Cytotoxic effect of Rosa canina extract on human colon cancer cells through repression of telomerase expression
2018-12-10IbrhimTurnSelimDemirKgnKilincSerpOzerYmnSemMisirHnieKrBernGencAhmetMenteseYukselAliyziciogluOrhnDeger
Ibrhim Turn,Selim Demir,Kgn Kilinc,Serp Ozer Ymn,Sem Misir,e,Hnie Kr,Bern Genc,Ahmet Mentese,Yuksel Aliyzicioglu,Orhn Deger
aDepartment of Genetic and Bioengineering,Faculty of Engineering and Natural Sciences,Gumushane University,29100 Gumushane,Turkey
bMedicinal Plants,Traditional Medicine Practice and Research Center,Gumushane University,29100 Gumushane,Turkey
cDepartment of Nutrition and Dietetics,Faculty of Health Sciences,Karadeniz Technical University,61080 Trabzon,Turkey
dDepartment of Medical Biochemistry,Faculty of Medicine,Karadeniz Technical University,61080 Trabzon,Turkey
eDepartment of Biochemistry,Faculty of Pharmacy,Cumhuriyet University,58140 Sivas,Turkey
fProgram of Medical Laboratory Techniques,Vocational School of Health Sciences,Karadeniz Technical University,61080 Trabzon,Turkey
Keywords:Apoptosis Colon cancer Cytotoxicity Rosa canina Telomerase
A B S T R A C T Rosa canina is a member of the genus Rosa that has long been used for medical objectives.Several studies have reported cytotoxic effects of different Rosa species,but there has been only limited investigation of the cytotoxic effect of R.canina.The purpose of the current study was to examine the potential effect of R.canina extract on cell viability,the cell cycle,apoptosis,and the expression of telomerase in human colon cancer(WiDr)cells.The cytotoxic effect of the extract was determined using MTT assay.The mechanism involved in the cytotoxic effect of the extract was then evaluated in terms of apoptosis and the cell cycle using flow cytometry.Mitochondrial membrane potential(MMP)was investigated using the fluorometric method,and expression levels of telomerase were studied using RT-PCR.R.canina extract exhibited a selective cytotoxic effect on WiDr cells compared with normal colon cells.The extract induced cell cycle arrest at the S phase and apoptosis via reduced MMP in WiDr cells.R.canina extract significantly repressed telomerase expressions at treatment times of 48 and 72 h in WiDr cells.Our results suggest that R.canina may have considerable potential for development as a novel natural product-based anticancer agent.
1.Introduction
Rosa canina is a member of the genus Rosa and the family Rosaceae.The genus Rosa contains more than 100 species widely spread over Asia,Europe,the Middle East,and North America[1,2].
Approximately 25%of all rose species are reported to grow in Turkey,especially in the regions of central and North-East Anatolia,and particularly in Gumushane and neighboring cities.R.canina contains many beneficial compounds,including vitamins,phenolic acids,proanthocyanidins,tannins, flavonoids,pentacyclic triterpenes,and minerals[1,3,4].It exhibits numerous biological properties,such as antioxidant,anti-inflammatory,anti-ulcerogenic,anti-obesity,antidiabetic,diuretic,antimutagenic,anticarcinogenic,anti-arthritic,neuroprotective,and antimicrobial effects[3–6].R.canina is one of the most commonly used medicinal plants in traditional medicine in both Western and Asian countries for the treatment of colds,asthma,hemorrhoids,infections,chronic pains,arthritis,and inflammatory diseases[1,2,4].The fruits of R.canina are consumed as a natural source of vitamin C in the forms of tea,snacks,jam,nectar,and dried pulp.There is also consider able interest in this speciesin various commercial spheres,especially the food,pharmaceutical,and cosmetic industries[2,3].
Cancer is a pathological condition characterized by excessive cell growth and deriving from loss of control over the cell cycle and/or decreased apoptosis[7].Cancer cells exhibit characteristic features,such as avoidance of apoptosis,impaired cell cycle control,self-sufficiency in growth signaling,and telomerase activation[8,9].Telomerase is a ribonucleoprotein consisting of human telomerase reverse transcriptase(hTERT)and human telomerase RNA(hTR)[8].Its principal function is to synthesize telomeres using the RNA template instead of telomeric series lost during DNA replication in order to overcome the problem of end-replication[10].Telomerase reactivation in the cell is believed to be associated with carcinogenesis and is a significant step in tumor immortalization.Telomerase is reported to be active in more than 85%of all tumors but is present at low or undetectable levels in normal cells[8].Telomerase plays an important role in the unlimited growth of cancer cells,and inhibition of telomerase activity potentially represents a highly selective target for the treatment of cancer[11].
