Long-term stability of gentamicin sulfate-ethylenediaminetetraacetic acid disodium salt(EDTA-Na2)solution for catheter locks
2018-12-10AnneSophieFioletEliseJndotPulineDouceyCorlieCrtetliBrunelChristinePivotJenMrcGhigoChristopheBeloinDvidLeeuxFricePirot
Anne-Sophie Fiolet,Elise Jndot,Puline Doucey,Corlie Crétet,Céli Brunel,Christine Pivot,Jen-Mrc Ghigo,Christophe Beloin,Dvid Leeux,c,d,Frice Pirot,e,*,2
aService Pharmaceutique,Plateforme FRIPHARM,Groupe Hospitalier Centre Edouard Herriot,Hospices Civils de Lyon,5,Place d′Arsonval,F-69437 Lyon Cedex 03,France
bUnité de Génétique des Bio films,Département de Microbiologie,Institut Pasteur,28 rue du docteur Roux,F-75724 Paris Cedex 15,France
cService de Microbiologie,Unité Mobile de Microbiologie Clinique,Assistance Publique-Hôpitaux de Paris,Hôpital Européen Georges Pompidou,20 rue Leblanc,75015 Paris,France
dUniversité Paris Descartes,12 rue de l′Ecole de Médecine,75270 Paris Cedex 06,France
eLaboratoire de Recherche et Développement de Pharmacie Galénique Industrielle,UMR 5305,Plateforme FRIPHARM,F-69373,Faculté de Pharmacie,Université Claude Bernard Lyon 1,8,avenue Rockefeller,F-69373 Lyon Cedex 08,France
Keywords:Gentamicin-EDTA-Na2loaded antimicrobial lock solution Pharmaceutical compounding Stability indicating HPLC assay method Totally implantable venous access ports
A B S T R A C T A lock solution composed of gentamicin sulfate(5 mg/mL)and ethylenediaminetetraacetic acid disodium salt(EDTA-Na2,30 mg/mL)could fully eradicate in vivo bacterial biofilms in totally implantable venous access ports(TIVAP).In this study,fabrication,conditioning and sterilization processes of antimicrobial lock solution(ALS)were detailed and completed by a stability study.Stability of ALS was conducted for 12 months in vial(25°C ± 2°C,60% ± 5%relative humidity(RH),and at 40°C ± 2°C,RH 75% ± 5%)and for 24 h and 72 h in TIVAP(40°C ± 2°C,RH 75% ± 5%).A stability indicating HPLC assay with UV detection for simultaneous quantification of gentamicin sulfate and EDTA-Na2was developed.ALS was assayed by ion-pairing high performance liquid chromatography(HPLC)needing gentamicin derivatization,EDTA-Na2metallocomplexation of samples and gradient mobile phase.HPLC methods to separate four gentamicin components and EDTA-Na2were validated.Efficiency of sterility procedure and conditioning of ALS was confirmed by bacterial endotoxins and sterility tests.Physicochemical stability of ALS was determined by visual inspection,osmolality,pH,and sub-visible particle counting.Results confirmed that the stability of ALS in vials was maintained for 12 months and 24 h and 72 h in TIVAP.
1.Introduction
Central venous catheters(CVC)are frequently used in oncology,nephrology and intensive care units to administer medication or fluids to patients[1].The use of CVC is complicated by the risk of colonization by pathogenic microorganisms on their surface,causing infectious complications such as catheter-related bloodstream infections(CRBSI)[2].Optimal management of CRBSI involves systemic antimicrobial therapy and CVC removal,which might be questionable in specific clinical situations(e.g.,limited vascular access or bleeding disorders)[3].Therefore,direct instillation of a small volume of highly-concentrated antimicrobial solution into the lumen of the catheter,known as antimicrobial lock therapy(ALT),might be favored as an alternative strategy for both prevention and treatment of CRBSI[4,5].Several studies reported the use of antibiotics such as aminoglycosides,e.g.,gentamicin,[3],beta-lactams, fluoroquinolones,folate antagonists(sulfamethoxazole/trimethoprim), glycopeptides,glycylcyclines,lipopeptides,oxazolidinones,polymyxins,and tetracyclines in ALT[6].However,surface of medical device might be colonized by a reservoir of pathogenic microorganisms forming a biofilm,a complex microbial consortium surrounded by an heterogeneous matrix composed of water,polysaccharides,proteins and deoxyribonucleic acid[6],decreasing the antibiotic efficacy[7].Potential additives(e.g.,ethylenediaminetetraacetic acid disodium salt,EDTA-Na2,and sodium citrate)have shown their capacity to disrupt biofilm and to enhance bactericidal effect of current antibiotics[6,8–11].Recent in vitro and in vivo study demonstrated the beneficial effect of EDTA-Na2against Pseudomonas aeruginosa biofilm and the concomitant decrease of minimal inhibitory concentration of ciprofloxacin and ampicillin(30-fold)and gentamicin(two-fold)[12].An earlier in vivo study reported that an antimicrobial lock solution(ALS)composed of gentamicin(5 mg/mL)and EDTA-Na2(30 mg/mL)in association with systemic antibiotics completely eradicated biofilms formed by Escherichia coli,Pseudomonas aeruginosa,Staphylococcus epidermidis or Staphylococcus aureus on the surface of totally implantable venous access ports(TIVAP)[13].
Gentamicin produced by Micromonospora purpurea is used as gentamicin sulfate which is a complex mixture of five structurally related components:C1,C1a,C2 and C2a and a minor component C2b[14].Previous basic stability study showed that gentamicin sulfate-EDTA-Na2antibiotic lock solution stored at 25 °C or 37 °C was visually stable for at least 72 h[3].
In the present study,a process of pharmaceutical compounding and conditioning of gentamicin sulfate-EDTA-Na2lock solution is detailed and then completed by a study of stability according to the technical requirements of International Conference on Harmonization(ICH)for registration of pharmaceuticals for human use.The physicochemical stability of ALS conditioned in glass vial was analyzed(i)over 12 months of storage at 25°C ± 2 °C(relative humidity(RH)60%± 5%)and 40°C± 2°C(RH 75%± 5%),and(ii)after 24 h and 72 h of contact time in TIVAP at 40°C ±2°C(RH 75% ± 5%),by high performance liquid chromatography(HPLC)evidencing the impurities and degradation products of gentamicin sulfate and EDTA-Na2.
