Hepatoprotective Effects of Folium syringae Extracts Against Ethanolinduced Acute Liver Injury
2019-01-09HeJingshanLinYuexiaLiChangwenLiRuiChangYicongLiYingShiChenxiMaXinLiZhiandLiuFangping
He Jing-shan, Lin Yue-xia, Li Chang-wen, Li Rui, , Chang Yi-cong, Li Ying, Shi Chen-xi, Ma Xin, Li Zhi, and Liu Fang-ping, *
1 College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
2 Heilongjiang Key Laboratory for Animal Disease Control and Pharmaceutical Development, Harbin 150030, China
3 Harbin Veterinary Research Institute of Chinese Academy of Agricultural Sciences, Harbin 150069, China
Abstract: The aim of the study was to investigate the hepatoprotective effects of Folium syringae (FS) extracts against ethanolinduced acute liver injury. Mice and primary hepatocytes were pretreated with FS extracts at different dosages before ethanol administration. Transaminases, glutathione S-transferase A1 level and hepatic biochemical indices (malondialdehyde, superoxide dismutase, glutathione and glutathione peroxidase) were determined. Pretreatment with FS extracts significantly inhibited the damage caused by ethanol and the hepatoprotective effects of FS were almost similar to Silymarin that was used to treat alcoholic liver injury.GSTA1 contents in all the FS extract-treated groups were significantly different from those in the ethanol-induced acute liver injury model group (p<0.01), and similar trends were observed in transaminases and hepatic indices level both in vitro and in vivo. The results showed that FS extracts had hepatoprotective effects against ethanol-induced injury. Those effects might be related to the enhancement of antioxidant capacity of liver cells, and FS extracts could reduce the release of liver GSTA1, which contributed to improve liver detoxification.
Key words: ethanol, Folium syringae extract, GSTA1, liver injury, primary hepatocyte, mouse
Introduction
Ethanol abuse and ethanol dependence have become an important social and medical problem in modern society. It indicates that acute ethanol intoxication has adverse effects on many organs both in experimental and clinical researches (Biros et al., 1999; Young Cho et al., 2012; Chuang et al., 2016). Excessive ethanol intake can cause oxidative stress to the cell membrane and mitochondria. As a result, liver cells are damaged owing to a large number of free radicals (Kim et al.,2007). Reactive oxygen species (ROS) produced during ethanol metabolism can be eliminated by the body antioxidant (Albano, 2006). Liver is the main rgan of ethanol metabolism in the body, and excessive ethanol intake causes various degrees of liver damages.
Alpha class glutathione S-transferases (GSTAs)have been shown to protect hepatocytes from reactive oxygen species caused by various physico-chemical agents (Sharma et al., 2011). In human liver, GSTA1 accounts for a large percentage of GSTs at 65%-75%. GSTA1-1 is the main member of GSTA family(Oakley, 2011). GSTA1 plays a major protective role against oxidative stress damage, which catalyzes the combination of glutathione (GSH) with carcinogens,drugs and toxins of oxidative stress (Adnan et al., 2012).Earlier experimental results showed that GSTA1 appeared earlier than those of alanine aminotransferase(ALT) and aspartate transaminase (AST) in liver injuries, and could be an early marker of liver injuries(Liu et al., 2014; Chang et al., 2017; Li et al., 2017).
Studies have shown that Chinese herbal medicine is useful in the treatments of liver diseases (Wang et al.,2012; Liu et al., 2016). Folium syringae (FS) are the dried leaves of Syringa vulgaris Linn., Syringa dilatata Nakai. and Syringa oblata Lindl., belong to the family Oleaceae., which has a wide range of natural distribution across Eastern Europe to temperate Asia. FS leaves are traditional Chinese folk herbs and possess significant broad-spectrum antimicrobial and antiviral effects. Syringopicroside, an iridoid glycoside, is the major active ingredient in FS by investigating its chemical constituents, and possesses significant antiin flammatory, broad-spectrum antimicrobial, antiviral and immune enhancement effects (Oh et al., 2003; Liu and Wang, 2011; Zhou et al., 2014).
