a near-resonance process.Secondly,the energy level arrangements near the 5d[3/2]1state and the 6s′[1/2]0state are unique.As shown in FIG.5,both of these two states have big energy diflerences from the adjacent states:

Once one 6p[1/2]0atom reaches the 6s′[1/2]1state,another 6p[1/2]0atom strongly tends to reach the 5d[3/2]1state.

Ar and Kr atoms can accelerate the transfer of 6p[1/2]0→5d[1/2]1[10,11,18].Therefore,they can switch ASE channel from 3408 nm(6p[1/2]0-6s′[1/2]1)to 3680 nm(5d[1/2]1-6p[1/2]1)by collision[18].Accordingly,ASE spectra in bufler gas of Ar or Kr have two peaks at 3408 and 3680 nm,as shown in FIG.4(b)and(c).The intensity of ASE at 3680 nm decreases and new peaks at 1732,2026,3464,and 4052 nm emerge under the focused condition. The probability of collision between one 6p[1/2]0atom and another atom is described as Eq.(4):

where z is the collision probability between A and B,dABis the sum of radius of A and B,µis the reduced mass of A and B,T is the temperature,nAand nBare the concentration of A and B,respectively.

FIG.5 Schematic diagram of the energy levels of Xe∗related to this work.Each state is marked with its energy(in cm−1)in reference to the ground state S0.
Obviously,collision probability between A and B is proportional to the concentration of bufler atoms.Although the density of the 6p[1/2]0atoms near the focal point is high,it must be much lower than the density of Ar or Kr atoms(DRg=∼2.57×1017cm−3).Consequently,the probability of collision between two 6p[1/2]0atoms is much lower than that between one 6p[1/2]0atom and one bufler gas atom. However,FIG.4(b)and(c)reflect that the primary kinetic process under the focused condition is the energy-pooling collision instead of collision relaxation.It indicates that the collision between two 6p[1/2]0atoms is more eflective than that between a 6p[1/2]0atom and a bufler gas atom(Ar or Kr),because 6p[1/2]0atoms are more active than ground state Ar and Kr atoms.
Based on the analysis above,if the density of 6p[1/2]0atoms holds constant,the rate of energy pooling is constant.Some semi-quantitative deductions are given here.The pressure of Xe and excitation power hold constant.We suppose that the rate of energy-pooling is V.Then the Ar is filled into the cell.The probability of collision between Xe∗and Ar increases.The rate of relaxation can be expressed as×p.Ar can switch the ASE channel from 3408 nm(6p[1/2]0-6s′[1/2]1)to 3680 nm(5d[1/2]1-6p[1/2]1).It is attributed to the high value of.With the increasing pressure of Ar,the ASE at 3680 nm should gradually increase,the ASE at 3408 and 1732 nm should gradually decrease.As shown in FIG.6,the actual phenomenon precisely follows this prediction. Then the intensity of 3680 nm is proportional toas Eq.(5).

Then we can introduce a parameter α to modify the intensity of 3680 nm.Eq.(5)can be rewritten as Eq.(6).


FIG.6 Plot of ASE at(a)1732 nm,(b)3408 nm,and(c)3680 nm against pressures of Ar.The pressure of Xe is 6.0 Torr.The energy of excitation laser is 2.30 mJ(3.54×1010W/cm2).

FIG.7 Plot of 1/I3680against 1/p.The pressure of Xe is 6.0 Torr.The bufler gas is Ar.The energy of excitation laser is 2.30 mJ(3.54×1010W/cm2).The line is the result of linear fitting.

When the laser prepared state is the 6p[1/2]0state,the time-resolved fluorescence lines of 6p-6s are shown in FIG.8.The intensity of 828 nm under the focused condition is much stronger than that under the unfocused condition.It results from the high density of the 6p[1/2]0atom under the focused condition.The restfive fluorescence lines can reflect the populations of thefive secondary 6p states.Under the unfocused condition,the primary mechanism generating the 6p[3/2]2,6p[3/2]1,6p[5/2]3,and 6p[5/2]2atoms is collisional relaxation.The intensities of these fluorescence lines are weak.Obviously,the intensities of fluorescence lines of 823,916,882,and 904 nm are much stronger and their rising edges are much steeper under the focused condition.Therefore,a new mechanism,energy-pooling collision,should emerge under the focused condition described as reaction(8).