2.Materials and methods
2.1.Reagents
Gentamicin sulfate and EDTA-Na2dihydrate physico chemical properties are reported in Table 1.Ready-to-use gentamicin sulfate sterile solutions(40 mg-2 mL and 80 mg–2 mL),and EDTA-Na2dihydrate (pharmaceutical grade) were purchased from Panpharma®(Beignon,France)and Inresa(Bartenheim,France)laboratories,respectively.Nitrilotriacetic acid and trifluoroacetic acid(HPLC grade,TFA)were purchased from Carl Roth(Lauterbourg,France)and Rhône Poulenc(Lyon,France),respectively.Hydrochloric acid(HCl)and sodium hydroxide(NaOH)were provided by Carlo Erba Reagents(Peypin,France).Hydrogen peroxide solution(3%)was purchased from Gifrer(Décines,France).Gentamicin for peaks identification(CRS,European Pharmacopoeia reference standard),gentamicin USP standard,sisomicin sulfate,EDTA disodium salt standard,ortho-phtalaldehyde(OPA),thioglycolic acid,boric acid and potassium hydroxide(KOH)were obtained from Sigma-Aldrich(Saint Quentin Fallavier,France).Acetonitrile and methanol(HPLC grade)were purchased from Merck Millipore(Molsheim,France).Copper sulfate was obtained from Cooper(Melun,France).Peptone water(0.1%)was purchased from Oxoid(Basingstoke,United Kingdom).Culture media for anaerobic bacteria( fluid thioglycollate medium),aerobic bacteria and fungi(soya bean casein digest medium),and neutralizing pharmacopoeia diluent used for the gentamicin neutralization were obtained from Biomérieux(Lyon,France).Water for injection was delivered by Lavoisier(Paris,France).
2.2.Pharmaceutical compounding and conditioning of ALS
EDTA-Na2solution(40 mg/mL)was prepared by dissolution of 22.5 g of EDTA-Na2in 562.5 mL of water for injection.Then,EDTANa2solution(40 mg/mL)was mixed to 187.5 mL of gentamicin sterile solution for injection(40 mg–2 mL).Finally,the pH value of the ALS was adjusted to 8.5 by adding few drops of 5 M NaOH solution.ALS was filtered through 0.22 μm sterile filter(Millex®GS,Millipore®,Molsheim,France),and then sampled(5 mL)in 150 individual amber type 1 glass injection vials(15 mL)hermetically sealed by bromobutyl stoppers in cleanroom.Finally,ALS vials were autoclaved at 121°C for 20 min[15,16].Final concentrations of gentamicin(5 mg/mL)and EDTA-Na2(30 mg/mL)in ALS were assayed by HPLC(see Section 2.6).
2.3.Storage stability testing
The environmental factors(temperature,RH)and time of storage upon degradation of gentamicin sulfate and EDTA-Na2in ALS conditioned in vials and then stored at 25°C± 2°C,RH 60% ± 5%and 40°C ± 2°C,RH 75% ± 5%in climatic test chambers(Froilabo,Meyzieu,France)for 12 months were investigated using HPLC(see Section 2.6)[17].Complementary physicochemical andmicrobiological controls of ALS were conducted over the period of storage(see Section 2.4).TIVAP,supplied by Perouse Medical(polysite 4000,4008 IPS,Ivry le Temple,France),was used to test compatibility between ALS and implantable port.This medical device is made up of a chamber in titanium/polyoxymethylene and a silicone catheter.Total dead space of implantable port was 1.38 mL and internal surface of silicone catheter was 6.6 cm2.One mL of ALS was injected into the device and left for 24 h and 72 h at 40 °C ± 2°C,RH 75% ± 5%.To detect a potential water loss through polymeric wall,implantable port was weighted before and after filling throughout storage.Gentamicin and EDTA-Na2contents after 24 h and 72 h of contact time in chambers(n=3)were assessed by HPLC(see Section 2.6).
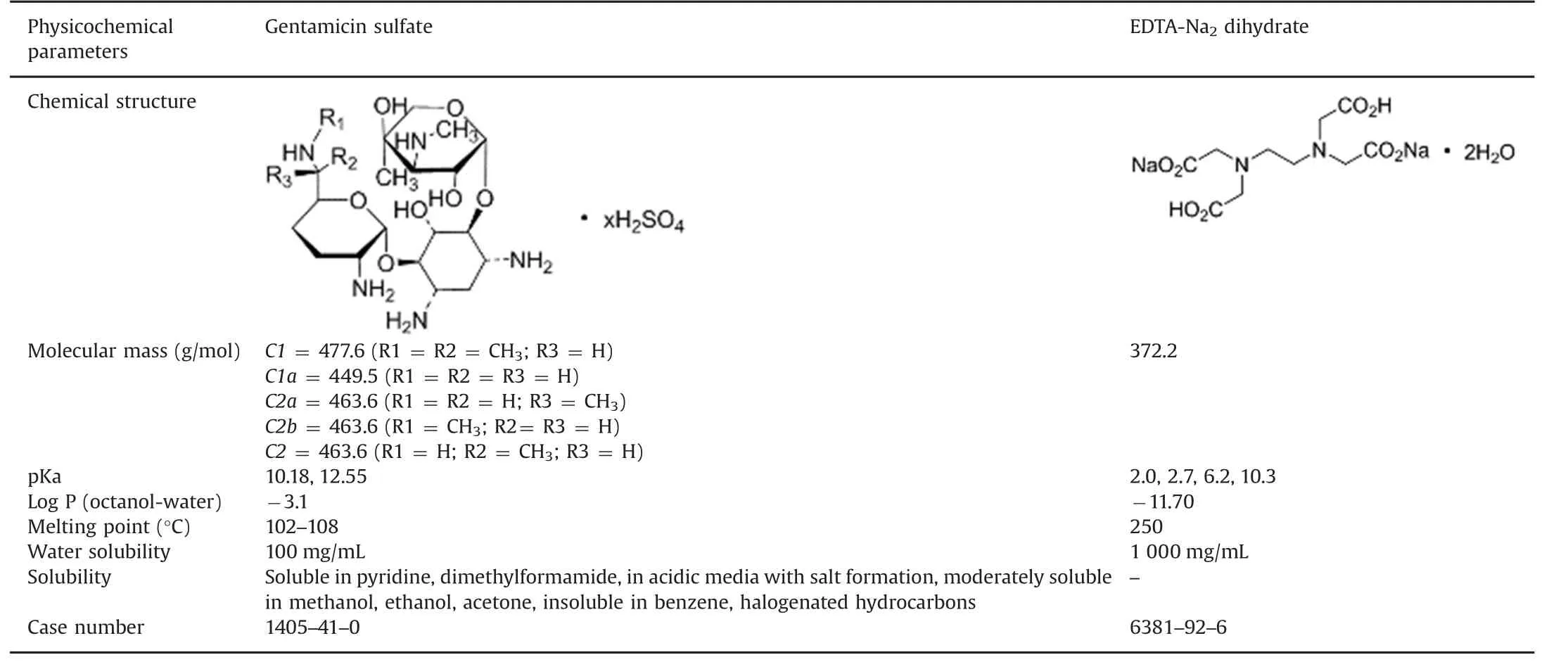
Table 1 Physicochemical properties of gentamicin sulfate and EDTA-Na2dihydrate(from Pubmed open chemistry database,National Institute of Health).