Colon cancer is one of the most common types of cancers worldwide.While chemotherapy is one of the most widely used therapeutic strategies against colon cancer,it also has some limitations,such as normal cell toxicity and gradually increasing resistance in cancer cells.The discovery of new drugs for use in alternative strategies in cancer treatment is therefore highly desirable.Plants are regarded as very promising from this perspective,since they represent substantial sources of substances with various therapeutic uses.Most anticancer drugs are today produced from plants[12–14].
Various studies have investigated the cytotoxic effects of different Rosa species.Olsson et al.[15]demonstrated that ethanolic extract of rose-hip has a cytotoxic effect on human colon(HT-29)and breast(MCF-7)cancer cells,while Fujii et al.[16]reported that ethanolic extract of R.canina exhibits a cytotoxic effect on mouse melanoma cells by inhibiting tyrosinase activity.Zamiri-Akhlaghi et al.[17]reported that ethanolic extract of R.damascena has a concentration-dependent cytotoxic effect against human cervix cancer(HeLa)cells.Recently,Jiménez et al.[18]demonstrated that R.canina extracts exhibit antiproliferative effects on human colon cancer(Caco-2)cells by increasing the number of apoptotic cells and cell cycle arrest at the S phase.However,no studies have examined the relationship between telomerase and R.canina extract in any cancer cell lines.The objective of this study was therefore to determine the cytotoxic effect of acidified dimethyl sulfoxide extract of R.canina on colon cancer(WiDr)cells,coupled with the mechanism of action.
2.Experimental
2.1.Chemicals
Gentamicin and trypsin solutions were obtained from Biological Industries(Kibbutz Beit Haemek,Israel),Eagle's minimum essential medium(EMEM)from Lonza(Verviers,Belgium),and fetal bovine serum(FBS)from Biochrom(Berlin,Germany).All flow cytometry kits were purchased from Becton Dickinson(San Diego,CA,USA).All kits used for gene expression studies were purchased from Roche Diagnostics(Mannheim,Germany).The other principal chemicals used were obtained from Sigma(St.Louis,MO,USA).
2.2.Sample collection and extract preparation
Fully mature fruits of R.canina were harvested from Gumushane in the Eastern Anatolia region of Turkey.Samples were preserved in cool bags for transportation to the laboratory.These fruits were air-dried at 25°C and converted into a fine powder using a blender and milling procedures.The fruit powder(1 g)was extracted with 20 mL of dimethyl sulfoxide(DMSO)plus 0.5%(v/v)hydrogen chloride(HCl)in a mechanical shaker(Shell Lab,Cornelius,OR,USA)in the dark for 24 h at 45°C.The prepared 50 mg/mL stock acidified DMSO extract of R.canina was filtered with Whatman No.1 filter paper and a 0.2μm filter and then stored at-20°C until used in further experiments.
2.3.Drug preparation and treatment
Cisplatin was dissolved in DMSO and used as a reference compound in cytotoxicity experiments due to its use in colon cancer treatment[19].Final solvent concentrations of compounds were no higher than 0.5%in culture media in any experiment.That concentration was not sufficient to affect cell morphology or viability.
2.4.Cell culture
Human colon adenocarcinoma(WiDr,ATCC-CCL-218)cancer and human colon normal epithelial cell lines(CCD 841 CoN,ATCCCRL-1790)were supplied by the America Type Culture Collection(Manassas,VA,USA).Both cells were cultured in EMEM supplemented with 10%heat inactivated FBS and 1%antibiotic solution with a 5%CO2supply at 37°C.
2.4.1.Cytotoxicity experiments
MTT assay with a 72 h treatment time was employed to measure the cytotoxic effects of R.canina extract and cisplatin on colon cancer and normal cells[20].Brie fly,cells were seeded into a 96-well cell culture plate at 1×104cells per well.The cells were then treated with varying concentrations of R.canina extract(0–500 μg/mL)and cisplatin(0–10 μg/mL)for 72 h in a quadruplet manner.Subsequently,10 μL of MTT dye(0.25 mg/mL)was placed inside each well.The crystals that emerged were then dissolved in DMSO.Finally,absorbance was measured at 570 nm with the help of a microplate reader(Molecular Devices Versamax,California,USA).Optical densities were employed to calculate percentage viabilities in treated cells compared to untreated control cells.Logconcentrations versus%cell viabilities were plotted with a logarithmic graph,which was then used to determine the IC50values.