Collision of this type between two 6p[1/2]0atoms can lead to one atom reaching a higher state and the other one reaching a lower state.Besides,the ASE at 1732 nm(5d[3/2]1-6p[5/2]2)and 2026 nm(5d[3/2]1-6p[3/2]1)can also populate the 6p[5/2]2state and the 6p[3/2]1state,respectively.This is another reason why the intensities of 916 and 904 nm become stronger.The dominant mechanism of populating 6p[1/2]1atoms is a series of processes related to the ASE at 3408 nm(6p[1/2]0-6s′[1/2]1)and 3680 nm(5d[1/2]1-6p[1/2]1)under the unfocused condition[17,18].According to FIG.4,the intensity of ASE at 3408 nm increases and that at 3680 nm decreases under the focused condition.Thus the population of the 6p[1/2]1atoms should be mainly owed to the processes related to the ASE at 3408 nm.Another phenomenon shown in FIG.8 is that all the fluorescence lines are serious trailing under the focused condition.The lifetimes of these states are∼30 ns[10,11,17].However,even at∼1500 ns,all these fluorescence lines are still observed.It means that there still exist some channels populating these 6p atoms even at∼1500 ns.Maybe the channel populating these 6p atoms be radiation trapping.This phenomenon was widely reported in high-lying states of alkali metals[20]and some states of rare gases[21].The mechanism of this phenomenon is that radiation near a resonance line can be absorbed and emitted many times before escaping.Then,the apparent radiative lifetime of the higher state can be obviously extended by this eflect.However,the prerequisite of radiation trapping is that the population density of the lower state is high enough.If the population density of the lower state is relatively low,the radiation cannot be eflectively absorbed.Then,the radiation cannot be trapped by absorbing and emitting many times.The lifetimes of alkali metal states and 6s states of Xe are usually aflected by this eflect[20,21],since the lower state is the ground state.However,the 6p states of Xe is ever hardly aflected for the lower state being the 6s state.The situation may be diflerent in our work.Not only the density of the 6p[1/2]0state but also those of the 6s states should be very high in the area near the focal point.Then the radiation lines with the higher states being the 6p states can be trapped.The trailing of thefluorescence line is probably due to this reason.

FIG.8 Time-resolved fluorescence lines of the six 6p states under the focused and unfocused conditions.The laser prepared state is the 6p[1/2]0state.The gases contain 6.0 Torr Xe and 8.7 Torr Ar.The energy of excitation laser is 2.30 mJ(3.54×1010W/cm2).Note:828 nm(6p[1/2]0-6s[3/2]1),823 nm(6p[3/2]2-6s[3/2]2),916 nm(6p[3/2]1-6s[3/2]1),882 nm(6p[5/2]3-6s[3/2]2),904 nm(6p[5/2]2-6s[3/2]2),and 980 nm(6p[1/2]1-6s[3/2]2).
When the laser prepared state is the 6p[3/2]2state,time-resolved fluorescence lines of 6p-6s under both the focused and unfocused conditions are shown in FIG.9.Under the unfocused condition,the fluorescence lines of 916,882,904,and 980 nm were observed,while that of 828 nm could not be observed.It indicates that the 6p[3/2]2atoms can reach the lower states including the 6p[3/2]1,6p[5/2]3,6p[5/2]2,but the 6p[1/2]1state cannot reach the higher state(6p[1/2]0).Collision relaxation usually cannot cause endothermic transfer with big energy diflerence.However,under the focused condition,the fluorescence at 828 nm is observed.The 6p[1/2]0atoms cannot be produced by collision relaxation.The mechanism is probably an energy-pooling process between two 6p[3/2]2atoms.Besides,intensities of all the fluorescence lines are much stronger and the rising edges of all the fluorescence lines are much steeper under the focused condition. It should also owe to the energy-pooling collision.Similar to the phenomenon shown in FIG.8,the fluorescence lines are serious trailing under the focused condition.The reason is attributed to the radiation trapping.When the laser prepared state is the 6p[5/2]2state,time-resolvedfluorescence lines of 6p-6s under the focused and unfocused conditions are shown in FIG.10.The phenomena are similar to those shown in FIG.9.The collision relaxation can cause the endothermic transfer of 6p[5/2]2→6p[5/2]3for small energy diflerence,but the endothermic transfers for big energy diflerences are hard to generate by collision relaxation.Therefore,the primary mechanism for producing the 6p[1/2]0,6p[3/2]2,6p[3/2]1,and 6p[5/2]3atoms is the energy-pooling collision instead of collision relaxation under the focused condition.And the serious trailing is probably owed to the radiation trapping.
IV.CONCLUSION

FIG.9 Time-resolved fluorescence lines of the six 6p states under the focused and unfocused conditions.The laser prepared state is the 6p[3/2]2state.These plots were obtained in pure Xe.And the pressure of Xe is 6.0 Torr.The energy of excitation laser is 2.30 mJ(3.54×1010W/cm2).

