Gas chromatography-mass spectrometry method for determination of β-propiolactone in human inactivated rabies vaccine and its hydrolysis analysis
2018-12-10ShuoLeiXunGoYngSunXingyongYuLongshnZho
Shuo Lei,Xun Go,Yng Sun,Xingyong Yu,Longshn Zho,*
aSchool of Pharmacy,Shenyang Pharmaceutical University,Shenyang 110016,China
bLiaoning Medical Device Test Institute,Shenyang 110179,China
cShenyang Wellwolf Pharmaceutical Science and Technology Co.Ltd,Shenyang 110022,China
Keywords:β-propiolactone Inactivated human rabies vaccine GC-MS Hydrolysis
A B S T R A C T A simple method was established for the determination ofβ-propiolactone(BPL)in human inactivated rabies vaccine by gas chromatography-mass spectrometry(GC-MS).The determination was performed on an Agilent HP-INNOWAX(30 m × 0.32 mm i.d.,0.25 μm)capillary column at the temperature of 80 °C.Electrospray ionization(ESI)was used by selective ion detection at m/z 42.The temperature for ESI source and inlet was set at 230 °C and 200 °C,respectively.Helium was used as the carrier gas at a flow rate of 25.1 mL/min.The total run time was 8 min.Acetonitrile and other components in the sample did not interfere with the determination of BPL.The results showed good linearity of BPL in the range of 0.50–10.01μg/mL,with the limit of detection and the limit of quantification of 0.015μg/mL and 0.050μg/mL,respectively.Satisfactory precision was achieved for the current developed method.The method was applied to detect 6 batches of vaccine samples,and the results indicated that the target analyte BPL was present in three batches of unpurified samples,but was not detected in the purified samples,indicating the test samples were qualified.The established method was proved to be simple,versatile and sensitive,which can meet the requirements of quality control of BPL in human inactivated rabies vaccine.
1.Introduction
Vaccines represent great triumphs of medicine against various infectious diseases[1]such as rabies,measles,diphtheria,whooping cough and hepatitis B.Among them,rabies is considered as a highly lethal encephalomyelitis caused by rabies virus(RABV)[2,3].Although it is highly lethal with few effective therapies,rabies can be prevented by vaccination[4].However,vaccines made from pathogenic microorganisms and their metabolites always show safety risks[5,6].In order to ensure their safety and effectiveness,it is of great significance to choose appropriate virus inactivator.β-propiolactone(BPL),an excellent virus inactivation reagent,can act directly on viral nucleic acid[7,8]to cause mutation and block replication of virus[9].Although BPL has a powerful reactivity with virus nucleic acid,it does not damage structure and function of the fusion protein on the surface of virus[10]and has no effect on the viral capsid protein or immunogenicity of inactivated virus[9].What's more,BPL can be easily hydrolyzed to 3-hydroxypropionic acid which is non-toxic[7,9,11].Therefore,it has been widely applied in inactivation of various vaccines[12–14].However,it is reported that BPL is a carcinogen[7,11],which should be paid more attention to in clinic.From the structure of BPL(top of Fig.1B),it can be seen that BPL contains two types of activated carbon atoms[15],carbonyl carbon atom andβ-carbon atom,which can be reacted with a variety of nucleophiles[14,16,17]and may cause toxicity in human body.Thus it is necessary to establish a high-sensitivity method to detect BPL in vaccines.
