Synthesis of S-doped Sb2O3 Visible Light-driven Photocatalyst and Its Facets-dependent Performance for the Degradation of Methyl Orange①
2018-11-22XUEHunLINXinYiCHENYiLanCHENQingHuaLIUXinPingQIANQingRongCollegeofEnvironmentalSieneandEngineeringFujianNormalUniversityFuzhou350007ChinaFujianKeyLaoratoryofPollutionControlResoureReuseFuzhou350007ChinaFujianProvini
XUE Hun LIN Xin-Yi CHEN Yi-Lan CHEN Qing-Hua ② LIU Xin-Ping QIAN Qing-Rong ②a (College of Environmental Siene and Engineering,Fujian Normal University, Fuzhou 350007, China) (Fujian Key Laoratory of Pollution Control & Resoure Reuse, Fuzhou 350007, China) (Fujian Provinial Key Laoratory of Eology-toxiologial Effets & Control for Emerging Contaminants, Putian University, Putian, Fujian 351100, China)

S-doped Sb2O3nanocrystals were synthesized using SbCl3and thioacetamide (TAA)as starting materials by a hydrothermal method.TAA dosage can affect the preferential growth direction of Sb2O3, and further influences its photocatalytic activity.The preferential growth of {130} facet is unfavorable for the photocatalytic activity of Sb2O3.
1 INTRODUCTION
Semiconductor photocatalysts have received extensive attention because of their abilities to utilize solar energy and reduce environmental pollution.However, many semiconductors, such as TiO2, ZnO and SrTiO3, can only be excited by ultraviolet (UV)light due to their broad bandgaps, which thus lowers the solar energy utilization efficiency[1-3].Efforts have been made to prepare visible light photocatalysts by modifying the wide-bandgap semiconductors, such as various metal and non-metallic element doping, surface coating with precious metals, semiconductor complexation, surface photosensitization,and inorganic-organic hybridization[4-12].sulfur (S), a non-metal element, is often used to dope widebandgap semiconductors like TiO2, ZnO and Zn2SnO4and so on, to achieve visible light responsive photocatalysts[4-7].
Photocatalytic reaction is a series of physical and chemical processes taking place on the surface of a catalyst.Different crystal faces possess different atomic arrangements and electronic structures, which directly affects the energy level structures of valence band and conduction band, as well as the migration of photo-generated carriers and surface energy of the semiconductor, and consequently influences the adsorption and activation of the molecules involved in the reaction on catalyst surface[13-15].Therefore, in addition to its instinct properties, the photocatalytic property of a photocatalytic semiconductor also depends on the exposed facets to different degrees,e.g.the facet effect[16-18].For example, it has been reported that the high-energy crystal face of anatase TiO2, {001} facet, contains more coordination unsaturated titanium ions and more active sites,which is favorable for photocatalytic reactions[19,20].Monoclinic BiVO4exhibits high photocatalytic activities due to the preferential growth of {010}facet[21].
Sb2O3has been used as flame retardants, fillers,catalysts, photocatalysts, optical materials, etc[22-25].It is also a wide-bandgap semiconductor with the ability to degrade organic contaminants under UV light illumination[25].In the present work, S-doped Sb2O3nanocrystals were synthesized by a hydrothermal method using SbCl3and thioacetamide (TAA)as the raw materials.The doping amount of S and the preferential growth direction were tuned by adjusting the amount of TAA.The visible light photocatalytic properties of the S-doped Sb2O3nanocrystals were investigated with the photocatalytic degradation of methyl orange (MO) as a model reaction.
2 EXPERIMENTAL
2.1 Synthesis
The S-doped Sb2O3nanocrystals were synthesized by a hydrothermal method using SbCl3and thioacetamide (TAA) as the precursors.In a typical procedure, 3 mmol SbCl3was added to a 100 mL Teflon-lined stainless-steel autoclave reactor containing 70 mL deionized water, mechanically stirred for 15 min, and mixed with a set amount (2,3, 4, 5, or 6 mmol) of TAA under constant stirring.The pH of the precursor solution was adjusted to 12 using a NaOH solution.The precursor solution was heated at 120 ℃ in an oven for 12 h.The produced precipitate was washed with distilled water and absolute ethanol for several times and dried in air at 70 ℃.Finally, samples Sb2O3–S–n were obtained,where n stands for the starting TAA amount.A control Sb2O3sample was prepared in the absence of TAA using the similar procedure.Briefly, 3 mmol SbCl3was dissolved in 50 mL deionized water and 20 mL absolute ethanol in a 100 mL Teflon-lined stainless-steel autoclave reactor under vigorous stirring and heated at 120 ℃ for 12 h.