2.4.2.Flow cytometry analysis for cell cycle distribution
WiDr cells in the exponential growth phase were treated with 135,270,405 and 540 μg/mL concentrations of R.canina extract for 72 h,then harvested by trypsinization,and washed twice with buffer solution(containing sodium citrate,sucrose,and DMSO).After that procedure,250 μL of trypsin buffer was added to each tube and incubated for 10 min.Next,200 μL of trypsin inhibitor and RNase buffer was added to each tube and incubated for 10 min.Finally,200 μL of cold PI stain solution was added to each tube and incubated for 10 min in the dark on ice.Data from 30,000 cells per sample were collected and analyzed on a flow cytometer(BD Accuri C6,MI,USA).The percentage of cells in cycle phases was determined using MODFIT 3.0 verity software.The results were finally compared with the untreated control cells.
2.4.3.Measurement of apoptosis using flow cytometry
WiDr cells in the exponential growth phase were treated with 135,270,405 and 540 μg/mL concentrations of R.canina extract for 72 h,then harvested by trypsinization,and washed twice with icecold PBS.The cells were then resuspended with 100 μL of the binding buffer.In the next stage,5 μL of FITC Annexin V and 5 μL of propidium iodide(PI)were added to each tube and incubated for 10 min at room temperature in the dark.Finally,400 μL of the binding buffer was added to each tube and data from 10,000 cells per sample were collected and analyzed on a flow cytometer(BD Accuri C6,MI,USA)within 1 h.The results were compared with the untreated control cells.
2.4.4.Determination of mitochondrial membrane potential(MMP)
DiOC6(3),a lipophilic cationic dye,was used to determine the changes in MMP in this study.Normally,it tends to remain in the mitochondria.A decrease in dye intensity indicates disruption of MMP[21].Brie fly,cells were seeded into a 96-well black-walled plate at 1×103cells per well.The cells were then treated with different concentrations of R.canina extract(135–540 μg/mL)for 72 h.After treatment,the cells were washed twice with PBS and loaded with 10 nM DiOC6(3)for 30 min at 37°C in the dark.Finally,the fluorescence measurement was performed on a plate-reading fluorometer(Molecular Devices SpectraMax Paradigm Multi-Mode,Sunnyvale,CA,USA)with an excitation wavelength of 484 nm and an emission wavelength of 525 nm.Results were given as relative MMP compared to untreated control cells.
2.4.5.Gene expression analysis
Based on data from the cytotoxicity studies,the extract IC50value(270 μg/mL)was used to investigate gene expression.WiDr cells were treated with this concentration of extract for 24,48,and 72 h.After incubation,the cells were harvested for RNA isolation.
2.4.6.RNA isolation and RT-PCR
Total RNA isolation was performed using a highly pure RNA isolation kit in line with the manufacturer's recommmendations.RNA samples were converted to complementary DNA using a first strand cDNA synthesis kit as recommended by the manufacturer.A total of 100 ng/μL total RNA was employed for all reaction samples.Reaction compounds were incubated at 25 °C for 10 min,50 °C for 60 min,and 85°C for 5 min.
The RT-PCR method using UPL probes was employed to measure hTERT expression.hTERT and GAPDH amplicon dimensions were 60 and 127 bp,respectively.Forward and reverse primers of hTERT and GAPDH were 5′-CCCCGGTTTCTATAAATTGAGC-3′,5′-CACCTTCCCCATGGTGTCT-3′, 5′-AGCCACGTCTCTACCTTGACA-3′,and 5′-CAGGTGAGCCACGAACTGT-3′,respectively.The reaction mixtures were incubated under the following conditions:pre-incubation 95 °C 10 min;amplification 95 °C 10 s 60 °C 30 s,72 °C 1 s,45 cycles and cooling 40°C 30 s,in a Roche Light Cycler 480-II device(Rotkreuz,Switzerland).Measurements were calculated using an advanced relative quantification module with mRNA levels being compared against the negative control samples(cells with no test compound)following normalization to GADPH mRNA levels.