2.4.Physicochemical and microbiological controls of ALS
Physicochemical controls of ALS included pH measurement(EcopHtest2,Thermo Fisher Scientific,Massachusetts,USA)and determination of osmolality(Fiske®,Norwood,MA,USA).Controls of visual aspect,particulate contamination of ALS by visible particles(2.9.20 European Pharmacopoeia Monograph,2016)and subvisible particles(2.9.19 European Pharmacopoeia Monograph,2016,method 1:light obscuration particle count test,Hiac/Royco 9703+,Pacific Scientific Instruments,IL,USA)were carried out after autoclaving.Microbiological assays of ALS were carried out by membrane filtration using Steritest™closed filtration device[18]with special low-binding Durapore®membranes for products with inhibitory properties,e.g.antibiotics(Millipore,Merck KGaA,Darmstadt,Germany),peptone water(0.1%)as rinse fluid,neutralizing pharmacopoeia diluent and both soya-bean casein digest medium and fluid thioglycollate medium(2.6.1 European Pharmacopoeia Monograph,2016).
2.5.Preparation of gentamicin sulfate and EDTA-Na2standard and ALS assay solutions
A derivatization reaction of gentamicin sulfate with OPA allowing a detection gentamicin OPA derivative was adapted from previous reports[19–21].A stock solution of derivatizing agent was prepared by dissolving 250 mg of OPA in 1.25 mL of methanol,then addition of 23.75 mL of boric acid solution(2.47%,m/v;pH adjusted to 10.4 by 8 M KOH solution)and 0.5 mL of thioglycolic acid.Finally,the pH value of derivatizing agent solution was adjusted to 10.4 using 8 M KOH solution,for pre-column derivatization.The stock solution of derivatizing agent,stored at 4°C and protected from light,was stable for three days.Therefore,2.5 mL of gentamicin sulfate standard solution or ALS was then mixed with 0.5 mL of stock solution of derivatizing agent and 2.75 mL of methanol( final volume:5.75 mL).Although usually derivatization was carried out at 60°C constant temperature[20,22],no heating was applied to gentamicin–OPA based solution,taking into account the lack of stability of such chemical reaction and the short half-life of reaction products,as reported elsewhere[23].
The determination of EDTA-Na2in ALS was allowed by conversion to Cu(II)EDTA complex[24]using copper sulfate containing solution and subsequent separation from gentamicin,formulation excipients,impurities,and potential degradation products by HPLC(see Section 2.6).Therefore,2.5 mL of EDTA-Na2standard solution or ALS were then mixed with 2.5 mL of copper sulfate solution(10 mg/mL)and 0.75 mL of water for injection( final volume:5.75 mL).
Gentamicin sulfate-standard EDTA-Na2solutions were prepared daily by dissolving the appropriate amount of EDTA-Na2in water for injection,and then mixing with gentamicin sulfate sterile solution for injection(40 mg–2 mL or 80 mg–2 mL)to obtain,after gentamicin sulfate derivatization orEDTA-Na2complexation, final concentrations of gentamicin sulfate and EDTA-Na2in the range of 87-260 μg/mL and 0.52-1.56 mg/mL,respectively.
2.6.Stability indicating HPLC assay
An HPLC Agilent®(Agilent®1290 In finity Quaternary LC System,Les Ulis,France),equipped with a binary pump with integrated vacuum degasser,a thermostated column compartment(40°C),an autosampler and a diode array detector,was used for gentamicin and EDTA dosages.The separation of gentamicin and EDTA-Na2was accomplished using Security Guard™cartridge and column Kinetex™ (C18100 Å,100 mm × 4.6 mm,2.6 μm)Core-Shell Technology(Phenomenex®,Le Pecq,France).The detection wavelength was set at 330 nm and injection volume was 10 μL.The mobile phase was a binary mixture of 100 mM TFA,used as pairing reagent in water(phase A,the pH value was adjusted to 3 by adding 5 M NaOH solution)and acetonitrile(phase B)in a gradient elution mode at a flow-rate of 1 mL/min[25,26].The mobile phase A was filtered and degassed through nylon membranes(0.20-μm pore size)under vacuum before use.A linear gradient elution was programed as 95%A-5%B(v/v;0 min),95%A-5%B(v/v;2 min),50%A-50%B(v/v;10 min),and 40%A-60%B(v/v;15 min).Therefore,the resolved peaks of gentamicin sulfate OPA derivatives,C1,C1a,C2a and C2,and EDTA-Na2were identified from gentamicin for peaks identification(CRS,European Pharmacopoeia reference standard),gentamicin(216 μg/mL)USP standard solutions,and EDTA(1.3 mg/mL)standard solution.Sisomicin and nitrilotriacetic acid,as gentamicin sulfate and EDTA-Na2respective impurities,were identified from sisomicin(4.65 mg/mL)and nitrilotriacetic acid(43.5 μg/mL)standard solutions.