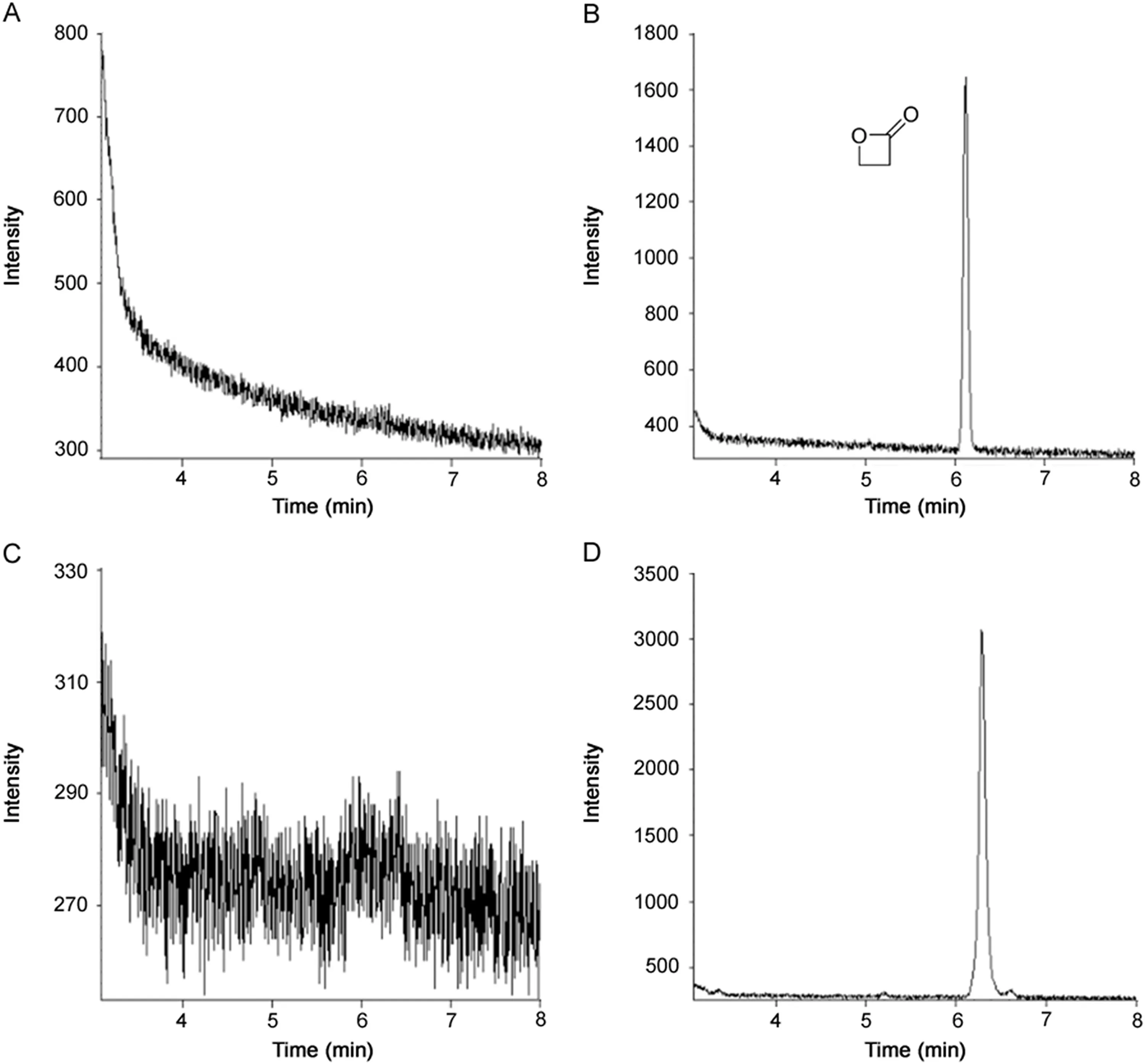
Fig.1.Chromatograms of blank solvent,BPL reference and samples for(A)blank solvent(acetonitrile),(B)BPL reference substance,(C)sample MPV20170401-G25,and(D)sample MPV20170401-Mie.
It has been reported that there are several analytical methods for the determination of BPL in vaccines,such as high performance liquid chromatography(HPLC)[18,19]and gas chromatography(GC)[7,9,11,20,21].However,due to weak ultraviolet(UV)absorption of BPL[9],it is not advocated to be determined by HPLC with UV-detector.Afterwards,it was reported that GC method for determination of BPL could achieve more faithful results[7,11].In order to avoid hydrolysis,the thinning water should be kept at 2–8°C,and the investigation required rapid operation in ice boxes,which was tedious and had a high demand for experiment operators.To overcome the drawbacks mentioned above,a modified method was developed for the determination of residual BPL in rabies virus concentrate by gas chromatography[9],using acetonitrile as reference attenuant dissolvent.In order to further optimize the determination of trace amount of BPL,an analytical method with much lower limit of detection based on gas chromatography-mass spectrometry(GC-MS)was developed and validated in this study.In addition,when BPL is used as a sterilizing agent,more attention should be paid to ensure that it is rapidly and completely hydrolyzed into its decomposition product 3-hydroxypropionic acid,which is perfectly compatible and harmless.In order to confirm the safety of vaccines and provide a reference for the research of vaccine safety,hydrolysis experiment was also carried out to verify the degradation of BPL in lyophilized human inactivated rabies vaccine(LHIRV).