2.5.Statistical analysis
All experiments were performed at least three times,the results being expressed as mean±standard deviation.Normal distribution was determined using the Kolmogorov-Smirnov test.One-way ANOVA was used to analyze intergroup differences.p<0.05 was regarded as significant.
3.Results
R.canina extract exhibited selective cytotoxicity in the colon cancer cell line compared to normal colon cells(Table 1 and Fig.1).
The results of the cell cycle analysis are presented in Fig.2.All the concentrations of R.canina extract used(except for 135 μg/mL)significantly increased cell numbers at the S phase(p=0.0001).Additionally,these concentrations of R.canina extract significantly reduced cell numbers at the G0/G1phase compared to untreated cells(p=0.0001).
The results of the Annexin V analysis are presented in Fig.3.R.canina extract significantly reduced the number of viable cells and increased the number of late and early apoptotic cells compared to untreated cells in a dose-dependent manner(p<0.05).
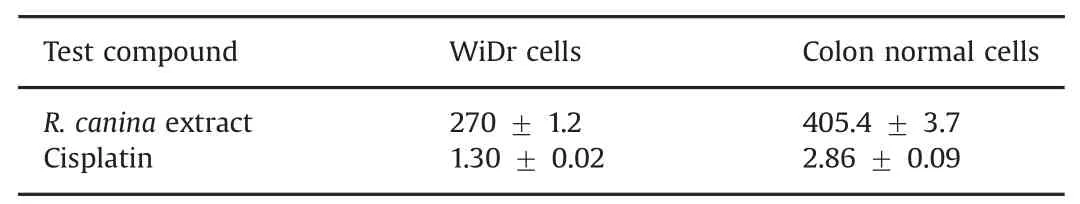
Table 1 Cytotoxic effects of test compounds and their IC50values(μg/mL)(n=4).
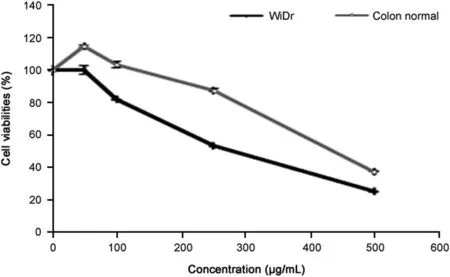
