Development and validation of an HPLC-UV method for analysis of methylphenidate hydrochloride and loxapine succinate in an activated carbon disposal system
2018-12-10PoojBkshiAndrewKoreyWillimFowlerAjyBng
Pooj Bkshi,Andrew Korey,Willim Fowler,Ajy K.Bng,*
Keywords:Methylphenidate hydrochloride Loxapine succinate Activated carbon Analytical method development FDA drug disposal
A B S T R A C T Unused medications have the possibility of being abused,causing serious harm to individuals who were not prescribed the drug.The Food and Drug Administration(FDA)recommends the proper disposal of unused prescribed medications to maintain safety and prevent environmental hazards.However,many of the current disposal techniques do not properly address safety.A drug disposal pouch containing granular activated carbon offers a unique disposal method to deactivate residual or expired medication in a convenient,effective,and safe manner.A robust and validated method for methylphenidate hydrochloride and loxapine succinate was developed using high-performance liquid chromatography(HPLC)and the deactivation efficiency of the disposal system was tested.Methylphenidate hydrochloride was analyzed on a C18analytical column(250 mm×4.60 mm,100Å)using acetonitrile-water(0.05%(v/v)trifluoroacetic acid)as the mobile phase at a flow rate of 1.0 mL/min with a run time of 15 min and retention time of 7.8 min.Loxapine succinate was separated on a C8 100Å (250 mm × 4.6 mm,5 μm)column maintained at 25 °C using a flow rate of 1.0 mL/min.The run time was 10 min and the retention time of the drug was around 4.6 min.Mobile phase was composed of acetonitrile and water(0.3%triethylamine)at pH 3.0 as 40:60(v/v).Reference standard solutions(100 μg/mL)for both drugs were prepared by dissolving in mobile phases.These methods provide good linearity(R2=0.999)over the range of 5–100 μg/mL for methylphenidate hydrochloride and 0.1–100 μg/mL for loxapine succinate.The assay methods were successfully applied to study the deactivation of these drugs.
1.Introduction
Proper disposal of unused prescription medications has become a significant problem.Storage of expired and unwanted medications can lead to either accidental exposure or intentional use or abuse of prescription medications.The potential for misuse and addiction to prescription medications,such as those for pain,is a national health concern that has social and economic implications.In 2015,over 33,000 Americans died as a result of opioid overdose or substance abuse disorders related to prescription of opioid pain medications,and 591,000 suffered from addiction to heroin[1,2].Although prescription medications play an important role in the treatment of severe and acute chronic pain conditions,due to their over prescription or prescription without adequate safeguards,their misuse can have devastating effects.According to the National Survey on Drug Use and Health,fewer than four percent of people who had used prescription painkillers non-medically started using heroin within five years[1].Thus,the proper disposal of prescription medication is important.In the present study,we focused on the disposal of two psychoactive medications,methylphenidate hydrochloride(MPH)and loxapine succinate.
MPH is a common prescription medication used for treating attention-deficit hyperactivity disorder(ADHD),and affects the dopamine balance in the brain by stimulating the nervous system[3].The pharmacological action of MPH through the intranasal route is similar to that of cocaine,which causes rapid release of dopamine[4].It is listed as a Schedule II,federally-controlled substance because of its high potential for abuse,which is similar to morphine and may lead to severe physiological dependence.This effect of intensely gratifying euphoria makes MPH very addictive[5].Another drug which has potential for abuse is loxapine succinate.It is a tricyclic,antipsychotic prescription medication,which is used for treating schizophrenia.Loxapine succinate exerts its action by blocking the action of dopamine,and is thus used to manage emotions and actions that are usually accompanied with schizophrenia.Loxapine succinate has a potential of being abused,as it only provides temporary relief and is used for the management of schizophrenia[6].Both drugs are prescribed frequently and thus have increased the potential for abuse.
Since MPH and loxapine succinate have a high potential for abuse,we wanted to investigate their deactivation profile using drug disposal system.The analytical accuracy of the method developed for both drugs was also tested.In the literature,there are few analytical methods reported for the determination of MPH[7,8]and loxapine succinate[9].All the available methods are time consuming and expensive and use liquid chromatography–mass spectrometry(LC–MS)or high-performance liquid chromatography(HPLC)with multiple solvents as mobile phases.Consequently,there is a need to develop a simple,sensitive,economical,and time-efficient method for the determination of MPH and loxapine succinate in dosage forms(tablets and capsules).Therefore,we developed an easy and reproducible reverse-phase HPLC(RP-HPLC)for the estimation of MPH and loxapine succinate in dosage forms by following the International Council for Harmonization(ICH)of Technical Requirements for Registration of Pharmaceuticals for Human Use validation guidelines[10].This method allowed us to investigate deactivation of MPH and loxapine succinate in the presence of activated carbon,as well as test the stability of the drug under different storage conditions.