2.7.HPLC assay validation criteria
The HPLC-UV analytical methods for gentamicin sulfate and EDTA-Na2were validated for specificity,precision(repeatability,intermediate precision),linearity,limits of detection(LOD)and quantification(LOQ),accuracy/recovery and robustness to include the essential requirements of ICH guidelines[27,28].The validation protocol was conducted on three consecutive days by the same operator and fresh solutions were prepared daily.Five standard gentamicin sulfate and EDTA-Na2solutions were prepared to enable the determination of drugs concentrations in ALS.Furthermore,forced degradation of ALS was investigated(i)by acidic(0.1–1 M HCl)and alkaline(0.1–1 M NaOH),(ii)heating(80°C for 1 h)treatments,(iii)under oxidative conditions using 3%H2O2then heating at 80 °C for 3 h,and(iv)from UVA(320–400 nm)irradiation for 6 h[29].
3.Results and discussion
3.1.Physicochemical and microbiological controls of ALS
Results of visual inspections for color,clarity,and particles completed by sub-visible particles counting,pH and osmolality assessments of ALS throughout the 12 months storage at 25°C ±2°C,RH 60% ± 5%and 40°C ± 2°C,RH 75% ± 5%are summarized in Table 2.No significant macroscopic and/or microscopic alterations of ALS were evidenced.Efficiency of sterility procedure and conditioning of ALS was confirmed by the absence of microbial growth and endotoxin.No impact of autoclaving either upon particle quality or colligative property of ALS was observed.Interestingly,ALS was isosmotic to serum compared to hypoosmotic commercial gentamicin sulfate solution(Panpharma®)in favor of further easy handling in TIVAP filling and blood compatibility.
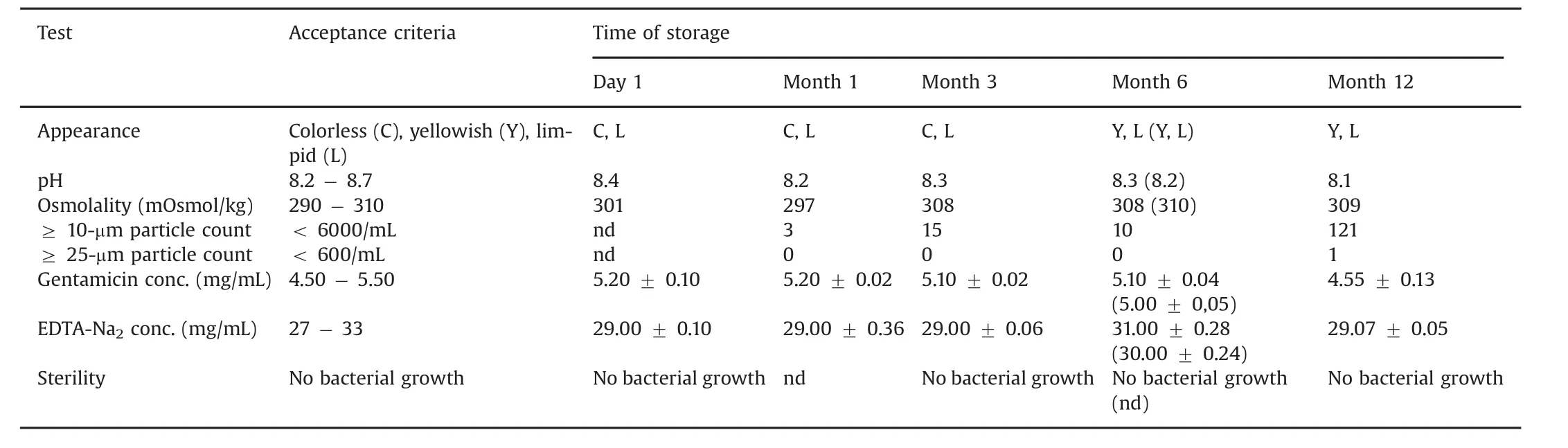
Table 2 Characterization of ALS stability over 12 months of storage at 25°C± 2°C,RH 60% ± 5%(at 40°C ± 2°C,RH 75% ± 5%).Each data is the mean± standard deviation of three experimental determinations.nd:Not determined.
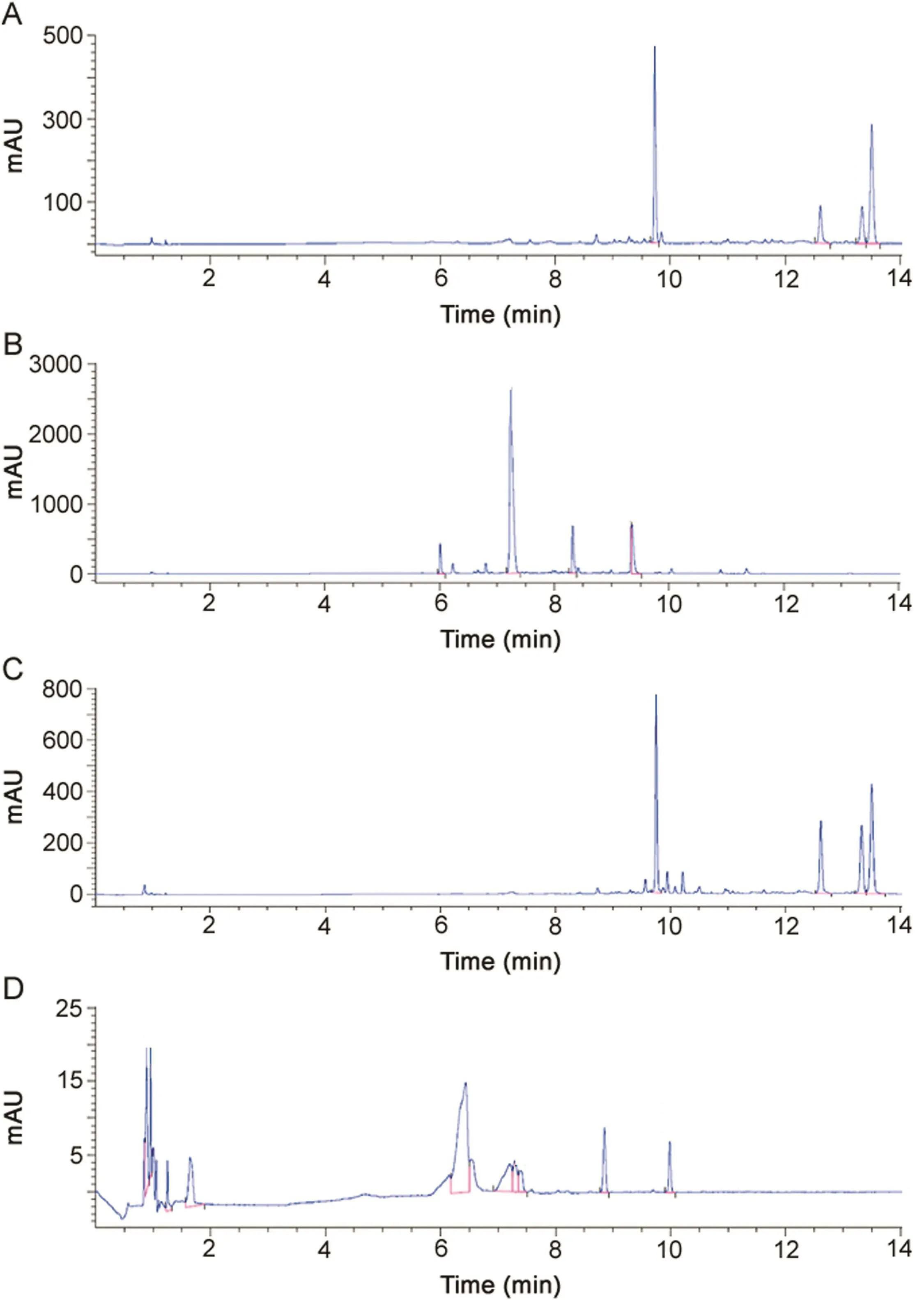
Fig.1.(A)A typical chromatogram of gentamicin standard solution(USP)(216 μg/mL)after OPA derivatization.(B)A typical chromatogram of sisomicin after OPA derivatization(4.65 mg/mL).(C)A typical chromatogram of ALS after OPA derivatization(gentamicin 217 μg/mL).(D)Analysis of ALS(gentamicin 217 μg/mL)under oxidative conditions(3%H2O2,80°C for 3 h)after OPA derivatization.
