Porcine Parvovirus Inducing Autophagy to Benefit Its Replication
2019-01-09ZhangYueLiXiaoxueZhongMingChenHuijieHuangXiaodanBaiYunyunCongYingyingZhangRuiliandLiGuangxing
Zhang Yue, Li Xiao-xue, Zhong Ming, Chen Hui-jie, , Huang Xiao-dan, Bai Yun-yun, Cong Ying-ying,Zhang Rui-li, and Li Guang-xing*
1 Key Laboratory for Laboratory Animals and Comparative Medicine of Heilongjiang Province, College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
2 College of Pharmaceutical Engineering, Jilin Agriculture Science and Technology College, Jilin 132101, Jilin, China
Abstract: Porcine parvovirus (PPV) is one of the major causes of reproductive failure in pigs, which poses a great threat to the pig breeding industry and results in tremendous economic losses worldwide. Autophagy is the biological process of cell self-defense and self-protection. Despite many viruses can cause cell autophagy, when they enter cell or copied, the relationship between autophagy and PPV infection has not been reported. In this study, impact of autophagy after swine testicular (ST) cells infected by PPV was studied.Autophagy was demonstrated by the effective replication of PPV through transmission electron microscopy, immuno fluorescence and western blot analysis. Moreover, autophagy was confirmed to benefit PPV replication by real-time fluorescence quantitative PCR and determination of median tissue culture infective dose (TCID50). For thefirst time, the complex interaction between PPV infection and autophagy was explored in this study. It indicated that PPV could induce autophagy in ST cells, which in turn facilitated its own replication, which might be one of the mechanisms of the virus infection. Thesefindings could facilitate the study of the pathogenesis of PPV infection and provide new insight into the development of effective therapeutic strategies.
Key words: autophagy, porcine parvovirus, virus replication, apoptosis
Introduction
Porcine parvovirus is an icosahedral capsid virus that is non-enveloped and has a diameter of 25 nm(Wilhelm et al., 2006; Cságola et al., 2012). PPV is a member of parvoviridae, containing a single stranded DNA genome structure containing approximately 4-6.3 kb (Shackelton et al., 2007; Oh et al., 2017). PPV outbreaks have occurred in many countries, in which swine industries have suffered serious economic losses (Kresse et al., 1985). PPV infection mainly causes reproductive failure in pregnant sows with the reproductive clinical syndrome of infertility, abortion,stillbirth, neonatal death, reduced neonatal vitality and delayed return to oestrus (Antonis et al., 2006; Wolf et al., 2008). Boars play an important role in PPV transmission. Intriguingiy, PPV is transmitted to sows through semen, and can cause damage of swine testicle in vivo. Therefore, in this study, ST cells were used to study autophagy in PPV. Previous studies found that PPV infection caused apoptosis in pig kidney cells line 15 (PK-15 cells) and ST cells (Zhang et al., 2010;Zhao et al., 2016). However, whether PPV induces ST cells autophagy and the role of autophagy in PPV replication are unknown.
Autophagy is a highly conserved degradation process that is ubiquitous in eukaryotic cells. It is a protective mechanism for cells to maintain homeostasis. Autophagosomes (double-membrane vesicles) will be formed in cells stimulated with starvation, endoplasmic reticulum (ER) stress, and viral infections. These vesicles transfer long-lived or misfolded proteins, or damaged organelles to lysosomes, andfinally degrade these proteins and organelles into small peptides or amino acids for cells as renewable resources (Kim et al., 2010; Sun et al., 2014). Cell autophagy plays an important role in innate and adaptive immunity to foreign pathogenic microorganisms, including response to viral infections(Kundu and Thompson, 2008). Many studies show that autophagy is an intrinsic host defense mechanism. In the virus-infected cells, the produces of autophagosomes with engulfed viruses inside move to lysosomes for degradation to inhibit virus replication.The autophagy is also the target which was used by some virus. It is important significance for virus replication, such as classical swine fever virus (CSFV),porcine reproductive and respiratory syndrome virus(PRRSV) and rotavirus (Pei et al., 2014; Sun et al.,2012; BerKova et al., 2006). In the latter part of autophagy; however, the virus prevents its degradation by inhibiting autophagy (Levine et al., 2011),such as human cytomegalovirus (HCMV) and herpes simplex virus type 1 (HSV-1) (Dreux and Chisari,2010; Chaumorcel et al., 2008; Talloczy et al., 2002).Thesefindings show that there is a complex relationship between autophagy and viral infection.