One of the ways by which accidental exposure of unneeded medicines can be avoided is through the“medicine take back program.”This program offers safe disposal of most types of unneeded prescription medicines[11].If no medicine takeback programs or DEA-authorized collectors are available,the easiest way to dispose of these medications in household trash is by mixing these medications with an unpalatable substance such as dirt,cat litter,or coffee grounds.Medications that pose a potential threat can be flushed down the toilet.To minimize the accidental exposure and misuse of these prescription medications,the Food and Drug Administration(FDA)has developed several guidelines to encourage the proper disposal of these medicines,as mentioned in the FDA recommendations for drug disposal[12].Still,there are some medicines,for example,fentanyl patches,that may be harmful,and,in some cases,fatal with just one dose,especially if they are used by someone other than the person for whom the medicine was prescribed[13].All the above mentioned procedures do not actually make the drug inactive,and have harmful effects on the environment as the mixing of these medications with the cat litter or coffee grounds cannot deactivate the drug,and can lead to contamination of the water system[14].
Activated carbon is one of the best alternatives to dispose of medications,as it attracts and holds the organic compounds by the adsorption process[15].Due to its property of material porosity,active pharmaceutical ingredient(API)easily sticks to the surface area[16].However,this technology has not been explored to address the drug disposal problem,and there is a pressing need for more research on effective disposal techniques for highly addictive prescription medications.
In the present study,we aimed to evaluate the deactivation efficiency of the activated carbon-based drug disposal system,Deterra®.This drug deactivation system is based on MAT12®Molecular Adsorption Technology,which deactivates the API by a physical adsorption process[17].The term “deactivates”is used to signify the irreversible physical adsorption process between active substance and activated carbon.We investigated the drug disposal of two model psychoactive prescription medications which have a potential of abuse,MPH and loxapine succinate.
The proposed drug deactivation system offers a unique disposal method to deactivate unused,residual or expired medications by using granular activated carbon within a pouch that is convenient,safe and effective.This study is aimed to investigate the deactivation profile of MPH and loxapine succinate using an activated carbon disposal system.Successful method development and validation of MPH and loxapine succinate was performed to test the efficiency of this system precisely.
2.Experimental
2.1.Chemicals and reagents
MPH and loxapine succinate were purchased from Sigma-Aldrich(St.Louis,MO,USA).Dosage forms:generic MPH(20 mg,CorePharma)tablets and loxapine succinate(20 mg,Lannett)capsules were provided by Verde Environmental Technologies Inc.(Minnetonka,MN,USA).The Deterra®drug deactivation system(the pouch containing 15 g of granular activated carbon within a water soluble film reservoir)was also provided by Verde Environmental Technologies Inc.Acetonitrile(ACN),methanol and trifluoroacetic acid(TFA),of HPLC grade,were obtained from Fisher Scientific(Pittsburgh,PA,USA).Nylon filters(0.22 μm)used for sample filtration were purchased from Medsupply Partners(Atlanta,GA,USA).Deionized water(DI)(MQ res:18.2 MΩ·cm,permC:7.4 μS/cm)was generated with a Milli-Q Direct 8(Millipore,Bedford,MA,USA).All other reagents used were of HPLC or ACS grade.
2.2.Instrumentation
The analysis was carried out using a Waters Alliance HPLC system(e2695 separating module)(Waters Co.,Milford,MA,USA)with photodiode array detector (Waters 2996)with an autosampler and column heater.Data were collected and processed using Empower™software(Version 2)from Waters.RP-HPLC methods were used for the quantification of all samples.
2.3.Chromatographic conditions
The assay method for MPH and loxapine succinate was developed,validated and applied to study the drug deactivation profile of both drugs.This method was also used to predict the storage stability of MPH and loxapine succinate in water.The mobile phase was filtered through a 0.2 μm filter(GNWP 0.2 μm;Millipore,Bedford,MA,USA)and degassed using sonication.
MPH was analyzed using a C18Phenomenex Kinetex,biphenyl(250 mm × 4.6 mm,100 Å)column set at 25 °C with methanol(0.1%formic acid(FA))and water(0.1%FA,pH 6.8 adjusted using ammonium hydroxide)(50:50 v/v)as the mobile phase.A flow rate of 1 mL/min with an injection volume of 25 μL and an absorption wavelength of 258 nm were used.The run time was 15 min and the retention time of the drug was around 7.8 min.
For the analysis of loxapine succinate,the compound was separated on a C8Phenomenex Luna(250 mm × 4.6 mm,5 μm)at an ambient temperature with acetonitrile(ACN)and water(0.3%(v/v),trimethylamine,pH 3)(40:60 v/v)as the mobile phase.A sample volume of 10 μL was injected at a flow rate of 1 mL/min and analyzed at an absorption wavelength of 211 nm.The run time was 12 min and the retention time of the drug was around 4.6 min.
2.4.Preparation of stock and working standards solutions
All standard solutions for MPH and loxapine succinate were prepared using deionized water to give a working standard in the range of 5–100 μg/mL and 0.1–100 μg/mL,respectively.Stock standard solutions of MPH and loxapine succinate were prepared at a concentration of 1 mg/mL in deionized water and stored at 4°C.Working standard solutions of MPH and loxapine succinate were prepared by diluting the standard stock solution with deionized water to yield concentrations of 0.1,0.25,0.5,1,2.5,5,10,25,50 and 100 μg/mL.Quality control(QC)concentrations were then prepared at 50,75 and 100 μg/mL for MPH and 25,50 and 100 μg/mL for loxapine succinate control samples.