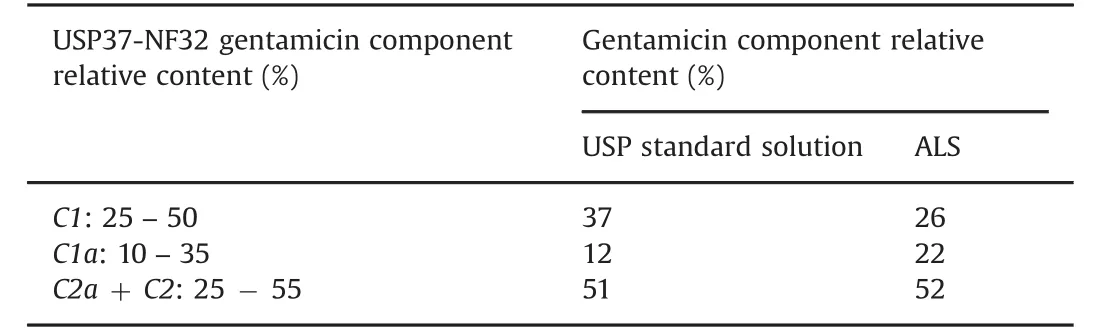
Table 3 Identification of the gentamicin sulfate components in gentamicin USP standard solution(217 μg/mL)and in ALS according to the requirements of US Pharmacopeia USP37-NF32 monograph.Relative content(%)of each component was calculated as the area of each individual peak divided by the sum of all peak areas.The sum of all peak areas(C1a,C2 C2a,C2b and C1)corresponds to 100%.The elution order is gentamicin C1,C1a,C2a and C2.
3.2.HPLC assay validation
Gentamicin sulfate and EDTA-Na2contents in ALS conditioned in amber type I glass vials were analyzed during the storage using HPLC method adapted,for a part,from US Pharmacopoeia Monograph[22]and earlier studies[20,21,24].The chemical structure of gentamicin reveals the lack of chromophore in the molecule,making the direct detection of the antibiotic difficult.Furthermore,besides methods based on microbiological assay[30],enzymeimmunoassay,polarization fluoroimmunoassay[31,32],direct detection methods,e.g.,electrochemical detection[14,33,34],evaporative light scattering detection[35,36],charged aerosol detection[26,37],direct capillary electrophoresis[38]require specific and costly instrumentation(e.g.,tandem mass spectrometer,[39])are not commonly found in quality control laboratories.In the present study,gentamicin sulfate components were assayed by UV-detection after pre-column derivatization with OPA based reagent which reacts only with three primary amines of gentamicin to form UV-absorbing fluorophores,ionpairing chromatographic gradient and separation by a combination of electrical(charge-charge between two underivatized secondary amines of gentamicin positively charged at acidic pH of mobile phase)and hydrophobic interactions with the stationary phase and ions of the mobile phase[25].No pre-heating of gentamicin-OPA based mixture was carried out,as described by previous authors[19],eliminating tedious manual procedures,reducing error,and thereby increasing method reproducibility.The column was maintained at 40°C,leading likely to more reproducible in situ derivatization of gentamicin components passing through the C18stationary phase.