Recent studies have shown that SAT protein of PPV can accelerate viral spreading through irreversible ER stress induction in porcine testis (PT) cell and thereby promote apoptosis (Mészáros et al., 2017). Moreover,PPV infection can induce apoptosis in PK-15 cells through activation of p53 and mitochondria-mediated apoptosis pathway. Further-more, PPV infection can trigger ST cell apoptosis via PPV-induced ROS accumulation. Another study shows that toll-like receptor (TLR) pathway participates in recognition of PPV and induction of NF-κB activation (Cao et al.,2017). Based on the above reports, PPV could cause endoplasmic reticulum stress, produce apoptosis, and damage the host innate immunity via NF-κB signaling pathway. These processes were all closely related to the occurrence of autophagy in cells. Therefore,it was hypothesized that whether PPV could induce autophagy to influence the virus replication in host cells or not. This study demonstrated for thefirst time that autophagy was triggered in ST cells during PPV infection to promote its replication.
Materials and Methods
Cells, viruses and plasmids
ST cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum at 37℃ with 5% CO2. PPV strain TJ(accession no. KX233726) was isolated from the liver of an aborted fetus in Tianjin of China and identified by physicochemical test, neutralization test, RT-PCR and sequence analysis (Shi et al., 2012). Virus titers were measured by 50% tissue culture infective doses(TCID50) assay according to Reed-Muench method(Reed et al., 1938), PPV TJ strain at a titer of 10-8.11TCID50/mL. The plasmid GFP-LC3 was maintained in the laboratory.
Virus infection
ST cells were infected with PPV at a MOI (multiplicity of infection) of 0.1. After 1 h incubation at 37℃,unbound viruses were removed by washing three times with PBS, infected cells were incubated in the complete DMEM at 37℃ for the relevant times in the conformity with experimental requirements.
Transmission electron microscopy
By observing autophagosomes under the transmission electron microscope, autophagy could be visually analyzed (Peng et al., 2016). In this assay, ST cells were treated with 100 nmol • L-1rapamycin (Sigma,USA), or were treated with DMEM, or infected with PPV at 1 MOI. Ultrathin sections were prepared as previously described by (Risco et al., 2012) and autophagosome-like vesicles were viewed under the transmission electron microscope (JEOL, Tokyo,Japan). The Autophagosome-like vesicles were defined as double- or single-membrane vesicles measuring 0.3 to 2.0 µm in diameter with clearly recognizable cytoplasmic contents.
lmmuno fluorescence assay (lFA)
MAP1LC3 (LC3), short for microtubule-associated protein one light chain three (orthologue of mammalian yeast Atg8), had been extensively studied as a marker of autophagy (Guo et al., 2017).According to LipofectamineTM2000 transfection reagent instructions (Invitrogen, USA), GFPLC3 eukaryotic expression plasmid contained the LC3 gene (gift from Dr. Norobu Mizushima) was transfected into ST cells to monitor LC3 gathering within the cells. After transfection for 24 h, the cells were infected with PPV, or treated with rapamycin(100 nmol • L-1), or treated with DMEM, respectively.Afterfixing, permeabilization and blocking, the cells infected with PPV were then incubated with anti-PPV polyclonal antibody and subsequently incubated with the rhodamine-conjugated secondary antibody.The cells nuclei of different treatment groups were stained with 4, 6-diamidino-2-phenylindole-dihydrochloride (DAPI, Invitrogen, USA). The fluorescent images were examined under a fluorescence microscope (Ti-S, Nikon, Japan), and the number of LC3 fluorescence signal gathering point was counted in 30 cells.