FIG.10 Time-resolved fluorescence lines of the six 6p states under the focused and unfocused conditions.The laser prepared state is the 6p[5/2]2state.These plots were obtained in pure Xe.And the pressure of Xe is 6.0 Torr.The energy of excitation laser is 1.50 mJ(2.31×1010W/cm2).
The kinetic processes of Xe atoms in the 6p[1/2]0,6p[3/2]2,and 6p[5/2]2states were studied under the focused condition.The density of the atoms in the laser prepared state under the focused condition is much higher than that under the unfocused condition.The atoms in the high-lying state are much more active than the atoms in the ground state.Then,the collision between two 6p[1/2]0atoms is more eflective than that between a 6p[1/2]0atom and a bufler gas atom(Ar or Kr).Therefore,primary mechanism is the energy-pooling collision instead of the collision relaxation under the focused condition.Since the Xe states are more complex than alkali metals states,the energy-pooling collisions are more abundant among high-lying Xe atoms.
The phenomenon observed from the side window is the energy-pooling ionization.The energies of these three laser prepared states are all high enough to trigger the ionization.When the laser prepared state is the 6p[1/2]0state,two new ASE peaks at 1732 nm(5d[3/2]1-6p[5/2]2)and 2026 nm(5d[3/2]1-6p[3/2]1)appear.Thanks to the unique energy level arrangements near the 5d[3/2]1state and the 6s′[1/2]0state,two 6p[1/2]0atoms strongly tend to pool their internal energy to produce one 5d[3/2]1atom and one 6s′[1/2]0atom.Based on the collision probability,the rate of energy-pooling between two 6p[1/2]0atoms is deduced(6.39×108s−1).The intensities of all the fluorescence curves increase and their rising edges are steeper under the focused condition.The atoms in the secondary states are mainly produced by energy-pooling collision.Another phenomenon is that even at∼1500 ns,all thesefluorescence lines are still observed,although the lifetimes of these states are∼30 ns.The densities of the 6s states should be very high in the area near the focal point.Radiation near a resonance line can be absorbed and emitted many times before escaping.Then the apparent radiative lifetime of the higher state can be obviously extended.The mechanism is probably due to the radiation trapping.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.11475177 and No.61505210)and Key Laboratory of Chemical Laser Foundation(KLCL 2017).
[1]M.H.Kabir and M.C.Heaven,J.Phys.Chem.A 115,9724(2011).
[2]J.Han and M.C.Heaven,Opt.Lett.37,2157(2012).
[3]J.Han,L.Glebov,G.Venus,and M.C.Heaven,Opt.Lett.38,5458(2013).
[4]J.Han and M.C.Heaven,Opt.Lett.39,6541(2014).
[5]J.Han and M.C.Heaven,Opt.Lett.40,1310(2015).
[6]W.T.Rawlins,K.L.Galbally-Kinney,S.J.Davis,A.R.Hoskinson,J.A.Hopwood,and M.C.Heaven,Opt.Express 23,4804(2015).
[7]Z.Yang,G.Yu,H.Wang,Q.Lu,and X.Xu,Opt.Express 23,13823(2015).
[8]P.A.Mikheyev,Quantum Electron.48,704(2015).
[9]J.Han,M.C.Heaven,P.J.Moran,G.A.Pitz,E.M.Guild,C.R.Sanderson,and B.Hokr,Opt.Lett.42,4627(2017).
[10]J.K.Ku and D.W.Setser,J.Chem.Phys.84,4304(1986).
[11]J.Xu and D.W.Setser,J.Chem.Phys.94,4243(1991).
[12]G.An,Y.Wang,J.Han,H.Cai,J.Zhou,W.Zhang,L.Xue,H.Wang,M.Gao,and Z.Jiang,Opt.Express 23,26414(2015).
[13]Z.J.Jabbour,R.K.Namiotka,J.Huennekens,M.Allegrini,S.Milosevic,and F.de Tomasi,Phys.Rev.A 54,1372(1996).
[14]C.Gabbanini,S.Gozzini,G.Squadrito,M.Allegrini,and L.Moi,Phys.Rev.A 39,6148(1989).
[15]W.H.Breckenridge,W.L.Nikolai,and J.Stewart,J.Chem.Phys.74,2073(1981).
[16]J.F.Kelly,M.Harris,and A.Gallagher,Phys.Rev.A 38,1225,(1988).
[17]S.He,Y.Guan,D.Liu,X.Xia,B.Gai,S.Hu,J.Guo,F.Sang,and Y.Jin,J.Phys.Chem.A 121,3430(2017).
[18]S.He,D.Liu,X.Li,J.Chu,J.Guo,J.Liu,S.Hu,F.Sang,and Y.Jin,J.Phys.Chem.A 122,5361(2018).
[19]T.O.Nelson,D.W.Setser,and M.K.Richmann,J.Phys.Chem.99,7482(1995).
[20]K.C.Brown and G.P.Perram,Phys.Rev.A 85,022713-1(2012).
[21]N.Sadeghi and J.Sabbagh,Phys.Rev.A 16,2336(1977).
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Imaging HNCO Photodissociation at 201 nm:State-to-State Correlations between CO(X1Σ+)and NH(a1∆)
- Ultrafast Investigation of Excited-State Dynamics in Trans-4-methoxyazobenzene Studied by Femtosecond Transient Absorption Spectroscopy
- Strong Current-Polarization and Negative Diflerential Resistance in FeN3-Embedded Armchair Graphene Nanoribbons
- Unexpected Chemistry from the Homogeneous Thermal Decomposition of Acetylene:An ab initio Study
- Direct Observation of Transition Metal Dichalcogenides in Liquid with Scanning Tunneling Microscopy
- Photo-Induced Intermolecular Electron Transfer-Eflect of Acceptor Molecular Structures