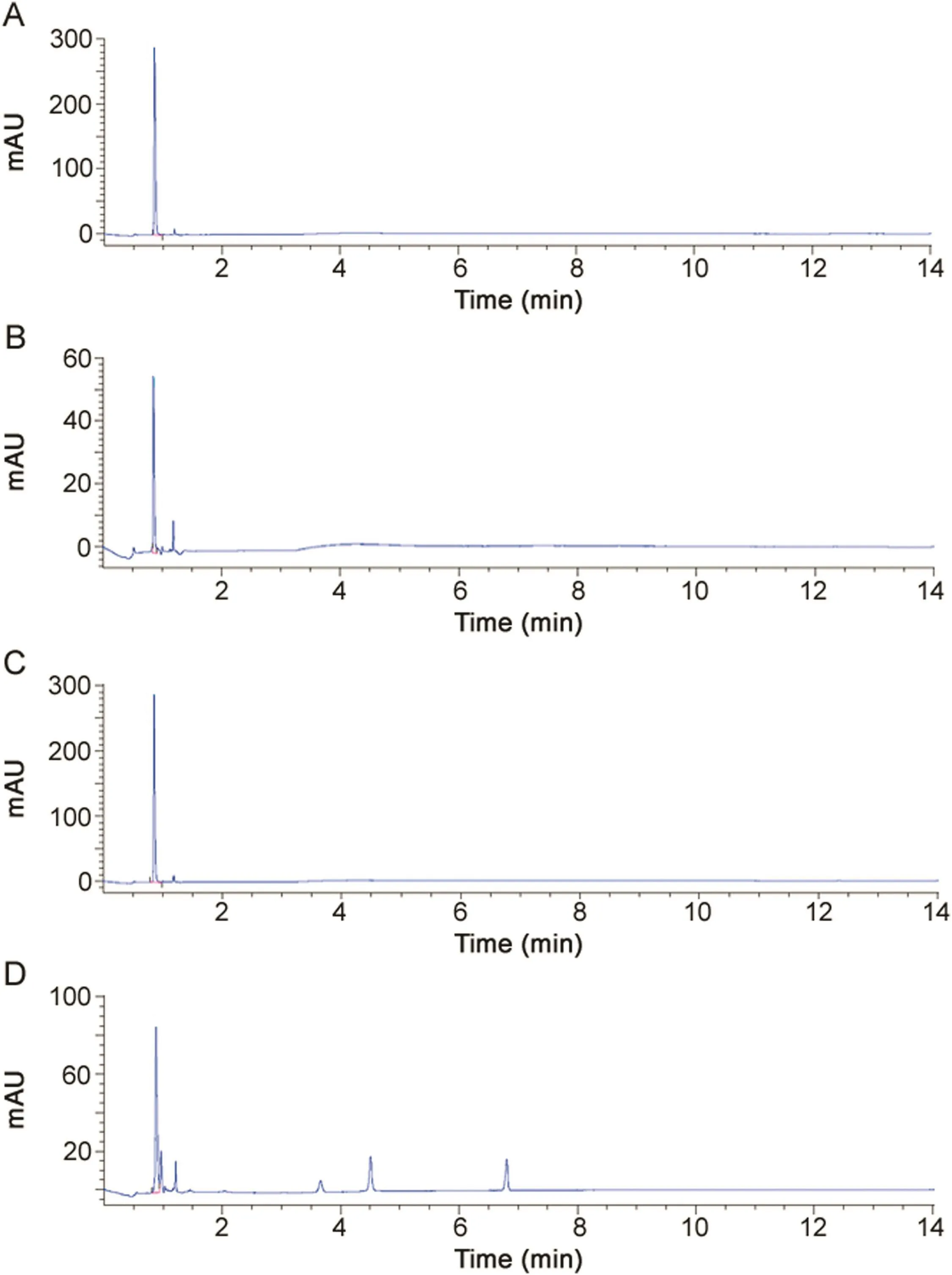
Fig.2.(A)A typical chromatogram of EDTA-Na2(USP)(1.3 mg/mL)after CuSO4complexation.(B)A typical chromatogram of nitrilotriacetic acid(43 μg/mL)after CuSO4 complexation.(C)A typical chromatogram of ALS(EDTA-Na2:1.3 mg/mL)after CuSO4complexation.(D)Analysis of ALS(EDTA-Na2:1.3 mg/mL)under stress oxidative conditions(3%H2O2,80°C for 3 h)after CuSO4complexation.
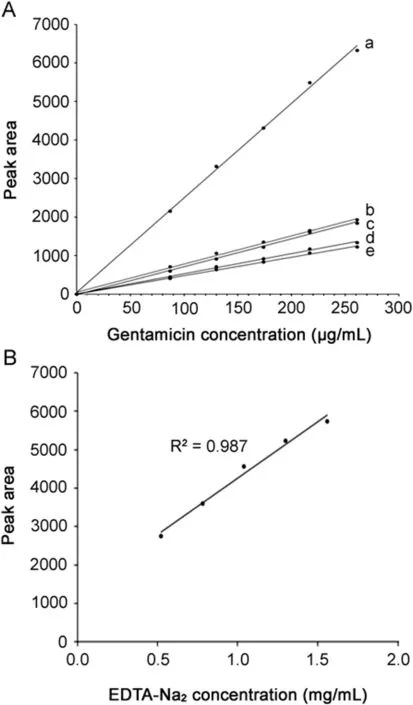
Fig.3.(A)A typical calibration curve of the cumulative peak area(C1,C1a,C2a,C2)of gentamicin sulfate(Panpharma®)(a)and four components(b,c,d and e)ranged from 87 to 260 μg/mL.(B)A typical EDTA-Na2calibration curve ranged from 0.52 to 1.56 mg/mL.