The objectives of this study were to determine the hepatoprotective effects of FS extracts against ethanolinduced acute liver injuries in mice and primary hepatocytes. Furthermore, the index changes were gauged to evaluate the hepatoprotective effects of FS extract against ethanol-induced liver injuries.
Materials and Methods
Chemicals and drugs
The ethanol was purchased from Shanghai Chemical Reagent Factory (Shanghai, China). The fast assay kits of ALT, AST, malondialdehyde (MDA), superoxide dismutase (SOD), GSH and glutathione peroxidase(GSH-Px) were purchased from Shanghai Chang Yi Biotechnology Co., Ltd. (Shanghai, China), mouse GSTA1 assay kit was obtained from SunBio Biomedical Technology Co., Ltd (Beijing, China). FS and Silymarin were purchased from Harbin Jiancheng Dispensary (Harbin, China). Type IV collagenase, dimethyl sulfoxide, insulin, transferrin, heparin, dexa-methasone and trypan blue were purchased from Sigma Chemical Co., Ltd (St Louis, MO, USA). All other chemicals were of analytical reagent-grade.
Extraction
FS was shattered then sifted out over 60 mesh screen to get 500 gfine powder. Thefine powder was immersed in 5 000 mL ethanol (70%), ultrasonic extraction.The extracted liquid wasfiltered with gauze, and concentrated by rotary evaporation instrument in 60℃,until paste was obtained. Then, the paste was dried in the vacuum drying oven at 35℃ and 150 g extracts were obtained. The extraction rate was 30%. Extract of FS was diluted with Dulbecco's Modified Eagle Medium (DMEM) in in vitro experiments.
Primary hepatocytes extraction and culture
All the Kunming mice were 40 days old. Mouse primary hepatocytes were isolated by an improved two-step perfusion method with collagenase (Shi et al., 2017). The anesthetized mice were accepted indwelling needle reversely at venae cava inferior.The primary hepatocytes were grown in 24-well plates(105/well) which were coated with rat tail collagen,cultured under the condition of 5% CO2at 37℃. After 6 h of seeding, the adherent culture medium was replaced with no-serum growth medium and cultured overnight for the next experiments.
Animals
Male Kunming mice of clean grade (weight ranging from 18 to 22 g) were purchased from the experimental animal center of Harbin General Pharmaceutical Factory Co., Ltd (Harbin, Heilongjiang Province,China). They were housed in well-ventilated room at 23±2℃, and a humidity of 40%-60%. All the mice were received a week of acclimation with a normal diet in the animal room until 40 days old. All the experimental protocols were in accordance with Northeast Agricultural University Animal Care and Use Committee, Heilongjiang Province, China.
Hepatoprotective effects of FS in vitro
The primary hepatocytes were assigned intofive groups (n=6): control group, ethanol model group,high-concentration FS extract group (FS-H, 200 μg • mL-1), middle-concentration FS extract group(FS-M, 150 μg • mL-1) and low-concentration FS extract group (FS-L, 100 μg • mL-1), respectively. The control and ethanol model groups were given an equal volume of culture medium. The culture medium was replaced after 12 h, and each group was treated with the culture medium with 100 mmol • L-1ethanol except the control group. After 8 h, the supernatant was collected for the determination of ALT, AST and GSTA1; the hepatocytes were collected for the determination of MDA, SOD and GSH.
Hepatoprotective effects of FS in vivo
Forty-eight mice were randomly assigned into six groups (n=8): control group, ethanol model group,high-dose FS extract group (FS-H, 200 mg • kg-1),middle-dose FS extract group (FS-M, 150 mg • kg-1),low-dose FS extract group (FS-L, 100 mg • kg-1)and positive drug group (Silymarin, 200 mg • kg-1),respectively. The control and ethanol model groups were treated with an equal volume of physiological saline. Drugs were administered orally to mice for 7 days. Then mice were administered orally 50% ethanol(14 mL • kg-1body weight) except the control group.After 8 h, the serum was collected, parts of liver tissues were dissected and preserved in 10% formalin for histopathological examination and the remnent livers were stored at –80℃.
Determination of indices
ALT, AST, MDA, SOD, NO, GSH, GSH-Px and GSTA1 were determined using detection kits, according to the manufacturer's instructions.