2.5.Method validation
HPLC methods were validated to ensure consistent,reliable,and accurate results to determine the levels of two psychoactive medications in all samples.The HPLC methods were validated in terms of sensitivity,linearity,accuracy,precision,specificity and robustness.Method validations for both drugs were performed over a 3-day period.
2.5.1.Determination of the limit of detection(LOD)and limit of quantification(LOQ)
The LOD was determined by injecting lower concentrations of MPH and loxapine succinate sequentially until a signal(peak)-to-noise ratio was obtained.The LOQ,which is the lowest quantifiable concentration,was also determined from the range of concentrations analyzed for the LOD determination.
2.5.2.Evaluation of linearity
Standard solutions were evaluated for the linearity within a concentration range of 5–100 μg/mL for MPH and 0.1–100 μg/mL for loxapine succinate.The peak area was plotted against drug concentration and the linearity was thus calculated by the linear regression equation y=mx+c,where y represents the peak area and x represents either the MPH or loxapine succinate concentration in μg/mL.A correlation coefficient of approximately 0.999 or more was considered as desirable for all calibration curves.
2.5.3.Determination of accuracy and precision
The inter-day validation was conducted with three sets of three QC samples of different concentrations for MPH(50,75 and 100 μg/mL)and loxapine succinate(25,50 and 100 μg/mL).These samples were evaluated for three days by generating a calibration curve for each day.As for the intra-day validation,six sets of three different drug samples were assayed and evaluated with reference to one calibration curve on the same run.The accuracy and precision values were calculated using a standard formula,as per the ICH guidelines.The accuracy and precision of the methods were determined for both intra-day and inter-day variations using multiple analyses of different concentrations of samples on three different days.
2.5.4.Specificity
The specificity of each assay was determined by comparing the chromatograms of the blank solution(water)with that of the drug standard solution(drug in water)of varying concentrations.Furthermore,the specificity of the improved HPLC method was determined by analyzing the MPH and loxapine succinate dosage forms in activated carbon.Observations were made for any interfering peaks generated during the analysis.
2.5.5.Robustness
Robustness is a measure of method's capacity to remain unaffected by small deliberate changes.The chromatogram resolution and retention behavior were evaluated for any changes in flow rate(±0.05 mL/min),organic solvent ratio(±5%methanol),and pH(±0.5).
2.6.Stability
The short-term stability of MPH and loxapine succinate under storage conditions was evaluated using three standard concentrations(10,25 and 50 μg/mL)(n=3)stored for one week at varying temperatures of 4 °C,25 °C,and-20 °C.All stored standard solutions were analyzed using freshly prepared calibration standards.The stability of MPH and loxapine succinate was assessed by comparing the concentration of both drugs in each solution before and after the storage period.
2.7.Deactivation of pharmaceutical dosage forms using an activated carbon disposal system
The assay method was applied to support the deactivation profile of MPH and loxapine succinate in the presence of an activated carbon drug disposal system.The system consisted of a pouch containing 15 g of granular activated carbon packaged within a water soluble inner film reservoir.The deactivation of tablets and capsules as dosage forms were examined over 28 days using the model psychoactive medications.Ten MPH and loxapine succinate tablets(20 mg each)were placed into individual pouches separately followed by addition of 50 mL of warm tap water at a temperature of about 43°C.To mix the activated carbon and warm water properly,pouches were shaken for 10 s at a rate of one shake per second.This was followed by a waiting period of 30 s to release the air bubbles from the charcoal.After ensuring that all of the medications settled to the bottom of the pouch,the pouches were sealed,stored upright,and left undisturbed at room temperature.Separate pouches were set up for each time point at 8 h,1,2,4,7,14,21 and 28 days and samples were collected from pouches to examine deactivation of drug during the study.Before taking samples,pouches were mildly shaken from side to side to ensure the medications were mixed homogenously in the pouch.Samples were then filtered with a 0.22 μm nylon filter and analyzed by the validated HPLC methods.The deactivation rate was calculated as follows:
%Deactivated=[(Initial amount of drug in pouch–Final amount of drug in pouch)/Initial amount of drug in pouch]×100.
2.8.Desorption study
At the end of the adsorption study(28 days),the pouch contents were transferred to 500 mL bottles,and 200 mL of tap water was added to each bottle.The samples were shaken for 1 h at 150 rpm,stored upright for 23 h at room temperature,then filtered and analyzed by HPLC.The water was then completely replaced with 250 mL of 30%ethanol,shaken for an additional hour,and stored for 23 h at room temperature.After that,samples were taken from the container, filtered and analyzed by HPLC.
3.Results
3.1.Method development and optimization
The most suitable isocratic condition to resolve MPH with a C18column,after the chromatographic conditions were optimized for specificity,resolution and retention time,was a mobile phase consisting of methanol(0.1%FA)and water(0.1%FA,pH 6.8)(50:50,v/v).For loxapine succinate,analyte was separated on a C8column and the mobile phase consisted of ACN and water(0.3%(v/v)triethylamine,pH 3)(40:60,v/v).When the pH of the mobile phase was increased or when a higher percentage of organic solvent was used,the resultant chromatogram had an increase either in background noise or peaks indicating the tailing effect.Thus,based on the above mentioned parameters,MPH and loxapine succinate eluted at a retention time of 7.8 and 4.6 min,respectively,as shown in Fig.1.Table 1 depicts the chromatographic parameters applied for the method.