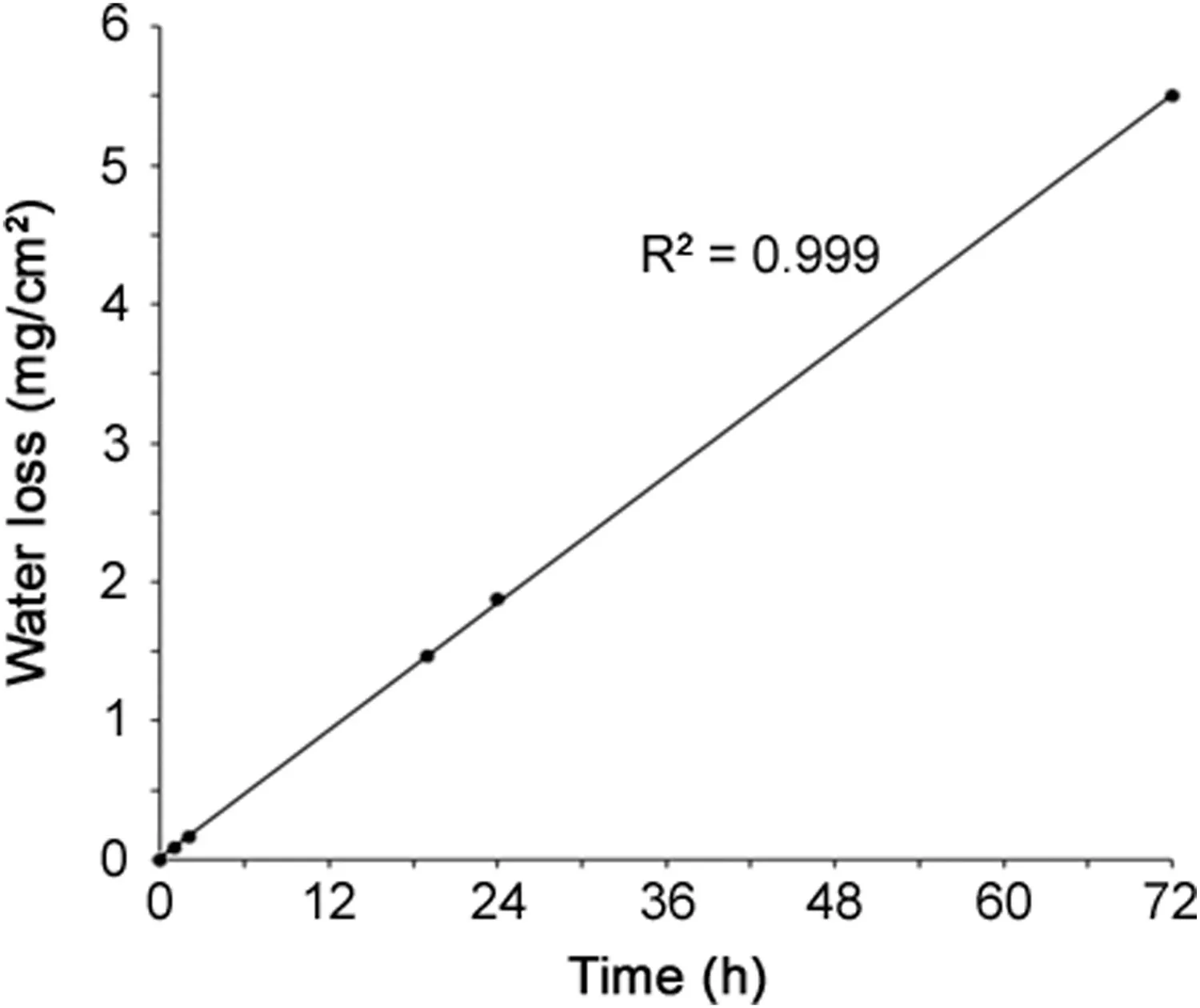
Fig.4.Linear relationship between water loss through TIVAP and time of storage at 40°C ± 2°C,RH 75% ± 5%.
Fig.1A shows typical chromatogram of gentamicin standard solution.The four components of gentamicin(C1,C1a,C2a and C2)were eluted as distinct peaks with retention time of 9.7,12.6,13.3 and 13.5 min,respectively in the order reported by US Pharmacopoeia Monograph[22].Gentamicin C2b component was below LOD.Furthermore,the relative content of each gentamicin component in gentamicin standard was found in agreement with US Pharmacopoeia Monograph requirements,as reported in Table 3.These findings were similar to those reported in previous study[20],in which the chromatogram of gentamicin sulfate solution(2 mg/mL)prepared from the gentamicin sulfate powder met the USP test specifications.The USP method describes the elution order as C1,C1a,C2a and C2 gentamicin with a 5 mm×10 cm column that contains 5μm packing L1(octadecyl silane chemically bonded to porous or nonporous silica or ceramic microparticles,1.5–10 μm in diameter,or a monolithic silica rod).In the present method,Kinetex™ (C18100 Å,100 mm × 4.6 mm,2.6 μm,Core-Shell Technology)column was preferred to Luna®C18(2)column used by earlier authors[20].Therefore,the elution of last gentamicin component(C2)was nearly 2.5 times shorter(13.5 min versus 31 min)in the present method than that reported previously[20],con firming that the combination of the small particle size and narrow particle size distribution coupled with the significantly shorter diffusion path resulted in a material that yielded significantly increased column efficiency and chromatographic resolution.Chromatographic separation was shortened mandatory,considering that isoindoles formed during derivatization are generally highly reactive compounds.As a result,it was considered that any derivative breakdown during chromatography would be time-dependent and that component derivatives with long retention time(C2a and C2)would be particularly affected by degradation prior to detection[40].Previous analysis of gentamicin in raw material used column(150 mm×4.6 mm)packed with Ul trasphere®ODS(C18)and elution gradient(A:5% acetic acid-25%water-70%methanol and B:100%methanol)for faster elution of C1(5 min)and similar elution time for C2(13.7 min)[41].In the present study,longer elution time for C1(~10 min,Fig.1C)was preferred to elute first the most hydrophilic components of ALS(e.g.,excipients from gentamicin sulfate sterile solution for injection,Panpharma®).Interestingly,it was noticed that the relative content of gentamicin components was proportional to molecular weight of C1,C1a,C2a and C2 as equimolar amounts,con firming that the sum of the areas of the four gentamicin components was a relevant measure of the gentamicin concentration.Sisomicin chromatogram shows one main peak surrounded by three minor peaks(Fig.1B).Possible partial derivatization of one,two or three primary amines of sisomicin might explain the presence of numerous peaks.
The determination of EDTA-Na2in ALS by direct UV detection was challenging since EDTA-Na2does not contain a significant chromophore.In the present study,EDTA-Na2in ALS was assayed by ion-pairing HPLC and metallocomplex formation by using TFA in mobile phase and copper sulfate in sample preparation.Typical chromatogram of EDTA-Na2eluted as EDTA-Cu2+is shown in Fig.2A.As reported in previous study assaying EDTA-Na2in pharmaceutical formulation,EDTA-Cu2+,as ionic analyte,showed minimal retention(Fig.2C,<1 min)through reversed-phase by using liquid chromatography with ion-pairing and elution gradient[42],and co-elution of nitrilotriacetic acid-Cu2+complex(Fig.2B).