Western blot analysis
LC3-I was phosphorylated to form LC3-II as a marker of active autophagy. In the meantime, p62,also known as Sequestosome1 (SQSTM1/p62), was a multifunctional protein involved in signal transduction,protein degradation and cell transformation which played an important role in autophagy (Bjorkoy et al.,2005; Pankiv et al., 2007). P62 degradation was required when autophagy was activated (Pursiheimo et al., 2009). According to lysis instructions, cells were washed two times with PBS and then lysed with RAPI lysis buffer (Solarbio technology Co., Beijing, China).Equal amounts of protein samples were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. The membranes were blocked with 5% (w/v) skim milk-TBST at room temperature for 2 h, and then incubated with the primary indicated antibodies at 4℃ overnight. After washing with PBST three times, the membranes were incubated with corresponding secondary antibodies.The membranes were again washed three times in PBST and then scanned using an Odyssey infrared imaging system (LI-COR Biosciences). The protein bands were quantified by gray value analysis with image J software (National Institutes of Health,Bethesda, MD, USA).
Quantitative real-time (qRT) PCR
Recent studies had shown that autophagy could promote viral replication (Monastyrska et al., 2013;Reggiori et al., 2010). To investigate whether autophagy affected PPV replication, real-time quantitative reverse transcriptase polymerase chain reaction (qRTPCR) was performed to analyze the virus copies. The cells were given the appropriate treatment or infection and DNA was extracted following virus genomic DNA rapid extraction kit (Bioteke Corporation, Beijing, China). A pair of specific primers (PPV-F: CAC GCATCAAGACTCATA/PPV-R: GTCCGCTGGAT TGAACCA) was used in the study for PPV detection,which was targeting a region to VP2 gene of PPV.Absolute quantitative real-time PCR was performed in an Applied LightCycler 96 Real-time PCR System(Roche, Switzerland).
Statistical analysis
All the data were expressed as the means with SD.The Student's t-test was used to perform statistical analyses. p<0.05 was considered as statistically significant, and p<0.01 was considered as highly significant.
Results
PPV-infected cells inducing formation of autophagosomes
It is well-known that double-membrane vesicles(DMVs) represent autophagy. In order to investigate whether PPV infection could activate the autophagy machinery, the cells were examined for the formation of autophagosome-like vesicles at 24 h post PPV infection through transmission electron microscopy (TEM). DMVs containing organelle and cytosolic components, which were analogous to autophagosomes, were visible in PPV infected cells (Fig. 1A), which were similar to rapamycin treated cells (Fig. 1B). However, autophagosome-like vesicles were rarely observed in the mock-infected cells (Fig. 1C).
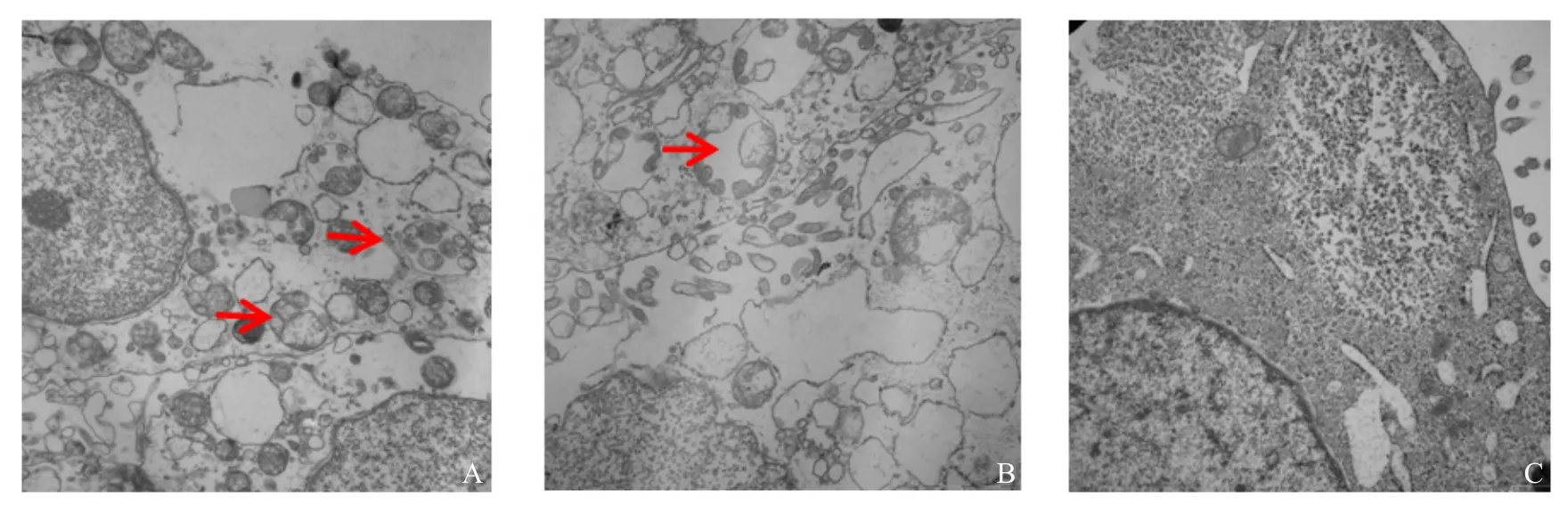
Fig. 1 Autophagosomes in porcine parvovirus-infected ST cells
Observation of positive GFP-LC3 fluorescence in PPV-infected ST cells