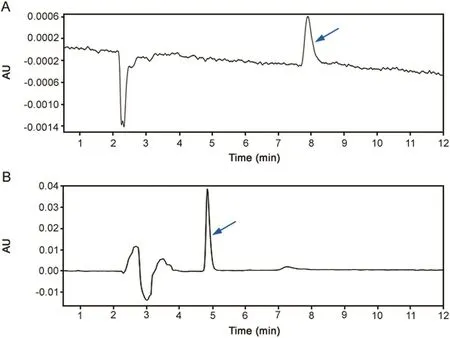
Fig.1.Representative chromatograms of(A)methylphenidate hydrochloride standard(25 μg/mL)and(B)loxapine succinate standard(25 μg/mL).Arrow indicates drug peak.
3.2.Method validation
The method was validated according to the validation of analytical procedures provided in the ICH guidelines and draft guidance for the industry:analytical procedures and methods validation.
3.2.1.Linearity and range
A linear relationship was obtained between the peak area for both drugs and corresponding concentrations.The mean standard calibration curves are presented in Fig.2.The calibration curves exhibit linearity over the concentration range of 5–100 μg/mL for MPH and 0.1–100 μg/mL for loxapine succinate with regression coefficient values greater than 0.999.The methods(R2=0.999)provided a good correlation between the peak area and drug concentration.
3.2.2.Sensitivity
The LOD was evaluated by determining the minimum levels of concentration for MPH and loxapine succinate that could be detected using this analytical method.The LOQ was studied by estimating the minimum concentration that could be quantified with acceptable accuracy and precision.The LOD values for MPH and loxapine succinate were determined to be 1.38 μg/mL and 0.07 μg/mL,and the LOQ values were 4.17 μg/mL and 0.20 μg/mL,respectively.
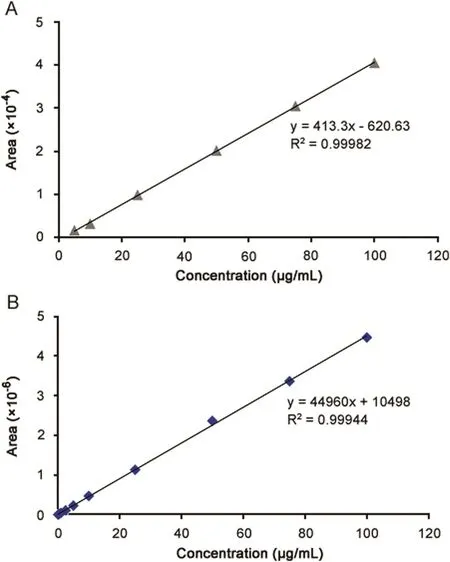
Fig.2.Linearity of the HPLC method for analysis of(A)methylphenidate hydrochloride and(B)loxapine succinate.
3.2.3.Accuracy and precision
The intra-day and inter-day accuracy and precision of the assay method were studied by analyzing replicates at 3 different concentration levels:50,75 and 100 μg/mL(MPH)and 25,50 and 75 μg/mL(loxapine succinate)(Table 2).The intra-day and interday variation was found to be within 0.8%–6%.The intra-day and inter-day accuracy was found to be within 90%–110%.
Under the stated experimental conditions,the precision(RSD)values were a maximum of 6%and the accuracy values were within a range of 94%–99%for MPH and the precision(RSD)values were at a maximum of 4.31%and the accuracy values were within a range of 98%–105%for loxapine succinate.
3.2.4.Robustness
The robustness of the method was determined by deliberately changing the experimental conditions.The resolution of MPH and loxapine succinate was evaluated and the effects of changes in flow rate±0.05 mL/min,mobile phase composition±5%(for methanol),and pH±0.5 were evaluated.Both the analytes,MPH and loxapine succinate,were adequately resolved under varied chromatographic conditions.Tables 3 and 4 demonstrate all the varied chromatographic conditions performed in the methods,and the%recovery for the MPH and loxapine succinate standardconcentration,25μg/mL,was found to be within an acceptable range of 80%–120%.
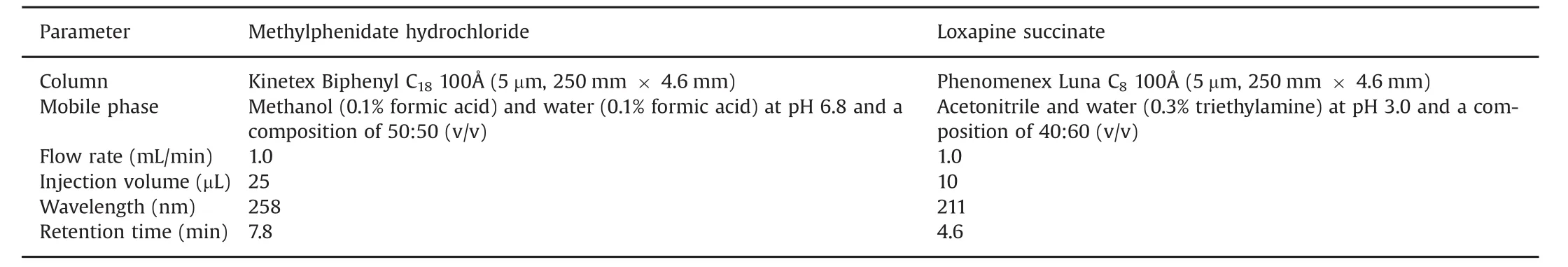
Table 1 HPLC isocratic method for methylphenidate hydrochloride and loxapine succinate.