Fig.1.The anti-growth effect using MTT assay after treatment with R.canina extract for 72 h against WiDr cells and colon normal cells(n=4).
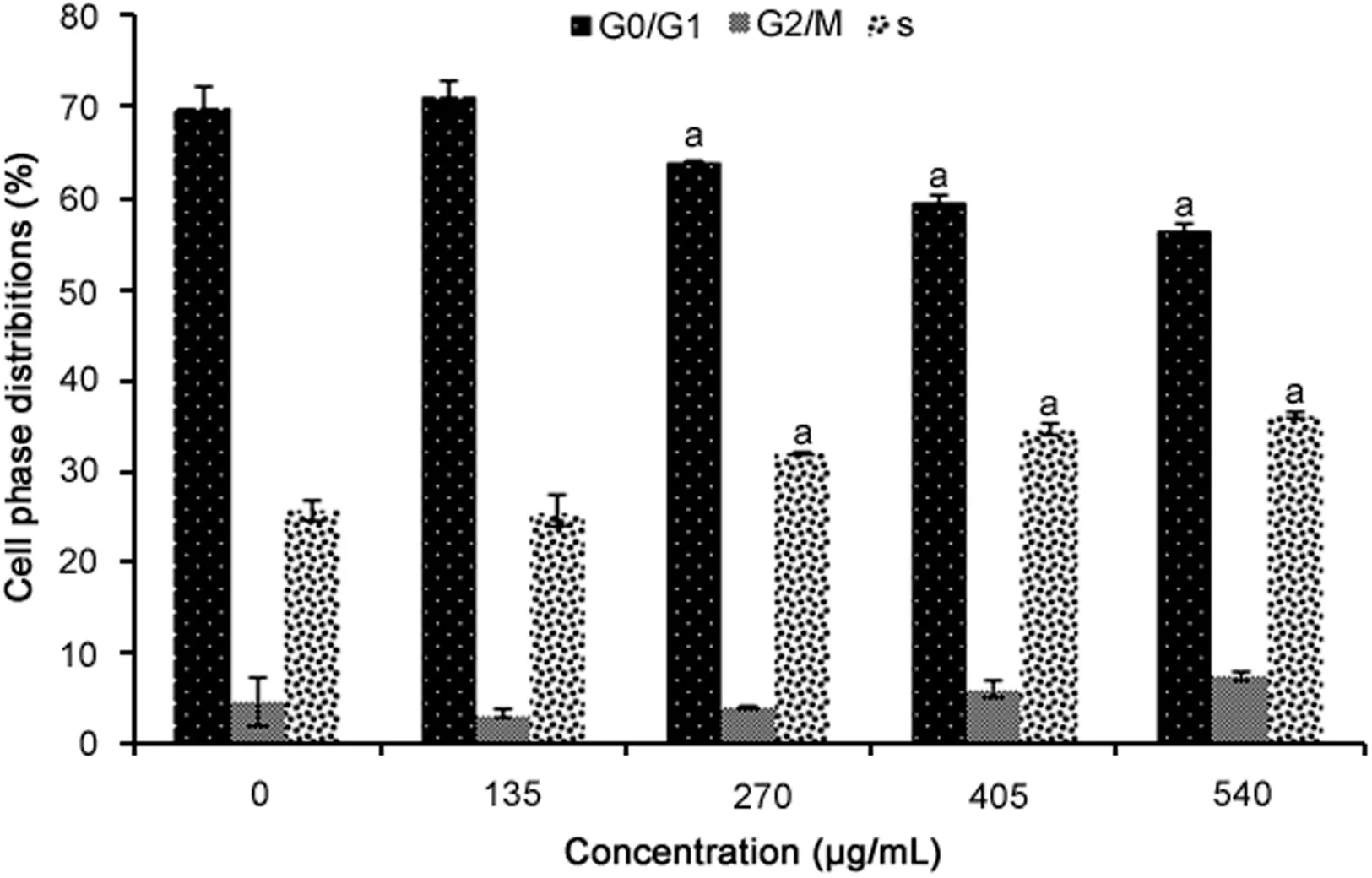
Fig.2.Cell cycle analysis of WiDr cells treated for 72 h with R.canina extract at different concentrations(n=4).aRepresents significant results(p<0.05)compared with untreated WiDr cells.
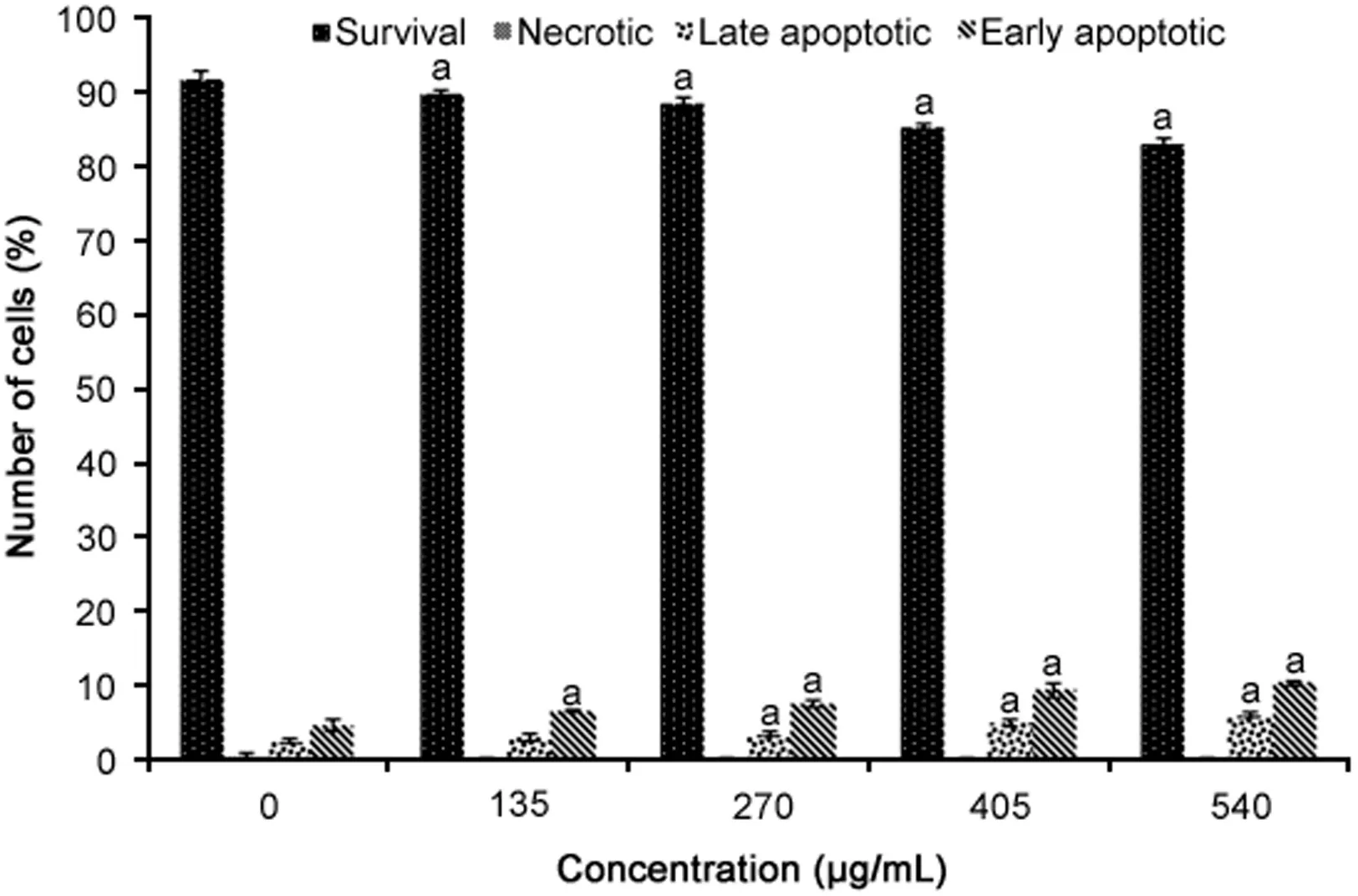
Fig.3.Apoptosis analysis of WiDr cells treated with different concentrations of R.canina extract for 72 h using Annexin-V FITC and propidium iodide staining(n=4).aRepresents significant results(p<0.05)compared with untreated WiDr cells.
MMP analysis results are presented in Fig.4.All concentrations of R.canina extract significantly reduced the MMP in WiDr cells(p=0.001).The percentage reductions in MMP caused by R.canina extract were 12.7%,23.7%,57.9%,and 62.9%for concentrations of 135,270,405 and 540 μg/mL of R.canina extract,respectively.
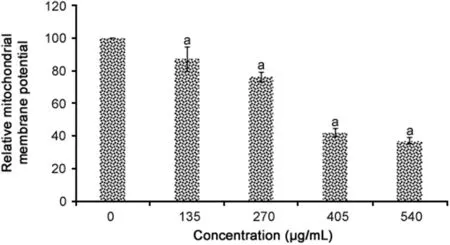
Fig.4.DiOC6staining for R.canina extract-induced dissipation of mitochondrial membrane potential in WiDr cells(n=4).aRepresents significant results(p<0.05)compared with untreated WiDr cells.