2.Experimental
2.1.Chemicals and reagents
BPL and LHIRV were supplied by Chengda Biological Technology Co.,Ltd(Shenyang,China).Acetonitrile of HPLC grade was bought from Fisher(Fair lawn,NJ,USA).Chromatographic-grade water was prepared with a Milli-Q Reagent Water System(Millipore,Bedford,MA).Sodium hydroxide of analytical grade was purchased from Tianjin Aopusheng Chemical Reagent Co.,Ltd.(Tianjin,China).
2.2.Instrumentation and conditions
An Agilent 6890N-5973 System was applied with an HP-INNOWAX capillary column(30 m × 0.32 mm i.d.,0.25 μm)at the temperature of 80°C.Helium was used as carrier gas with a total flow rate of 25.1 mL/min.The temperature for ESI source and inlet was set at 230 °C and 200 °C,respectively.Electrospray ionization(ESI)was used by selective ion scanning(SIM)at m/z 42.
2.3.Preparation of standard solution
BPL standard stock solution(1001μg/mL)was prepared in acetonitrile(HPLC grade)and kept in amber bottles at-20°C,which was used for the preparation of working solutions and calibration curves by further dilution with acetonitrile.
2.4.Preparation of samples
Unpurified LHIRV included MPV20170401-Mie,MPV20170402-MieandMPV20170501-Mie,while purified LHIRV included MPV20170401-G25,MPV20170402-G25 and MPV20170501-G25.The samples were diluted by acetonitrile to concentration of about 3.0 μg/mL and then filtered through 0.45 μm nylon membrane syringe filters.
2.5.Method validation
In order to prove the reliability of the method,this experiment verified the methodology from selectivity,linearity,recovery and other aspects.The selectivity of the method was investigated by analyzing blank solvent(acetonitrile),BPL standard solution,sample MPV20170401-Mie and sample MPV20170401-G25.The blank solvent and sample MPV20170401-G25 should have no interference peaks around the peak of the analyte.Linearity of the method was established at 0.50,1.00,2.00,5.00 and 10.01μg/mL.Calibration curve was generated using linear regression analysis.The linearity obtained was assumed satisfactory with the correlation coefficients(r2)higher than 0.99.The analytical limits were shown on the basis of limit of detection(LOD)and limit of quantification(LOQ)for BPL,which were calculated at the lowest concentration as3 and 10 times of signal-to-noise(S/N),respectively.
The precision was evaluated by measuring the standard solution for six times,and was expressed as the relative standard deviations(RSDs)of retention time and peak area.The standard solution was prepared by diluting the stock solution to 3.00μg/mL.To evaluate the stability of BPL,BPL standard solution and samples spiked with BPL were placed at room temperature for 4 h and determined every 2 h.Stability was checked by comparing the measured results with the results obtained at 0 h,expressed as RSD.The BPL in standard solution and samples could be regarded as stable with RSDs lower than 3.0%.The repeatability of the method was assessed by the determination of RSD values of BPL content in samples.The recovery was evaluated by determining spiked LHIRV samples at three levels(2.4,3.0 and 3.6μg/mL)in triplicate.
2.6.Application of the established method in LHIRV injection
The established method was applied in LHIRV injection,including LHIRV purified samples and unpurified samples.
2.7.BPL hydrolyzation experiments
In our research,in order to accelerate the hydrolysis of BPL,the aqueous solution was heated by water bath at 37°C.The aqueous solution was prepared at concentration of 1.00μg/mL.The amount of BPL was determined per 30 min,and hydrolysis curve of BPL was made.
3.Results
3.1.Method validation
3.1.1.Specificity
From Fig.1,it can be seen that the retention time of the target compound was6.12min.Theblank solventand sample MPV20170401-G25 had no interferences,suggesting that the specificity of the developed method was satisfactory.
3.1.2.Linearity
The calibration curve of BPL was y=49220×+3469.2(n=5)in the range of 0.50–10.01μg/mL and the correlation coefficients(r2)was 0.9999,indicating the obtained linearity was satisfactory.
3.1.3.LOD and LOQ
The analytical limits were shown on the basis of LOD and LOQ for BPL.The LOD and LOQ of BPL were 0.015μg/mL and 0.050 μg/mL,respectively,which was sufficient to determine trace amount of BPL in matrix.