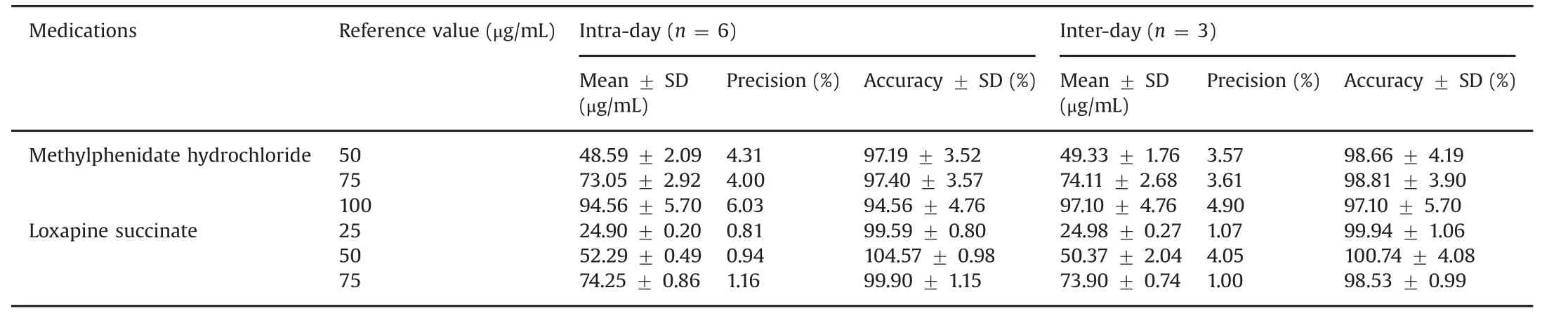
Table 2 Intra-day and inter-day accuracy and precision of HPLC assay for methylphenidate hydrochloride and loxapine succinate.
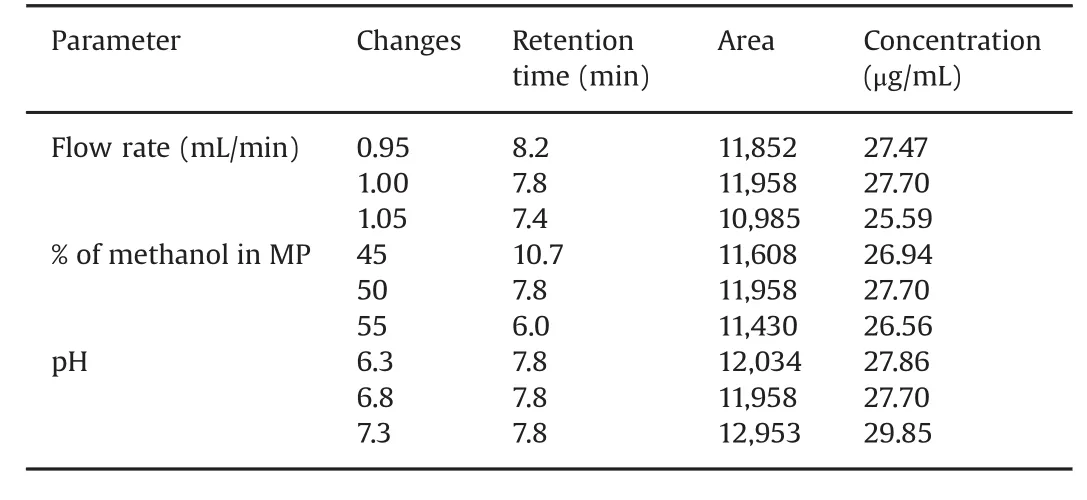
Table 3 Robustness of the method for methylphenidate hydrochloride.
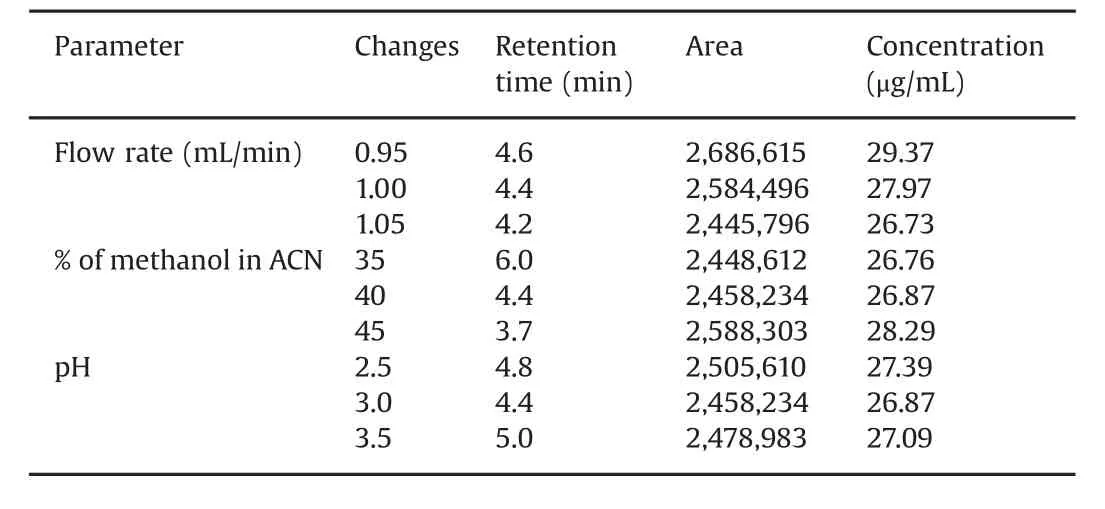
Table 4 Robustness of the method for loxapine succinate.