3.3.HPLC validation criteria
3.3.1.Specificity
The specificity of the assay was conducted to evaluate the assay for potential sources of interfering peaks from the matrices used in sample preparation.No interfering peaks were seen from waterused to prepare mobile phase,the mobile phase or a blank filter media.No significant macroscopic and/or microscopic alterations of ALS were evidenced in acidic(0.1–1 M HCl,at 80 °C for 1 h)and basic(0.1–1 M NaOH,at 80 °C for 1 h)conditions.ALS was found stable under heat conditions(at 80 °C)and under UVA(320–400 nm)irradiation for 6 h.Under oxidative stress conditions with 3%H2O2at 80°C for 3 h,EDTA-Na2was degraded to about 60%(Fig.2D)while all of gentamicin sulfate was degraded(Fig.1D).Four main degradation products were generated with retention time of 6.4,7.2,8.8 and 9.9 min that could correspond to the impurity A(e.g.,sisomicin,Fig.1B).
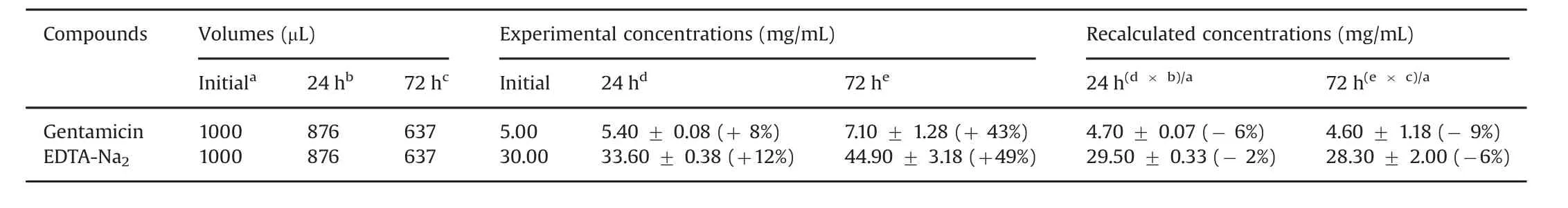
Table 4 Determination of gentamicin and EDTA-Na2concentrations in ALS after 24 h and 72 h of contact time in TIVAP.Each data is the mean±standard deviation of three experimental determinations of concentrations in three TIVAP.Relative standard deviation(RSD)(%)of concentrations was calculated as follows:RSD(%)=(initial concentration–experimental concentration)/initial concentration.
3.3.2.Precision
Repeatability and intermediate precision of gentamicin sulfate-EDTA-Na2assays were found to be 1.82%-1.23%,and 2.16%-1.47%,respectively,con firming the overall precision of HPLC methods involving pre-column derivatization and metallocomplex procedure.
3.3.3.Linearity
The linearity of assay was determined to have a correlation coefficient(r)for gentamicin sulfate of 0.999(in the range of 87-260 μg/mL)and for EDTA-Na2of 0.993(in the range of 0.52-1.56 mg/mL)as shown in Figs.3A and B,respectively.
3.3.4.Accuracy
The recovery of gentamicin sulfate and EDTA-Na2 was found to be in the range of 97.96%–99.81%,and 100.72%–102.72%over all concentration range,respectively.
3.3.5.LOD and LOQ
LOD and LOQ for gentamicin were 8 μg/mL and 24 μg/mL,respectively.The LOD and LOQ for EDTA were found to be 45 μg/mL and 136 μg/mL,respectively.
3.3.6.Robustness
The robustness of the assay in terms of varied injection volume(5–15 μL)and flow rate(0.5 mL/min)did not significantly change the time retention,asymmetry and the percentage of target amounts in the samples of gentamicin sulfate and EDTA-Na2.
3.3.7.Stability study of ALS in glass injection vials and in TIVAP
Stability of gentamicin sulfate and EDTA-Na2in vials stored for 12 months was confirmed by minimal variation of drug concentration in ALS,as reported in Table 2.A loss of ALS was highlighted by weighing the device throughout contact time in TIVAP.Loss solution per cm2of catheter is represented in Fig.4.Solution loss was perfectly linear in function of time.After 24 h and 72 h,124 μL and 363 μL of ALS were evaporated through polymeric wall,respectively.At the same time,HPLC assay of ALS after contact in TIVAP showed that gentamicin and EDTA concentrations increased compared to initial concentrations in ALS(Table 4).After recalculation considering the extent of water loss(124 μL for 24 h and 363 μL for 72 h),gentamicin and EDTA concentrations were in accordance with initial concentrations in ALS(Table 4).
4.Conclusion
In this study,a process of pharmaceutical compounding and conditioning of ALS was performed.Numerous physicochemical analysis,including the development of validated HPLC methods for dual gentamicin and EDTA-Na2assays,showed a stability of ALS conditioned in amber type I glass vials for 12 months at 25°C ±2°C(RH 60% ± 5%)and 40°C ± 2°C(RH 75% ± 5%).Furthermore,complementary findings reported satisfactory stability of ALS in TIVAP for 24 h and 72 h.At the outset,the present study confirmed the pharmaceutical relevance of gentamicin-EDTA-Na2combined solution as a new antimicrobial lock therapy.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was supported by Centre de Recherche Translationnelle de I′Institut Pasteur,grant Number S-PI15007-02A.JMG,CB and DL are supported by the French Government's Investissement d′Avenir program:Laboratoire d′Excellence ‘Integrative Biology of Emerging Infectious Diseases’(grant no.ANR-10-LABX-62-IBEID.),the Fondation pour la Recherche Médicale(grant no.DEQ.20140329508)and the Center for Translational Science of the Institut Pasteur(S-PI15007-02A).
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Novel ligand-based docking;molecular dynamic simulations;and absorption,distribution,metabolism,and excretion approach to analyzing potential acetylcholinesterase inhibitors for Alzheimer's disease
- Recovery rates of combination antibiotic therapy using in vitro microdialysis simulating in vivo conditions
- Evaluation of naproxen-induced oxidative stress,hepatotoxicity and in-vivo genotoxicity in male Wistar rats
- Cytotoxic effect of Rosa canina extract on human colon cancer cells through repression of telomerase expression
- Highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study
- Detection and determination of undeclared synthetic caffeine in weight loss formulations using HPLC-DAD and UHPLC-MS/MS