3.1.4.Precision
The precision was expressed as the RSD of retention time and peak area.The results showed good precision with the RSD of retention time less than 1%and RSD of peak area less than 3%,respectively.
3.1.5.Stability and repeatability
The results showed that the RSDs of BPL peak area were 4.0%and 6.6%,respectively,which indicated that it was unstable after being laid aside for long time at room temperature.Therefore,BPL solution should be stored at-20°C and measured immediately after being taken out.There was no BPL detected in LHIRV purified samples,while the average level was found 6.56μg/mL in unpurified samples with RSD of 4.0%,suggesting that relatively accurate results could still be obtained in case of minor fluctuations of the measurement conditions.
3.1.6.Accuracy
The accuracy was measured by determining the recovery of spiked samples.As Table 1 shows,the average recoveries of purified samples and unpurified samples were 103.46%and 102.65%,and the RSDs were 3.2%and 1.4%,respectively.
3.2.Application in LHIRV injection
To demonstrate the effectiveness and applicability of this method,the proposed method was applied for the analysis of LHIRV injection,including LHIRV purified samples(MPV20170401-G25,MPV20170402-G25 and MPV20170501-G25)and unpurified samples(MPV20170401-Mie,MPV20170402-Mie and MPV20170501-Mie).As shown in Table 2,BPL was not detected in three batches of purified samples and was found in three batches of unpurified samples.
3.3.BPL hydrolyzation experiments
BPL was unstable in aqueous solution and could be hydrolyzed easily to 3-hydroxypropionic acid at room temperature.The resultshowed that BPL in aqueous solution was hydrolyzed quickly and disappeared completely after 3 h(Fig.2).
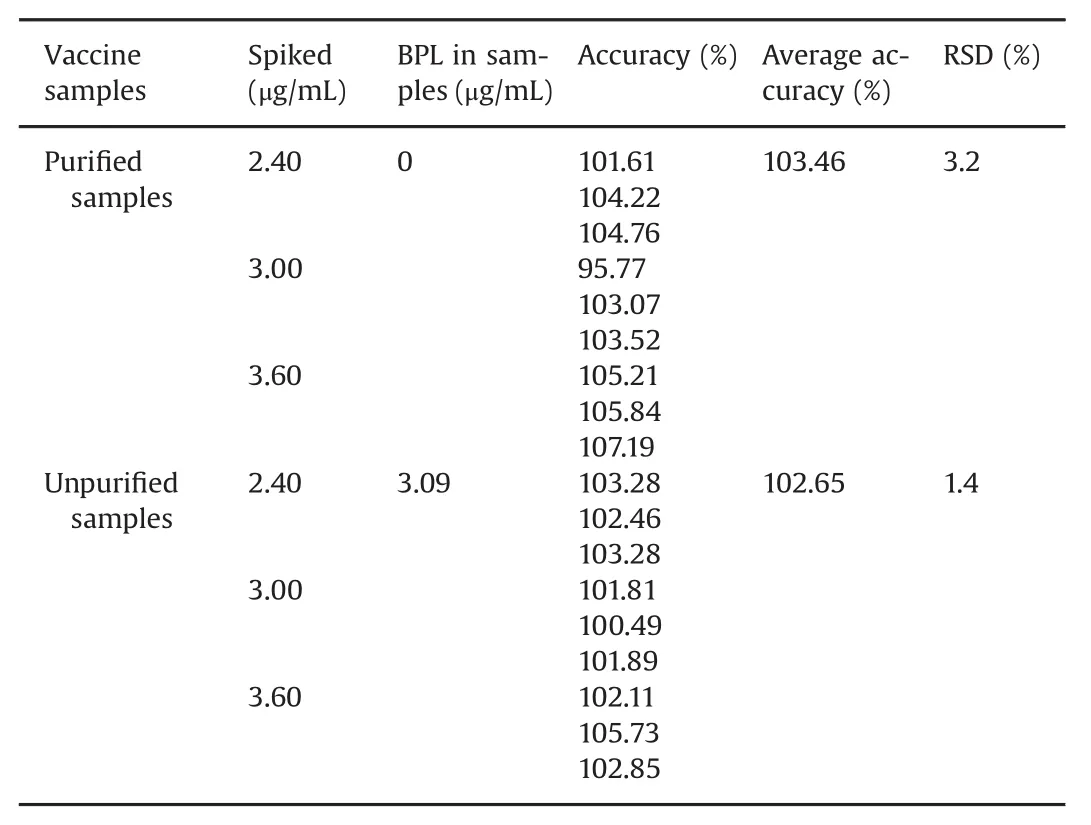
Table 1 Accuracy and precision of the method for analysis of BPL in vaccine samples at three spiked levels.