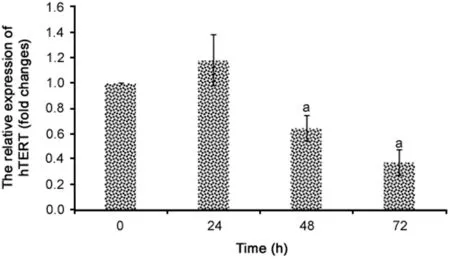
Fig.5.Relative mRNA levels of hTERT after treatment with R.canina extract(270 μg/mL)at different treatment times(n=3).aRepresents significant results(p<0.05)compared with negative control cells.
The IC50concentration(270 μg/mL)of R.canina extract significantly repressed hTERT expression at treatment times of 48 h and 72 h(p<0.05)in colon cancer cells(Fig.5).hTERT expression decreased by 36%and 63%after 48 and 72 h,respectively,compared to the negative control group.No statistically significant differences were regarded between the 48 and 72 h treatment groups(p>0.05).
4.Discussion and conclusion
The use of plants to treat diseases is known as herbal medicine and is regarded as a part of traditional treatment.Traditional medicine with plants is thought to go back to very early times in human history[22].Edible plants are rich in antioxidant molecules,such as vitamins,polyphenolic compounds and carotenoids.The fruits of R.canina have long been used for medical aims,and the biological functions of these fruits may be partly attributed to their phenolics and vitamin C contents[1,3].