Histopathological examination
Livers werefixed in 10% formalin solution, embedded in paraffin, sliced up infive microns thickness. Finally the hepatic pathology change was observed by hematoxylin and eosin stain under bright-field microscope.
Statistical analysis
Data were expressed as mean±standard deviation (SD).Statistical analyses were performed using one-way analysis of variance (ANOVA) and the group means were compared by Duncan test using SPSS software 19. 0. p<0.01 or p<0.05 was considered as statistically significant with the Tukey's multiple comparison tests. Statistical analyses were carried out using SPSS version 19.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Hepatoprotective effect of FS in vitro
ALT and AST activities in culture supernatant
In the model group, the activities of ALT and AST were significantly increased as compared to the control group (p<0.01), indicating that a successful model was duplicated. Both ALT and AST activities were significantly lower in the groups treated with FS extracts than those in the model group (p<0.01), and the activities of transaminase depressed with the increase of FS extract dose. The results are shown in Fig. 1.
Antioxidant index levels in hepatocytes
The changes of MDA, SOD and GSH levels in hepatocytes are presented in Table 1. The levels of MDA, SOD and GSH were significantly (p<0.01)altered in the model group. Compared to the model group, GSH and MDA level altered significantly (p<0.01)in all the FS extract groups (100, 150 and 200 μg • mL-1),while SOD increased markedly (p<0.05) in middle concentration of FS extract (150 μg • mL-1) group.
GSTA1 contents in culture supernatant
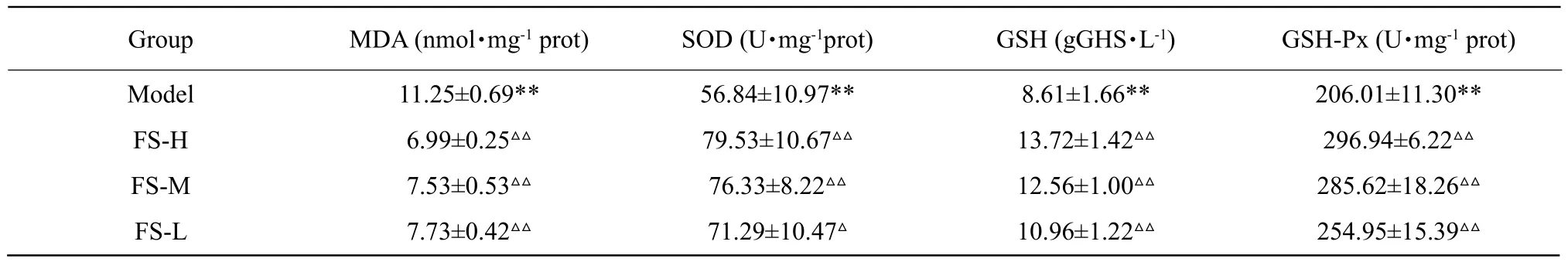
The changes of GSTA1 contents in hepatocyte culture supernatant are presented in Fig. 2. GSTA1 content in model group significantly increased (p<0.01) relative to the control group. Compared with the model group,there were significant decrease (p<0.01) in all the FS groups (100, 150 and 200 μg • mL-1). The contents of GSTA1 decreased gradually with the increase of FS extract concentration.
Hepatoprotective effect in vivo
ALT and AST activities in mice serum
As shown in Fig. 3, serum ALT and AST activities in model group were significantly increased (p<0.01)in comparison with the control group. It had been observed that ALT and AST activities significantly decreased (p<0.01) in mice serum administrated with FS extracts (100, 150 and 200 mg • kg-1) relative to model group. Moreover, the activities of ALT and AST in high dose group with FS (200 mg • kg-1) were nearly similar to the positive group (Silymarin).