3.2.5.Specificity
Specificity was used to test the ability of the assay method to eliminate the effects of all interfering substances on MPH and loxapine succinate peak results,specifically by comparing the chromatograms to the blank samples.The validated method showed that the drug contents eluted with no interfering peaks generated by the excipients in the marketed products.
3.3.Stability
Three concentrations(10,25 and 100 μg/mL)of MPH and loxapine succinate in water(n=3)were analyzed to assess the stability.The stability was assessed after storage for one week at different storage temperatures.Stability assessments indicated that both drugs were stable in water for 1 week at room temperature(25 °C),4 °C and-20 °C.The%accuracies for the MPH and loxapine succinate standard concentrations were found to be within acceptable ranges of 92%–107%and 95%–105%,respectively(Fig.3).
3.4.Deactivation study
The deactivation of MPH and loxapine succinate with a drug disposal system was observed over 28 days.After the addition of the dosage forms and water into the pouches,adsorption started immediately.As shown in Fig.4,96.9%of loxapine succinate and 99.9%of MPH were adsorbed and deactivated by the drug disposal system at the end of 8 h.Both drugs continued to be adsorbed over time,and at the end of 28 days,100%drug deactivation was achieved.The deactivation profiles for both drugs are presented in Fig.4.
3.5.Desorption study
A desorption or washout study was performed following the deactivation study in order to determine the potential for leaching of the active ingredients from activated carbon in the presence of water and alcohol.To test the robustness of the system,desorption was examined in the presence of a larger volume of water(250 mL)followed by 30%ethanol(250 mL).The results show that after 28 days,no drug leached out after one day of desorption in the presence of water,and only 1%of the drug was leached out from the activated carbon in the presence of the organic solvent ethanol(Table 5).
4.Discussion
Lack of awareness of the need for proper disposal of prescription medication leads to abuse and environmental contamination,and this problem has been increasing steadily[18].Thus,the potential for abuse of prescription medications should be addressed in the medical community and by primary care practitioners.
MPH and loxapine succinate are examples of two commonly abused drugs,and we investigated their deactivation efficiency by using an activated carbon disposal system.MPH and loxapine succinate were successfully detected with RP-HPLC,utilizing buffered water and organic solvents(Fig.1).
In the present study,as MPH(logP:2.2)and loxapine(logP:3.6)are lipophilic compounds,C18reverse-phase column was used for MPH analysis and C8for loxapine.MPH and loxapine succinate are weak bases with pKa values of 8.8 and 7.1,respectively.MPH was separated using methanol and water as the mobile phase,and the pH was adjusted to 6.8.Similarly,loxapine was separated using ACN and water as the mobile phase with the pH adjusted to 3.More than 99%ionization was achieved for both drugs at their respective pH values,with corresponding log D value of 0.The concentrations of methanol and acetonitrile were optimized to give a symmetric peak with a reasonable run time.A detailed layout of the HPLC parameters used in the developed method is discussed in Table 1.The reliability and sensitivity of the validated methods were ensured with good linearity,accuracy,and precision within the ICH and FDA limits for the method validation of analytical samples.