Colon cancer is one of the leading causes of death in the USA and the second most common form of fatal cancer worldwide.Almost half of all patients diagnosed with colon cancer are reported to die.Fewer than 10%of patients of with metastatic colon cancer survive for more than 5 years.Surgical treatment,chemotherapy,radiotherapy or combinations of these are generally applied in cancers of the digestive system[13].Chemotherapy is an effective means of treating several types of cancer,including colon cancer,but the cost represents an important public health problem in developing countries.In addition,gradual resistance developed in cancer cells to chemotherapeutic drugs and healthy cells being affected by them reduce the success levels of chemotherapy[14].New strategies are therefore required in order to solve these problems.More than 60%of anticancer drugs in use today are isolated from natural products of plant origin[23].Natural product research for cancer treatment is therefore one of these new strategies due to its effectiveness against cancer cells and the fact that it is harmless to normal cells[12].Although experimental studies have shown that phenolic compounds in particular exhibit anticancer properties,the entire mechanism involved is as yet unclear.The mechanisms that have been proposed in the context of the anticancer effect of phenolic compounds include their ability to scavenge free radicals,their ability to induce xenobiotic metabolism,their ability to regulate gene expression,and their ability to modulate DNA repair,apoptosis and cell signaling,such as cell replication and invasion[14].However,the number of cytotoxicity studies performed with Rosa species is very limited[15–18].The purpose of this study was therefore to determine the cytotoxic effect of R.canina extract in colon cancer cell lines,one of the most common forms of cancer worldwide,and to identify the probable mechanisms of action.In vitro analyses are first performed in determining the potential biological bene fits of any compound.If positive results are obtained from that analysis,then in vivo studies are performed in the second stage[7].The WiDr cell line is derived from a human colon carcinoma and is commonly used in cancer research involving in vitro colon cancer models[24,25].This study was therefore planned on a colon cancer cell line(WiDr)under in vitro conditions.An effective and acceptable anticancer compound has to meet various criteria,including exhibiting no harmful effects on healthy cells[7].Cytotoxicity experiments were therefore conducted in WiDr cells together with normal colon cells.In order to investigate the antiproliferative activity of R.canina extract,WiDr and colon normal cells were treated with different concentrations of extract for 72 h.R.canina extract exhibited reasonable selective cytotoxic effects against WiDr cells compared with normal colon cells.In terms of cytotoxic activity,Lee et al.[26]demonstrated that methanolic extracts of R.rugosa stem act as anti-prostate cancer agents,while Olsson et al.[15]demonstrated that ethanolic extract of rose hip has a cytotoxic effect on HT-29 and MCF-7 cells.Tumbas et al.[27]reported that quercetin,ellagic acid and vitamin C are the most abundant antioxidant compounds in R.canina.However,only vitamin C-free extract,which is rich in polyphenols,exhibits cytotoxic activity in three human cancer cell lines(HeLa,MCF-7 and HT-29 with IC50values of approximately 81,248 and 364 μg/mL,respectively).They concluded that vitamin C and flavonoids are responsible for the antioxidant activity of R.canina,while only polyphenols contribute to its cytotoxic activity.Olech et al.[28]reported that teas and tinctures prepared from R.rugosa exhibit cytotoxic effects against human ovarian,lung,cervix,and breast cancer cells without harming human skin fibroblast cells.In another study,Guimarães et al.[29]showed that methanolic extract of R.canina from Portugal exhibits cytotoxic effect against human lung,colon,cervix and liver cancer cells,while no effect was observed on breast cancer cells.Artun et al.[30]recently reported that methanolic extract of R.damascena exhibits selective cytotoxic effects on human cervix cancer(HeLa)cells compared to normal kidney epithelial(Vero)cells.Additionally,not only the extracts of Rosa species but also various compounds isolated from Rosa species(such as bee pollen polysaccharides,phenylethanoids,and essential oils)have been reported to exhibit cytotoxic effects in colon,leukemia,liver,breast and oropharyngeal epidermoid carcinoma cell lines[13,31,32].
Loss of control of the cell cycle is reported to play a role in the development of cancer.Therefore,halting the cycle of cancer cells at any stage is one of the basic aims in the approach to treating cancer[9].We performed cell cycle analysis to assess population cell death and to determine whether R.canina extract can induce cell cycle arrest.As shown in Fig.2,R.canina extract induced significant dose-dependent accumulation of cells at the S phase.Consistent with our results,Jiménez et al.[18]reported that R.canina extracts arrested the cell cycle of Caco-2 cells at the S phase.Cagle et al.[33]demonstrated that an 80%methanolic extract of R.canina exhibited antiproliferative effects on human glioblastoma cells by increasing cell cycle arrest at the G2/M phase and blocking both the MAPK and AKT signaling mechanisms.No findings were determined concerning apoptosis in that study.