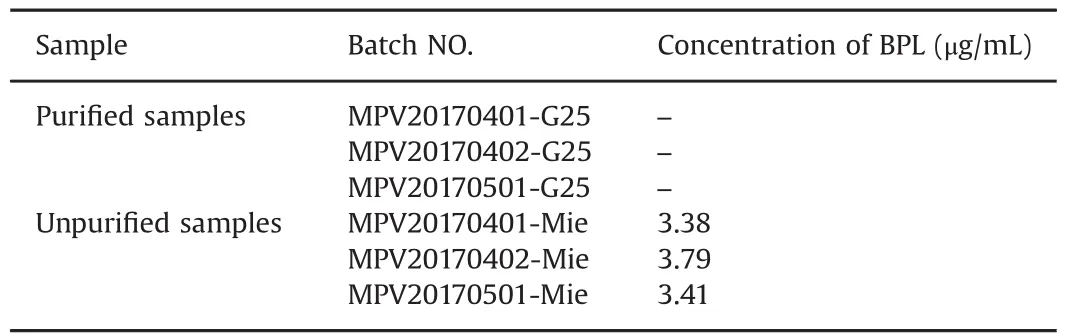
Table 2 Determination results of lyophilized human inactivated rabies vaccine samples.
4.Discussion
At present,many kinds of vaccines are inactivated by BPL for the following reasons:First,vaccines inactivated with BPL possess good immunocompetence on account of the reservation of the virus capsid protein[8].Next,the residual can be hydrolyzed and removed easily[8],which confirms the safety of vaccines.Finally,BPL can also destroy the DNA of stromal cells that cause contamination or remain in the vaccines[18].Therefore,it is very necessary to consider the factors that affect the experimental efficiency.
4.1.Column selection
At the beginning of the experiment,DB-624 and HP-INNOWAX were selected to determine BPL,according to the polarity of BPL and existing literature[7,9,11].The results showed that the analyte had a satisfactory symmetry factor and better resolution from interfering substances in samples when HP-INNOWAX capillary column was chosen and the temperature was set at 80°C.Therefore,the analysis of BPL was operated on HP-INNOWAX at the column temperature of 80°C.
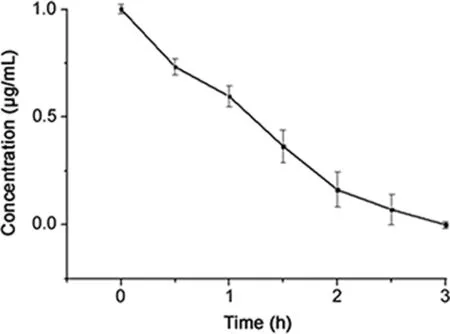
Fig.2.Hydrolysis curve of BPL in water solution.
4.2.Hydrolysis analysis
Initially,BPL was hydrolyzed directly without adjusting pH of the solution.After 4 h,BPL could still be detected.Then,we tried to explore the optimal hydrolysis condition by adjusting pH.It is reported that BPL was added to vaccines with pH of 7.2,7.4,7.6,7.8 and 8.0,respectively and hydrolyzed in water bath at 37°C for 2h[21].The results showed there was a decrease in content when pH increased:BPL was detected when hydrolyzed for 2h with pH of 7.2 and 7.4;when pH was above 7.6,BPL disappeared completely.Therefore,pH value was proved to be an important factor in determining whether BPL would be completely hydrolyzed within 2 h.In this study,hydrolysis of BPL was carried out with pH of 7.6 and the concentration of BPL decreased below LOQ of 0.050 μg/mL after hydrolyzing for 3h.