As it was well-known that LC3 protein was selectively recruited to autophagic vesicles, which could be considered as its redistribution from a diffuse cytoplasmic localization to a distinctive punctate cytoplasmic pattern during autophagy (Sun et al., 2012). GFP-LC3 positive cells treated with rapamycin showed high punctate LC3 accumulation (Fig. 2A Rapa). Additionally, large amounts of punctate GFP-LC3 proteins were observed in PPV-infected cells at 24 h post-infection(hpi) (Fig. 2A PPV), while GFP-LC3 was detected as a diffuse distribution in mock-infected cells (Fig. 2A Mock), indicating that the accumulation of GFP-LC3 dots was induced by PPV infection. The number of LC3 fluorescence signal was examined in each sample(Fig. 2B). The average number of GFP-LC3 fluorescence spots in PPV infected cells was 53, which was obviously higher than that in the mock-treated cells,and which was close to the rapamycin treated cells.
Western blot analysis of p62 and LC3-ll in PPV-infected ST cells
To further analyze whether autophagy was induced by PPV infection, the level of autophagy marker proteins in PPV-infected cells was examined by immunoblotting. The conversion from LC3-Ⅰto LC3-Ⅱwas monitored at 6, 12, 18, 24, 30, 36, 42 and 48 h post PPV infection. As shown in Fig. 3A, compared with the mock-infected cells, the conversion of LC3-Ⅰto LC3-Ⅱ was significantly increased in the positive control, as well as in the progression of PPV infection, which was tracked by PPV V protein.Meanwhile, the ratio of LC3-Ⅱ /β-actin gradually increased with prolonged PPV infection, but slightly decreased at 24 h but then gradually increased from 30 to 48 h. Furthermore, there was a significant difference between PPV group and the normal group at 24 h. The degradation of SQSTM1 (p62) was recognized as an indicator for assessing autophagy flux. Whether a complete autophagic process was triggered by PPV infection wasfirst determined by the degradation of p62 through immunoblotting analysis. As shown in Fig. 3A, compared with the mock-infected cells, the level of p62 was significantly decreased in the positive control, as well as in the progression of PPV infection. Additionally the ratio of p62/β-actin gradually decreased with prolonged PPV infection, but slightly increased at 30 and 36 h, but then gradually decreased from 42 to 48 h. Moreover,there was a significant difference between PPV group and the normal group at 24 h, too. The results further supported that autophagy was induced by PPV infection.
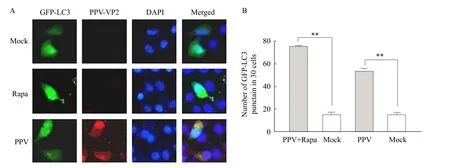
Fig. 2 Infection of ST cells with porcine parvovirus inducing formation of GFP-LC3 fluorescence spot
Effect of autophagy on PPV replication
As could be seen from Fig. 4, compared with PPV infection group, the expression of virus VP2 increased when autophagy promoter was added. However, when autophagy inhibitor was added, the expression of VP2 increased. These results showed that autophagy could increased viral protein expression, and therefore could promote PPV replication in ST cells.
Virus replication and titer were closely related, as the virus replication increased, the titer increased,and vice versa. To further elucidate the effects of autophagy on PPV replication, the virus titer was determined by TCID50. As could be seen from Fig. 5,compared with PPV infection group, after rapamycin treatment, PPV titer increased, conversely, after treatment with wortmannin, PPV titer decreased. This further demonstrated that the virus facilitated selfreplication by promoting autophagy in ST cells.