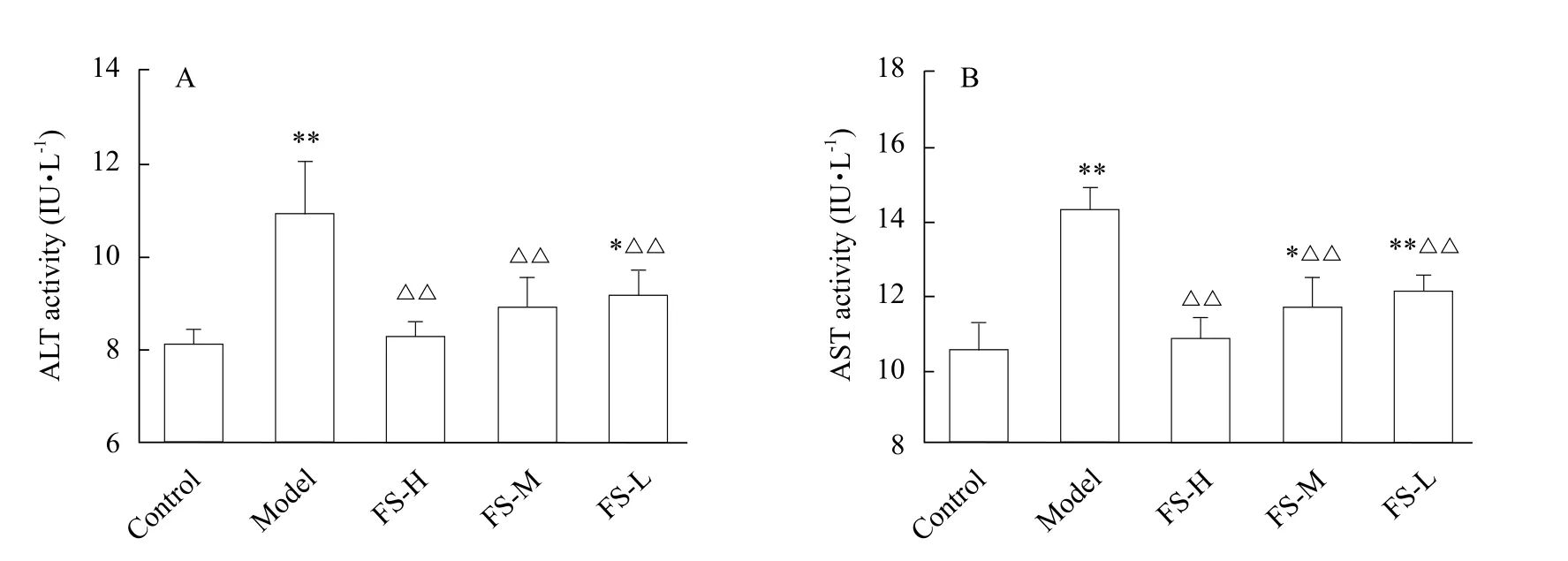
Fig. 1 Activity of ALT (A) and AST (B) in cell culture supernatant
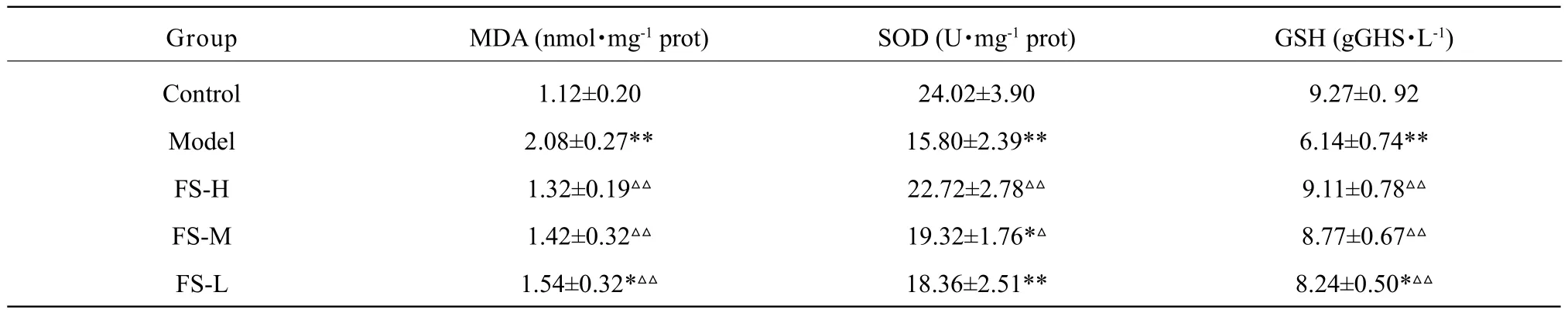
Table 1 Changes of MDA, SOD and GSH levels in cells
Antioxidant indices levels in mice liver
All the liver antioxidant indices in ethanol model group were significantly different from those in the control group (p<0.01). MDA contents in FS extract groups (100, 150 and 200 mg • kg-1) significantly decreased as compared to the model group (p<0.01).SOD and GSH-Px activities displayed significant enhancement in the ethanol group (p<0.01), and the same was true with the change of GSH content.The levels of MDA, GSH and GSH-Px changed extremely significantly (p<0.01) in the low-dose FS group (100 mg • kg-1). In addition, SOD was notably(p<0.05) increased in comparison to the model group.There was no significant difference between FS high-dose group (200 mg • kg-1) and the positive drug group(Silymarin group). Results are shown in Table 2.