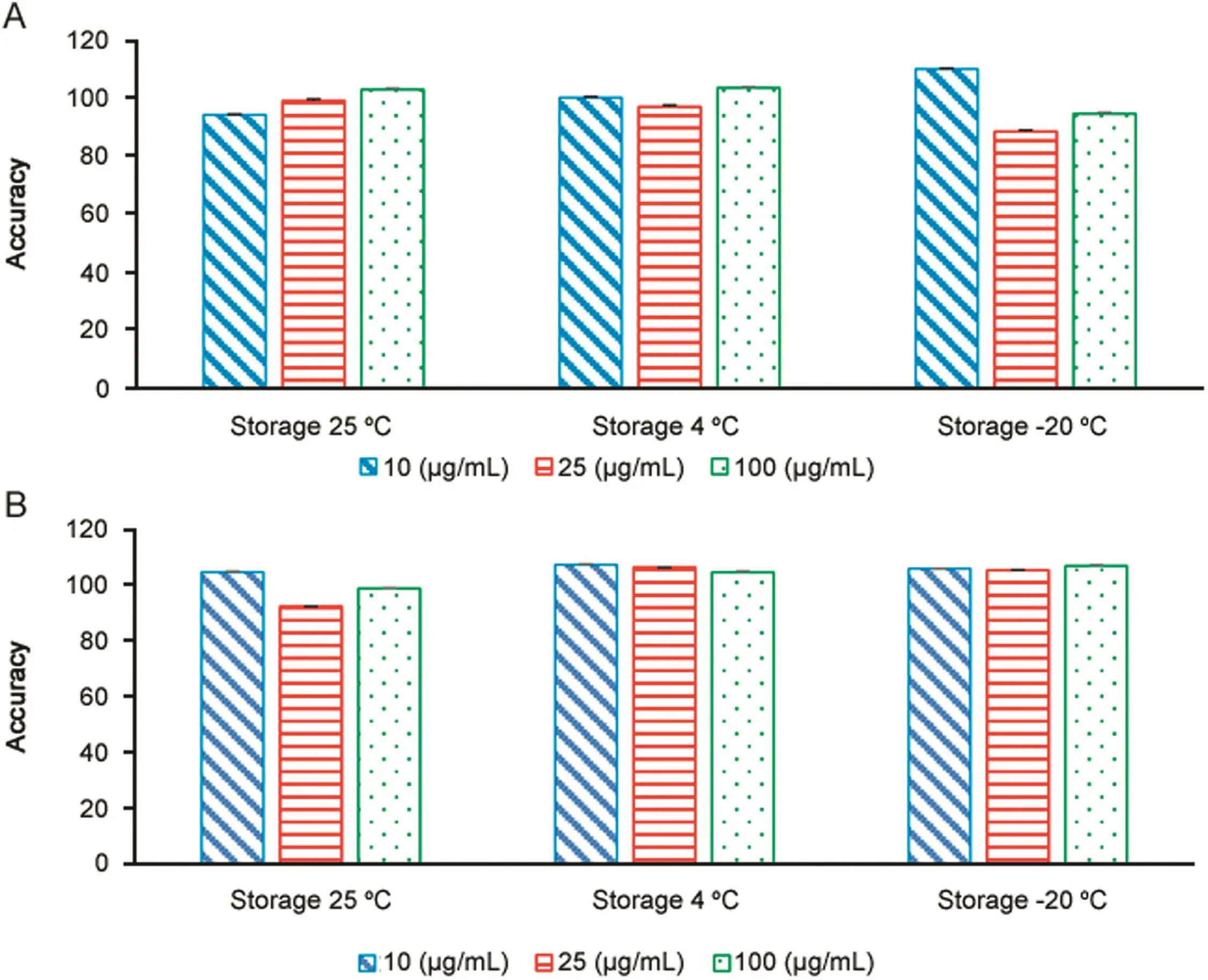
Fig.3.Stability of(A)methylphenidate hydrochloride standards(10,25 and 100 μg/mL)and(B)loxapine succinate standards(10,25 and 100 μg/mL)at different temperatures,25 °C,4 °C and-20 °C for one week.
In addition,analysis of the marketed preparation of MPH and loxapine succinate with the validated assay methods showed that the drug contents eluted with no interfering peaks generated by the excipients in the marketed products.Results for robustness are summarized in Tables 3 and 4,and the methods were found to remain unaffected by changing the method parameters.The study also presented that both MPH and loxapine succinate were stable in water at different temperatures,25 °C,4 °C and-20 °C,for the storage over the period of one week.Both validated methods were applied to examine the ability of the disposal system to deactivate two commonly abused prescription drugs,MPH and loxapine succinate.
According to the FDA guidelines,all medications being deposed of in household trash should be mixed with unpalatable substances such as cat litter or coffee grounds,or should be flusheddown the toilet[11].However,these procedures do not deactivate the drug,and the drug is still available in the active form;this can lead to contamination of the environment and the water system.Our studies were consistent with the studies performed by Harwadkar et al.[15],in which various deactivating agents were tested,and activated carbon was found to be the most efficacious deactivation agent,causing complete deactivation for various dosage forms of medications such as dexamethasone tablets and amoxicillin capsules.
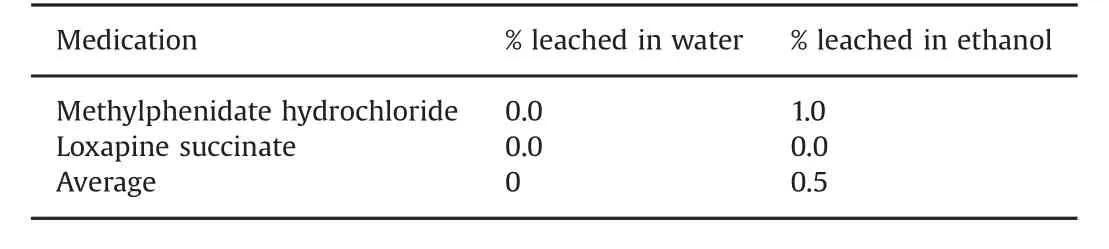
Table 5 Amount of the drug leached from activated carbon during desorption study.
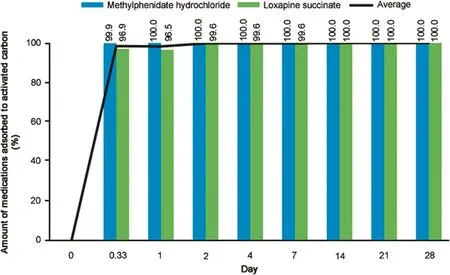
Fig.4.Deactivation profile of methylphenidate hydrochloride and loxapine succinate dosage forms.