In order to further verify that autophagy promoted PPV replication, the virus copy number was determined. As could be seen from Fig. 6A, there was significantly increase of PPV copy number in rapamycin-treatment of PPV infection group compared to that of only PPV infection group, demonstrating that PPV replication increased. Conversely, after treatment with wort-mannin, PPV copy number decreased, indicating that PPV replication decreased.As is displayed in Fig. 6B, compared with PPV-infected group, the rapamycin concentration caused an increase in the viral number. All the aforementioned data indicated that the autophagy mechanism was triggered by PPV infection to facilitate its replication.
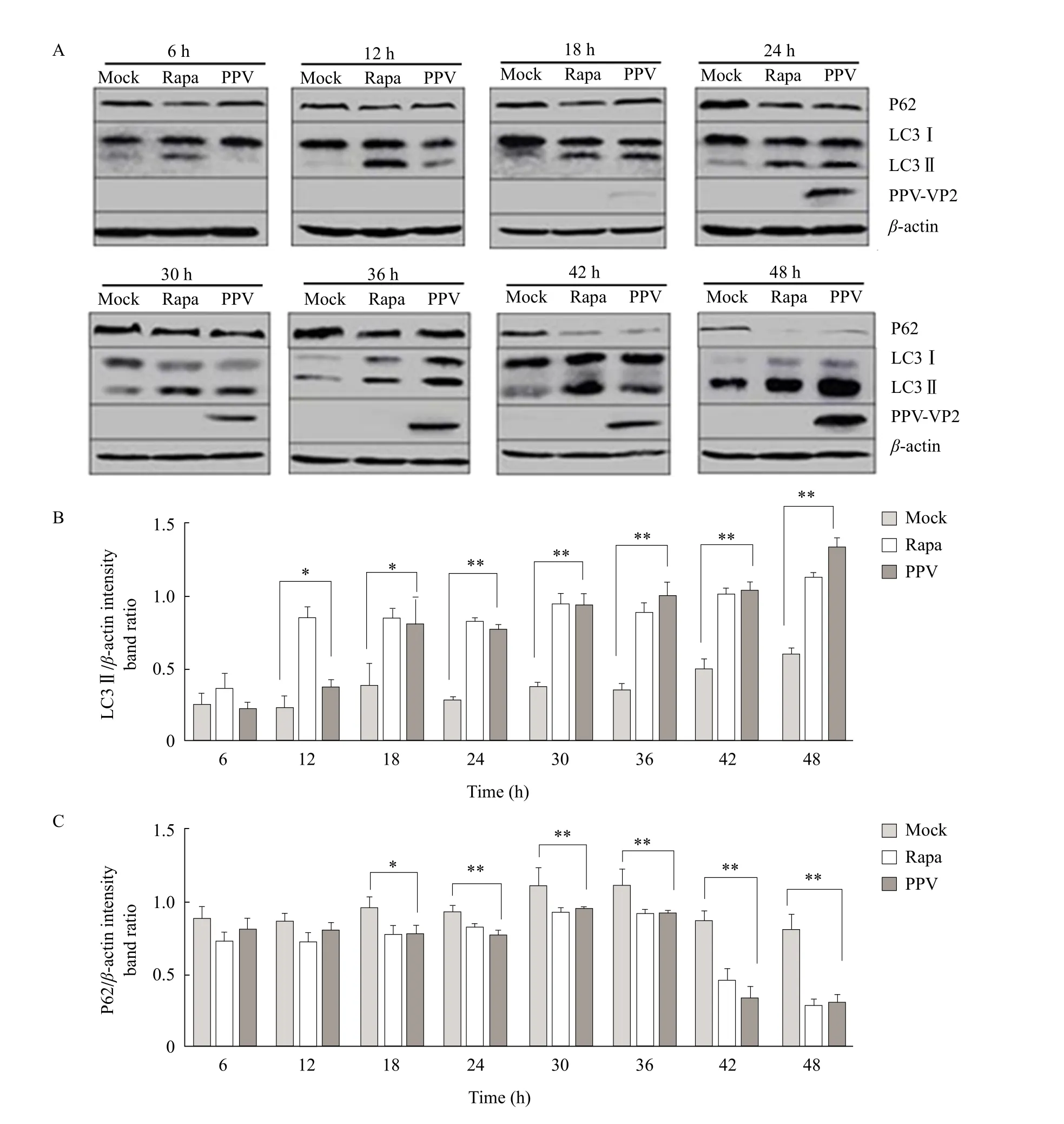
Fig. 3 Western blot analysis of p62 and LC3-II in PPV-infected ST cells
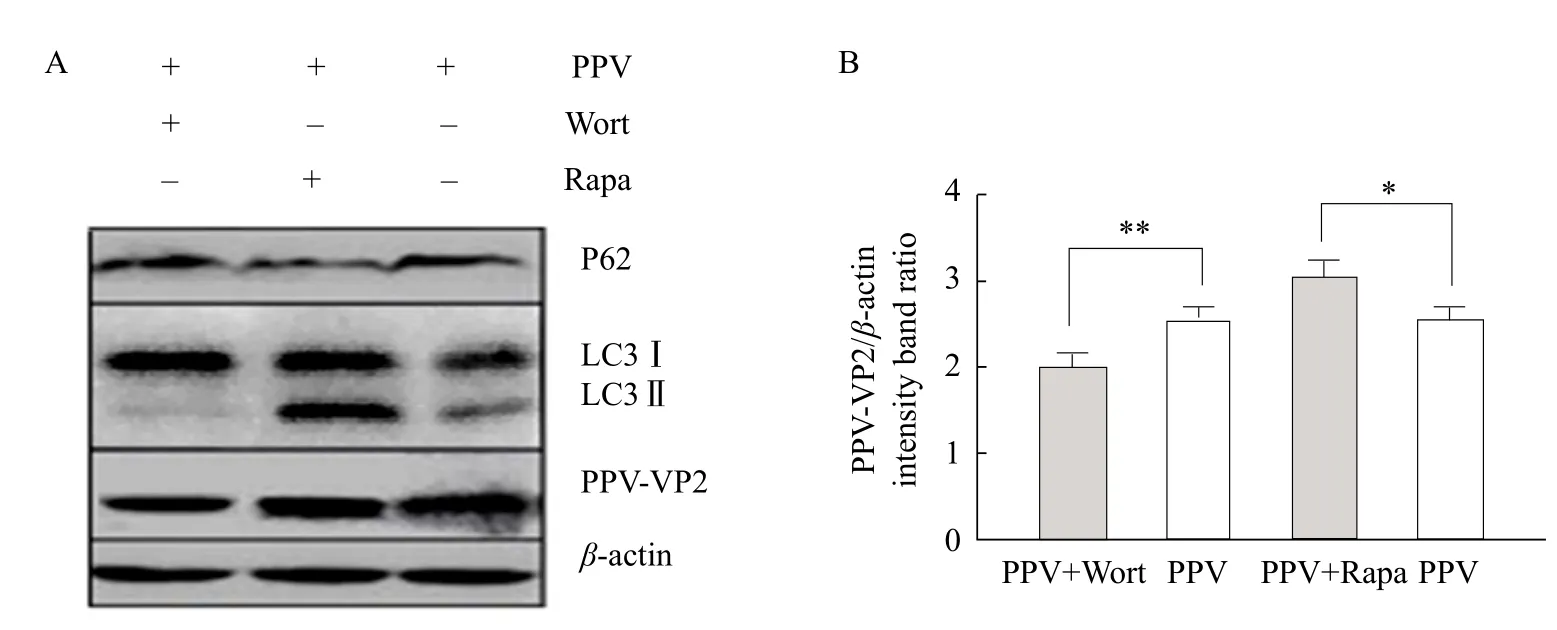
Fig. 4 Western blot analysis of autophagy on PPV replication

Fig. 5 PPV titers analysis of autophagy on PPV replication
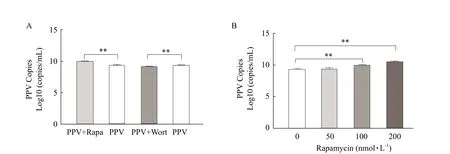
Fig. 6 PPV-VP2 copies analysis of autophagy on PPV replication
Discussion
In recent years, autophagy has been widely investigated due to its important role in the pathogenesis of many diseases (Ke and Chen, 2017). The relationship between autophagy and viral infection has attracted the attention of an increasing number of researchers(Dreux et al., 2009; Orvedahl et al., 2007). Autophagy played a vital role in the replication of DNA and RNA viruses (Dreux and Chisari, 2010). Many DNA viruses induced autophagy in order to produce the membraneassociated replication complexes. For instance, DEV induced autophagy via the endoplasmic reticulum stress related unfolded protein response (Yin et al.,2017). A further study revealed that HCMV infection in fluenced autophagy in THP-1 cells (Liu et al., 2017).In addition, herpes simplex virus 1 interfered with autophagy of murine dendritic cells and impaired their ability to stimulate CD8+T lymphocytes (Budida et al.,2017). However, the specific role of autophagy on PPV infection had not been reported until now. This study provided thefirst strong evidence that PPV infection could trigger autophagy to facilitate its replication.