GSTA1 contents in mice serum and liver
GSTA1 contents in model group were significantly different (p<0.01) from the control group both in serum and liver tissues. GSTA1 contents in tissue were significantly lower than those in normally treated tissue(p<0.01); however, there was an opposite trend of GSTA1 contents in serum. Additionally, the trend of GSTA1 contents in the high-dose FS group (200 mg • kg-1)was similar to Silymarin group and the control group.The results of GSTA1 contents in the serum and liver tissues are presented in Fig. 4.
Histopathological examination
Pathological changes were observed by hematoxylin and eosin stain (H&E stain). The protective effect of FS was further confirmed by morphological analysis(Fig. 5). As shown in Fig. 5A, normal hepatic cells were observed and showed even cytoplasmic staining.The structure of hepatic cord was clear. In the model group (Fig. 5B), hepatic cell cords were disordered along with erythrocytes exudation in sinusoidal and small vacuoles in cytoplasm. After administration with high-dose FS ethanol extracts (200 mg • kg-1,Fig. 5C), it was observed mild fatty degeneration,but the structure of hepatic lobules were clear. The Silymarin treatment group (Fig. 5D) appeared normal lobular architecture.

Fig. 2 Content of GSTA1 in cell culture supernatant
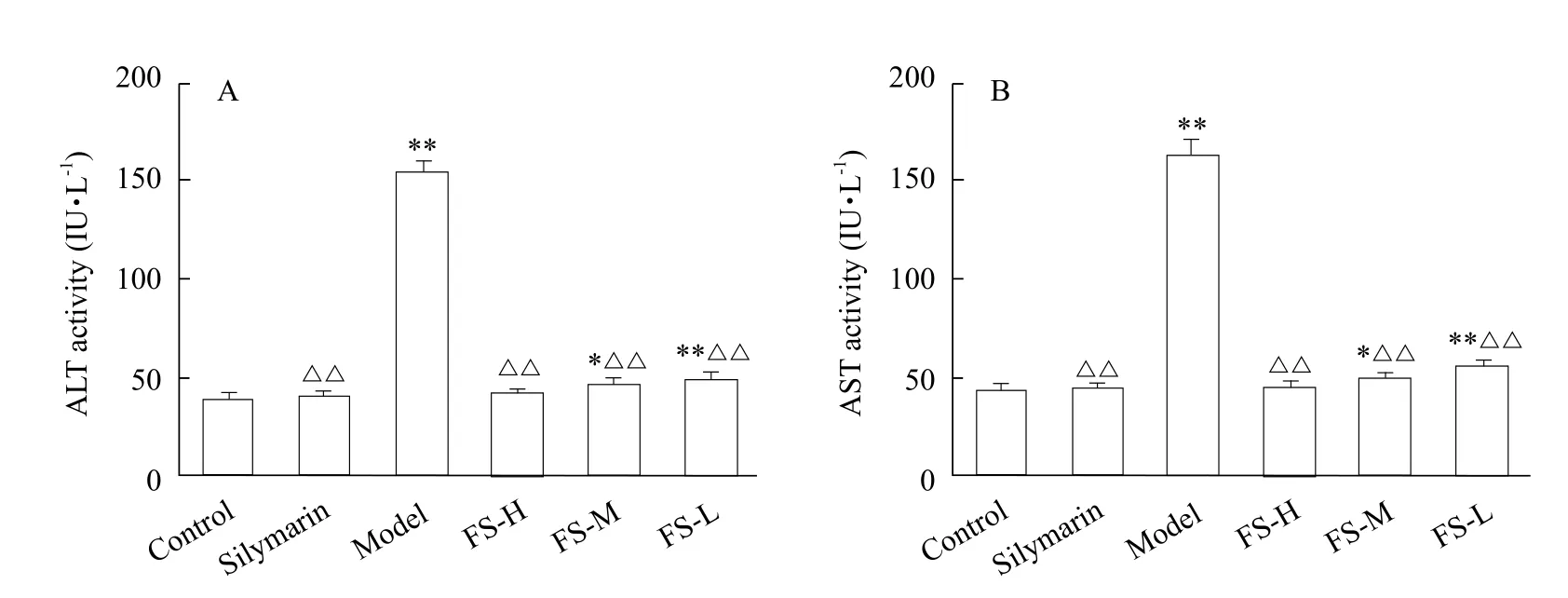
Fig. 3 Activity of ALT (A) and AST (B) in serum
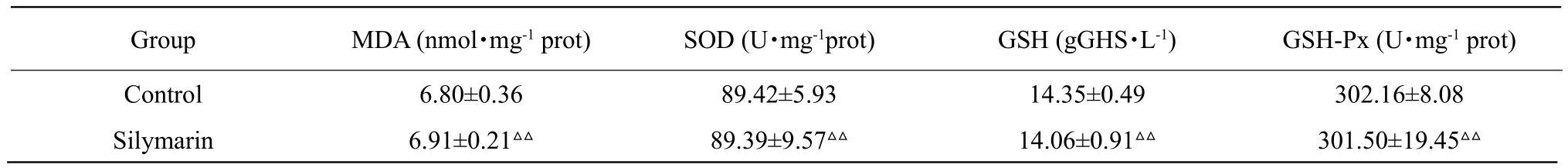
Table 2 Changes of SOD, MDA, GSH and GSH-Px contents in mice
Continued
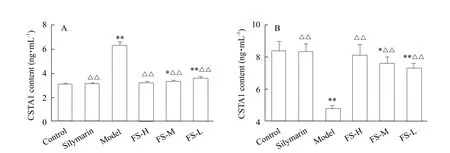
Fig. 4 Contents of GSTA1 in the serum and liver tissue
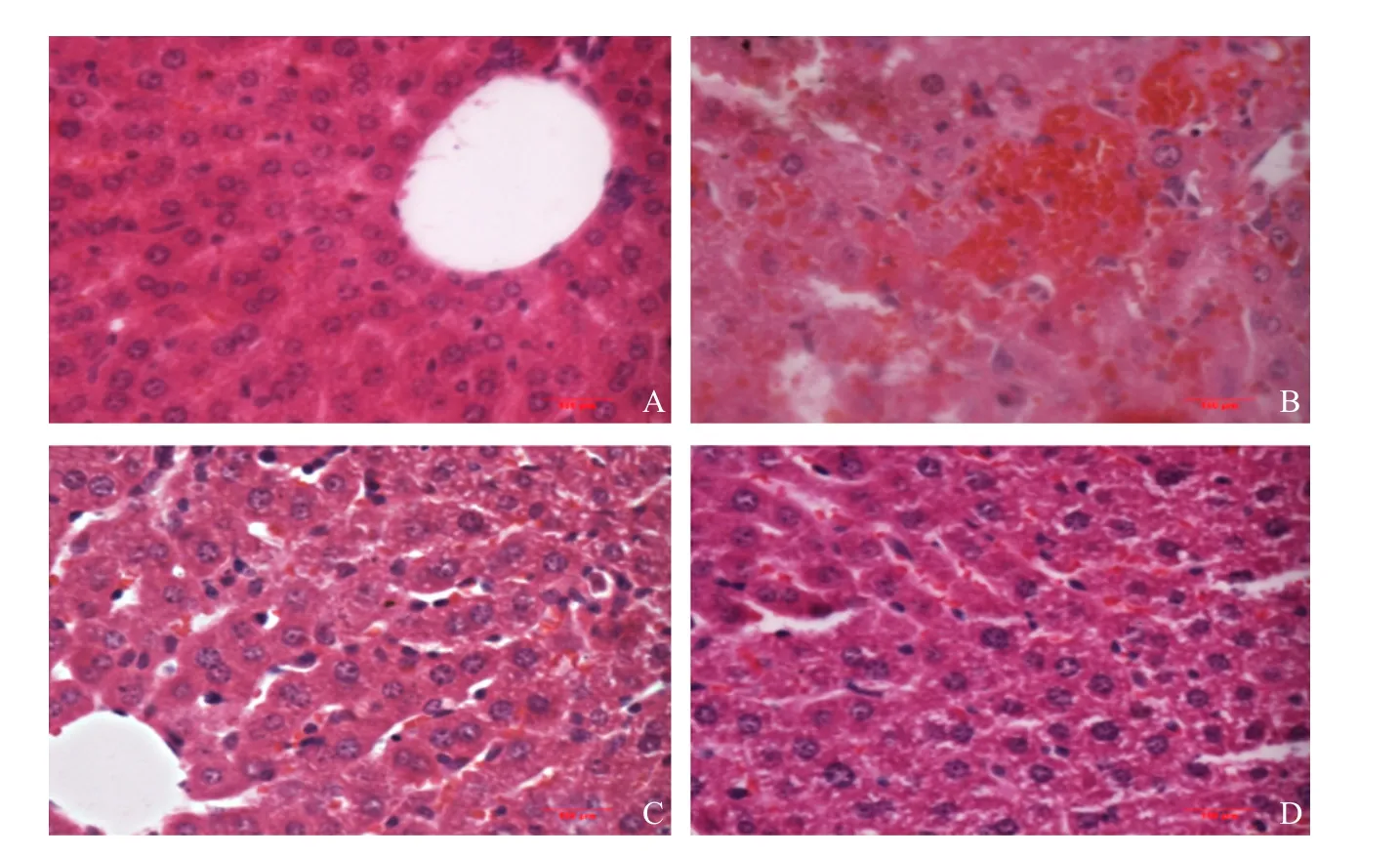
Fig. 5 Pathological changes of liver in mice
Discussion
Ethanol is an important factor to cause liver injury.The increase of free radicals and the reduction of antioxidants (such as GSH) are important reasons for lipid peroxidation. Free radicals are products of oxidative stress in ethanol metabolism, such as hydroxyl radical ( • OH), superoxide anion radicals(SAFR), ROS, and reactive nitrogen species (RNS)(Leung and Nieto, 2013). Previously, Folium syringae has shown indicated hepatoprotective properties against hepatotoxicity induced by acetaminophen (Shi et al., 2017). In the present study, mice and primary hepatocytes were used to test the hepatoprotective effects of FS extracts as primary hepatocytes could be used for investigations of liver functions and for evaluating the toxic effects of drugs, so that it had become the gold standard for drug toxicology experiments in vitro.
The hepatocyte membrane and organelles were damaged under the administration of ethanol which induced a released of transaminase into the extracellular space (Willner and Reuben, 2005). As a result, transaminase concentration increased in culture supernatant. ALT and AST activities were classic indicators of liver function for diagnosis, and the changes of transaminases reflect functional changes of hepatocytes. The activities of ALT and AST in the model group significantly increases both in vitro and in vivo relative to the control group (p<0.01), which indicated that the acute alcoholic liver injury models were successfully replicated. Compared with the model group, transaminases decreased significantly (p<0.01)in groups with FS extracts both in vitro and in vivo. It was clear that the administration of FS extracts could reduce liver membrane impairment and prevent the elevation of ALT and AST levels in serum of mice and culture supernatant of primary hepatocyte in ethanolinduced liver injuries. The results showed that FS extracts could significantly prevent or ameliorate liver injury induced by ethanol.