4.3.Comparison among different methods
According to the literature[7,9,11,18,21],residual BPL in vaccines was mostly determined by GC or HPLC.The comparison among different methods is shown in Table 3.First of all,acetonitrile was applied as reference attenuant dissolvent,which showed that BPL could remain stable within a short period in acetonitrile,avoiding the trouble of quick operation at low temperature with water as solvent.Moreover,the chromatographic behavior of BPL in acetonitrile and sample was the same,con firming that acetonitrile had no effect on the determination of BPL.What's more,in course of experiment,the study of Shan et al.[9]provided a reference but the present research was improved and supplemented.The advantages of the present study are summarized below.
Firstly,this study performed hydrolysis experiments,which ensured that as a common but virulent inactivator,BPL could be hydrolyzed into non-toxic 3-hydroxypropionic acid.The hydrolysis experiment made the process of inactivation and detoxification of vaccine more comprehensive and confirmed the safety of the preparation process of it.
Secondly,Shan et al.[9]determined BPL in vaccine by GC method.In this study,GC-MS was applied to obtain more accurate qualitative assay according to the structure.At the same time,matrix interference could be reduced and sensitivity could be improved with SIM module.
Thirdly,the sensitivity increased to a certain degree.Shan et al.[9]used GC method to obtain values of LOD and LOQ for 0.2μg/mL and 1.0μg/mL,respectively.In this study,the LOD and LOQ values obtained by GC-MS method were 0.015μg/mL and 0.050μg/mL,respectively,which means higher sensitivity.In the authors'opinion,an excessively large linear range had little significance for the determination of trace analytes.In this study,the linear range was narrowed appropriately without prejudice to the results,making the determination of trace analytes in biological samples morepractical.It is extremely important to increase the sensitivity for qualitative and quantitative determination of trace analytes in biological samples.
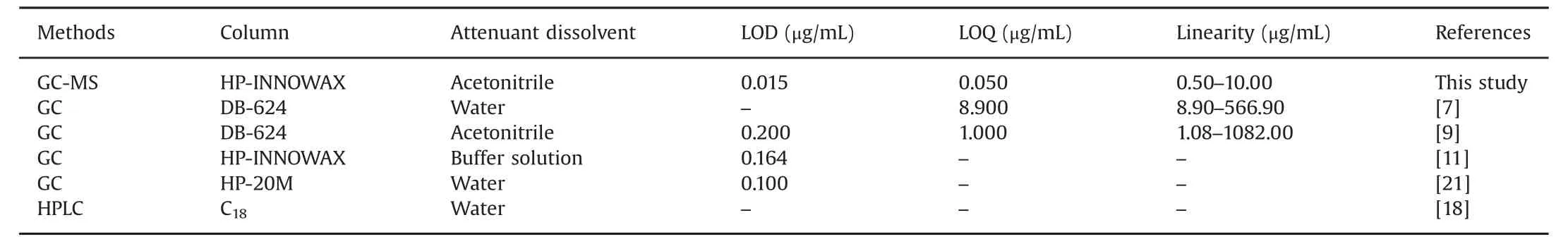
Table 3 The comparison of different methods for determination of BPL.
Lastly,BPL was measured by temperature-programmed GC in Shan's research[9].In the present study,column temperature was kept constant at 80°C.The improvement of this condition,on the one hand,simplified the instrument conditions and eliminated the process of cooling after running,and thereby shortened the running time.On the other hand,the service life of the capillary column was prolonged and the experimental cost was decreased to some extent.
5.Conclusion
In this paper,a simple,versatile and sensitive analytical method,based on GC-MS for determination of BPL in LHIRV,has been developed and validated to meet the needs of quality control of BPL in vaccines.To the authors’knowledge,such a method based on GC-MS is exploited for the first time,which has been applied to the trace determination of BPL in vaccines.
The method indicated that the purification process of the tested samples was favorable for removing BPL from the vaccine effectively.The established method provided means for validation of vaccine production process and effective experience and reference for research on the safety of biological products.
Conflicts of interest
The authors declare that there are no conflicts of interest.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Development and validation of an HPLC-UV method for analysis of methylphenidate hydrochloride and loxapine succinate in an activated carbon disposal system
- LC and LC–MS/MS studies for the identification and characterization of degradation products of acebutolol
- Detection and determination of undeclared synthetic caffeine in weight loss formulations using HPLC-DAD and UHPLC-MS/MS
- Highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study
- Long-term stability of gentamicin sulfate-ethylenediaminetetraacetic acid disodium salt(EDTA-Na2)solution for catheter locks
- Cytotoxic effect of Rosa canina extract on human colon cancer cells through repression of telomerase expression