Attenuate or extinct apoptosis capacity has been identified in a range of cancer types.Raising apoptosis levels through the use of different agents is therefore another approved target mechanism component of anticancer strategies[7].We therefore examined the capability of varying concentrations of R.canina extract to reduce the growth of WiDr cells by increasing levels of apoptosis using the Annexin V/propidium iodide double-staining assay.Our results showed that R.canina extract significantly induced cell apoptosis in a concentration-dependent manner(Fig.3).Mitochondrial depolarization is a major component of apoptosis induction[9].We therefore investigated alterations in MMP following R.canina extract treatment.As shown in Fig.4,R.canina extract significantly induced loss of MMP in a concentration-dependent manner.These results suggest that the type of cell death induced by R.canina extract is mitochondria-dependent apoptosis.Several studies have investigated the apoptotic properties of Rosa species in many different cancer cells.Khatib et al.[34]reported that essential oil from R.damascena exhibits cytotoxic effects against a gastric cancer cell line by inducing apoptosis.Erguven et al.[35]demonstrated that ethanolic extract of R.agrestis exhibits concentration-dependent cytotoxic effects on human endometrial adenocarcinoma cellsby inducing apoptoticcell death.Jiménez et al.[18]reported that R.canina extracts have cytotoxic effects on Caco-2 cells by increasing the number of apoptotic cells.
Telomerase activation is one of the characteristic features of cancer cells[8,11].Telomerase is reported to be active in more than 85%of all tumors,but it is present at low or undetectable levels in normal cells[8].Telomerase plays an important role in the unlimited proliferation of cancer cells,so inhibition of telomerase activity is regarded as a highly selective target for cancer treatment[11].Moreover,increased telomerase expression has been reported in several types of epithelial cancer,including colon cancer[36].To the best of our knowledge,no previous studies have investigated the effect of R.canina extract on telomerase expression in any human cancer cells.Our findings show that the IC50(270 μg/mL)concentration of R.canina extract significantly repressed hTERT expression at treatment times of 48 and 72 h.Inhibition of telomerase in cancer cells is known to result in induction of cell death.However,there is increasing evidence that telomerase plays a key role in apoptosis regulation by changing MMP or metal homeostasis in mitochondria in a manner unrelated to its classic role[37].Additionally,global expression profiling studies have stated that hTERT expression affects 284 genes,the function of which involves a wide range of cellular processes,including cell cycle regulation,metabolism,differentiation,apoptosis,and cell signaling[38].Suppression of hTERT expression also causes elevated p53 and p21 transcription,and p21 mediates p53-dependent growth arrest in cancer cells[37].Polyphenols are also capable of downregulating hTERT expression in various cancer cell lines.This con firms previous reports showing such downregulation with some polyphenols(resveratrol,apigenin,baicalein, chrysin,galangin,and kaempferol)in colon and esophageal squamous cancer cells[11,39].We therefore think that downregulation of telomerase by R.canina extract(rich in polyphenols)may contribute to its apoptotic and antiproliferative activity in WiDr cells.
One limitation of this research is that in vitro studies cannot be extrapolated to potential activity in vivo.Further studies are now necessary to understand the exact interaction of the involved signaling pathways.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors would like to thank the Foundation of Scientific Research of Gumushane University for financially supporting this research under Project No:13.F5119.02.1.The authors also wish to thank Professor Ersan Kalay from the Medical Biology Department,Karadeniz Technical University for professional assistance with the flow cytometry studies.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Novel ligand-based docking;molecular dynamic simulations;and absorption,distribution,metabolism,and excretion approach to analyzing potential acetylcholinesterase inhibitors for Alzheimer's disease
- Recovery rates of combination antibiotic therapy using in vitro microdialysis simulating in vivo conditions
- Evaluation of naproxen-induced oxidative stress,hepatotoxicity and in-vivo genotoxicity in male Wistar rats
- Long-term stability of gentamicin sulfate-ethylenediaminetetraacetic acid disodium salt(EDTA-Na2)solution for catheter locks
- Highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study
- Detection and determination of undeclared synthetic caffeine in weight loss formulations using HPLC-DAD and UHPLC-MS/MS