During the virus infection, LC3 could be recruited to form double-membrane vesicles (DMVs) from ER(Reggiori et al., 2010). These DMVs appeared to have a subcellular structure similar to the autophagosome,which was called a "virus factories" in a recent study.These viral factories provided a suitable platform for the replication and encourage autophagy, implying a covered connection between DMVs and autophagy(Blanchard and Roingeard, 2015). Here, the traditional TEM assay was performed, the effectively increased number of double- or single-membrane vacuoles are displayed in PPV infected cells for thefirst time, which revealed the formation of autophagosome. These data indicated that autophagy activity might be triggered by PEDV infection (Mizushima et al., 2010). In addition,accumulation of GFP-LC3 puncta in PPV-infected cells was observed by a fluorescence microscope. It further verified the induction of autophagy.
The term "autophagic flux" was used to denote the dynamic process of autophagosome synthesis, the delivery of autophagic cargoes to the lysosome, and the degradation of autophagic cargoes inside the lysosome and was a more reliable indicator of autophagic activity than measurements of autophagosome numbers. Some recent studies had demonstrated that the induction of autophagy not only increased autophagosome formation and expression of autophagy proteins, but also increased autophagic flux, which could be measured by detecting the levels of p62 (Pankiv et al.,2007). Using several means of measuring autophagic flux, including p62 degradation and LC3-Ⅱ turnover,confirmed that autophagic flux remained changed upon PPV infection. The cells were infected with PPV or Rapa treatment, the level of LC3-Ⅱ was increased and the level of p62 was suppressed. Thesefindings illustrated that a complete autophagy was activated in ST cells by PPV infection. Autophagy was often closely related to viral replication, and several viruses used autophagic substances or the autophagosome to escape the immune system in order to achieve viral proliferation. While others used the autophagosome or autophagic vesicle model structure as their own site for genome transcription and replication (Jackson et al., 2005). In the present study, the effect of autophagy modulation on PPV replication was further evaluated by the two essential components rapamycin and wortmannin. The yield of PPV-VP2 was found to be suppressed whenever the autophagy process was inhibited by wortmannin, and the yield was increased whenever the autophagy process was promoted by rapamycin. Additionally, the induction of autophagy by rapamycin also enhanced the viral titer and copy number. However, the induction of autophagy by wortmannin also weakened the viral titer and copy number. These results suggested that autophagy induction might benefit PPV replication.
Conclusions
It was thefirst report demonstrating that PPV infection triggered the formation of autophagosomes and induced autophagy in ST cells. Autophagy had an effect on PPV replication. It was not yet known that the mechanism of cellular autophagy induced by PPV or cell organelles associated with autophagy involved in replication of PPV. More in-depth studies should be focused on the mechanism of autophagy.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Laboratory Assessment of Susceptibility of Maize Varieties to Postharvest Infestation By Sitophilus zeamais (Mostchulsky) (Coleoptera:Curculionidae)
- Identification of Genes Associated with Clubroot Resistance in Chinese Cabbage
- Species Composition and Diversity Analysis of Lava Flow in Different Periods in Wudalianchi Nature Reserve, China
- FSH Promoting Proliferation of Calf Sertoli Cells Through Wnt/β-catenin Signaling Pathway with CDC25B Being Involved
- Function of RanGAP1 in Mouse Oocyte Fertilization
- Isolation and Characterization of E. Coli O157 : H7 from Infected Newborn Calves in Northeast China