Using activated carbon is an effective technique to remove contaminants or pollutants from the water or air,but various factors can influence the adsorption capacity.Generally,factors such as the pH of the solution,pKa,hydrophobicity and molecular weight of the compound,and type of the activated carbon used may influence the adsorption of molecules to the activated carbon,and thus affect the deactivation capability of the system.The activated carbon present in our disposal system pouch is specific for the molecular size,as it is based on MAT12®Molecular Adsorption Technology[19].This renders the drug irretrievable by binding to it through a physical adsorption process[20].
The pH of the drug disposal system,comprising of activated carbon in water,was close to neutral(pH 6.8),and was found to remain unaffected by the addition of drugs(MPH and loxapine succinate).It has been reported that the optimal pH for maximum adsorption capacity is near 7[21].The results obtained in our study are in accordance with this,as more than 95%deactivation of the drugs was achieved within 8 h.
The hydrophobicity of the compound is another factor that determines the adsorption efficiency of the activated carbon,and thus affects the hydrophobic interaction between the activated carbon and the adsorbent[22,23].Westerhoff et al.[24]observed that the removal efficiency of the contaminants was dependent on the logKowvalues,which are indicators of the hydrophobicity of the molecules.In addition,another study found that the hydrophobic character of the compound also influences the uptake rate of the compound[25].The study determined that the adsorbent(polar compounds)and adsorbate(activated carbon)displayed van der Waals force of interaction toward each other,thus leading to a better adsorption capacity.Thus,hydrophobicity not only determines the adsorption capacity,but also influences the rate of adsorption to the activated carbon.In our study,MPH(logP:2.2)[26]and loxapine(logP:3.6)[27]were both moderately lipophilic compounds,and hence showed more than 99%deactivation after 24 h of interaction with the activated carbon(Fig.4).Our results were consistent with the previous studies presented in the literature[28].
The MPH used were in tablet form;this could have led to faster adsorption to activated carbon compared to that of capsules.Solid dosage forms like capsules may require more dissolution time in water before adsorption can occur;this could cause a slight delay in the rate of adsorption of loxapine succinate capsules compared to that of MPH tablets.Previous research has noted the influence of molecular weight and hydrophobicity of the adsorbate on the adsorption capacity of activated carbon.In our study,we did not observe any significant differences in the adsorption capacity of the disposal system between these two model drugs.
The efficiency of the deactivation system to retain the adsorbed drug was further tested by examining the desorption.This study was aimed to simulate land fill situations which provide exposure to large volumes of water and some organic solvents.Our results showed that the activated carbon used in our study was efficient in adsorbing the drug,and did not release on exposure to these stress conditions.In the desorption study,we observed that no drug was leached out in the presence of water and,on an average,less than 1%of the drug was leached out in the presence of ethanol(Table 5).
The findings of the research indicated that the adsorption efficiency of the activated carbon was good,and it would not release the drug back into the environment when the contents of the pouch were present in the land fill,thereby providing a safer disposal method compared to other traditional alternative methods suggested by the FDA for drug disposal.This drug disposal pouch would therefore eliminate the risk of abuse of unused prescriptions,and also solve the problem of environmental and water pollution.Hence,the Deterra®activated carbon disposal system provides a simple and convenient way to dispose of these medications in normal trash,without causing any environmental or safety risks.
5.Conclusions
An isocratic RP-HPLC method for the determination of MPH and loxapine succinate was developed,and is precise and reliable.The regression line equation is capable of reliably predicting the drug concentration in the range of 5–100 μg/mL and 0.1–100 μg/mL for MPH and loxapine succinate,respectively,from the peak area obtained.The stability assessments revealed that both drugs were stable in water at 25 °C,4 °C and-20 °C for one week.The method was successfully validated and allowed the reliable,sensitive,robust,and specific detection of MPH and loxapine succinate in a common marketed preparation.
This method was then used to test the efficiency of an activated carbon-based drug disposal system for adsorption of MPH and loxapine succinate from dosage forms to activated carbon.The system was very efficient,with more than 99%drug deactivation achieved after 24 h,and less than 0.5%of the drug was released from activated carbon by an extraction protocol that mimicked a land fill situation.
Thus,this drug disposal system offers a simple and safe method to be used by patients.These results are encouraging,and provide the basis of an environmentally friendly method of drug disposal.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The current research project was funded by Verde Technologies(Minnetonka,MN,USA)as an SBIR Phase II contract from the National Institute on Drug Abuse(NIDA).Title:In-Home Deactivation System for Psychoactive Drugs(SBIR Phase 2),Contract no.HHSN271201400068C NIDA Reference no.N44DA-14-4420.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- LC and LC–MS/MS studies for the identification and characterization of degradation products of acebutolol
- Gas chromatography-mass spectrometry method for determination of β-propiolactone in human inactivated rabies vaccine and its hydrolysis analysis
- Detection and determination of undeclared synthetic caffeine in weight loss formulations using HPLC-DAD and UHPLC-MS/MS
- Highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study
- Long-term stability of gentamicin sulfate-ethylenediaminetetraacetic acid disodium salt(EDTA-Na2)solution for catheter locks
- Cytotoxic effect of Rosa canina extract on human colon cancer cells through repression of telomerase expression