A large number of studies have shown that oxidative stress was the main cause of liver injury induced by ethanol. Both of the direct effects of ethanol and ethanol metabolism were the causes of oxidative stress implicated in alcoholic liver injuries. Ethanol metabolism engenders reactive oxygen intermediates.The major systems for cellular metabolism of ethanol were production of acetaldehyde (Lieber, 1997).Acetaldehyde was a reactive substance that promoted lipid peroxidation and thereby increased oxidative stress (Wenzel et al., 2007). In addition, acetaldehyde could predispose the cell to lipid peroxidation and increased oxidative stress indirectly by forming protein adducts with enzymes, such as SOD, and thereby impairing the cell's defense systems against oxidative stress. MDA was one of the most important lipid oxidation products, which can aggravate membrane damage. MDA level in FS extract group significantly decreased as compared to the model group (p<0.01)both in vitro and in vivo. Increased amount of MDA in ethanol-induced liver injury signified the enhance degree of lipid peroxidation, while pretreatment with FS decreased MDA and degree of lipid peroxidation.
Moreover, SOD, GSH and GSH-Px levels in ethanol-induced group significantly decreased. But these indices in groups with FS extracts were significantly higher (p<0.01) than those in ethanol model group.SOD, GSH and GSH-Px were cell's defense systems against oxidative stress. GSH was a free radical scavenger that, if decreased, predisposes to lipid peroxidation by other factors. Acute ethanol intake inhibits GSH synthesis, and acetaldehyde decreases GSH activity by binding with cysteine in GSH (Daniell,1998). It indicated that FS could protect hepatocytes against ethanol-induced damage by enhancing antioxidant capabilities. Hence, FS had a protective effect in hepatocytes and mouse model of acute liver injury induced by ethanol in antioxidant activities.
GSTA1 was a key enzyme in the glutathione binding reaction, which played an important role in the metabolism of ethanol. Main functions of GSTA1 were to catalyze reduced glutathione combined with electrophilic substrates or by the non-enzymatic combination with a various potential toxicities in the body from some chemical carcinogens, dyes and lipotropy compounds in body, and to achieve the goal of detoxification. Liver injury induced by ethanol was mainly due to the changes of free radicals. According to the previous studies, an early emergence in the early stage of acute alcoholic liver injuries and a decrease of GSTA1 contents in livers were found (Ma et al.,2017). Compared with the model group, the contents of GSTA1 in FS group culture supernatant were significantly decreased and the same change was observed in the serum (from 4.88 to 3.34 ng • mL-1in supernatant, 6.30 to 3.21 ng • mL-1in serum), but it increased in liver (from 4.77 to 8.10 ng • mL-1). The contents of GSTA1 in mice with Silymarin, had no significant difference, when compared to FS high-dose groups (200 μg • mL-1in vitro, 200 mg • kg-1in vivo).In addition, all the targets of high-dose FS extract group (200 mg • kg-1) were almost similar to Silymarin group in mice. Silymarin was used as positive drug with hepatoprotective effects in this study which had a significant antioxidant effect, as used in clinical drug treatment of alcoholic liver injury (Crocenzi and Roma, 2006). According to the results above, it suggested that FS could not only reduce peroxidation and protect cell membrane, but also inhibit mitochondrial damage. The results indicated that FS had a good effect against ethanol-induced injuries in hepatocytes and reduced GSTA1 release. According to the pathological changes, it was clear that high-dose FS had a similar degree of hepatoprotective function to Silymarin in ethanol-induced acute livery injuries.
Conclusions
Taken together, the present study provided experimental evidence that FS ethanol-extracts had significant protective effects against liver injuries induced by ethanol via modulating detoxification enzymes(GSTA1 and GSH-Px) and antioxidative enzymes(SOD and GSH). It could be concluded that FS extracts could not only promote the metabolism of ethanol in vivo, but also eliminated the toxic effects of ethanol metabolism. Therefore, Folium syringae had obvious protective effects on acute ethanol-induced liver injuries.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Improvement of Temperature Uniformity of Instant Rice Inside Plastic Rectangular Container Under Microwave Reheating
- GIS Based Aroma Characteristic for Regional Distribution of Tobacco Using Kriging Interpolation Method Set in Henan Province of China
- Isolation and Characterization of E. Coli O157 : H7 from Infected Newborn Calves in Northeast China
- Porcine Parvovirus Inducing Autophagy to Benefit Its Replication
- Function of RanGAP1 in Mouse Oocyte Fertilization
- FSH Promoting Proliferation of Calf Sertoli Cells Through Wnt/β-catenin Signaling Pathway with CDC25B Being Involved