2.2 Characterization
Phase identification of Sb2O3and Sb2O3–S–n (n= 2, 3, 4, 5 and 6) was conducted with a Bruker D8 Advance X-ray diffractometer using CuKα radiation operated at the accelerating voltage of 40 KV and the applied current of 40 mA.The transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) images were taken by a JEOL model JEM 2010 EX instrument operated at the accelerating voltage of 200 kV.The sample powder was ultrasonically dispersed in ethanol and a drop of the suspension was dropped on a carbon film coated on a 3 mm diameter fine-mesh copper grid.The ultravioletvisible diffuse reactance spectra (UV-vis DRS)were collected on a UV-vis spectrometer (Cary 500 Scan Spectrophotometers, Varian, USA) using BaSO4as the reflectance standard.X-ray photoelectron spectra (XPS) were recorded on a PHI Quantum 2000 XPS System equipped with a monochromatic AlKα source and a charge neutralizer.The C1s peak at 284.8 eV of the surface adventitious carbon was used as the reference for all binding energies.Raman scattering spectra were obtained using a Renishaw inVia Raman microscope at room temperature.
2.3 Photocatalytic activity measurements
A 500 W halogen lamp (Philips Electronics) was positioned beside a cylindrical reaction vessel with a plane side as the visible light source.Two cut-off filters of 420 and 800 nm were placed between the lamp and vessel to ensure only visible light pass to reach the vessel.The vessel was maintained at room temperature by circulating water.The photocatalyst(80 mg) was powdered and added to the vessel containing 80 mL 3 × 10-5mol/L MO aqueous solution.The mixture was stirred in the dark for 1 h to reach the adsorption/desorption equilibrium on the photocatalyst and then exposed to the visible light.A 4 mL suspension was taken at certain time intervals and centrifuged.The supernatant was collected and measured with a Shimadzu UV-1750 UV-Vis-NIR spectrophotometer.The absorbance at the maximum absorption was recorded.
3 RESULTS AND DISCUSSION
The XRD patterns of both Sb2O3and S doped Sb2O3, Sb2O3–S–n (n = 2, 3, 4, 5 and 6), shown in Fig.1 are indexed to Sb2O3(JCPDS card 11–0689).The diffraction peak at 2θ value of 28.1º is attributed to the {130} facet of Sb2O3, and the diffraction peaks of {121} and {040} facets overlap at 2θ values of 28.5.The diffraction peak intensity of {130} facet decreases first with the increase of TAA dosage, reaches the minimum at 4 mmol TAA,and increases as the TAA dosage further increased.Table 1 lists the areas of these two peaks obtained at different TAA dosages, where A1and A2represent the peak area of {130} and the total peak areas of{121} and {040} facets, respectively.As can be seen, the lowest peak area ratio A1/A2is obtained at the TAA dosage of 4 mmol, and the ratio increases as the TAA dosage either increases or decreases,suggesting that the preferential growth direction of Sb2O3can be controlled by simply adjusting the TAA dosage.Meanwhile, the intensities of all characteristic XRD peaks of Sb2O3were higher than those of Sb2O3–S–n (n = 2, 3, 4, 5 and 6), indicating that TAA inhibited the growth of Sb2O3crystallite.
TEM image reveals that Sb2O3–S–n (n = 2 and 4)are rod-shaped nanoparticles with the diameter of 40 nm (Figs.2a and 2b).The lengths of Sb2O3–S–4 nanoparticles are in the range of 100~200 nm,shorter than those of Sb2O3–S–2 (150~250 nm).The HRTEM image revealed that the nanorods consisted of many randomly assembled nanoparticles and clear lattice fringes of these two samples were observed (Fig.2c and 2d).The lattice spacing of {200}, {131} and {130} facets of Sb2O3–S–2 was measured to be 0.25, 0.27 and 0.32 nm, respectively (Fig.2c).The lattice fringes of Sb2O3–S–4 with lattice spacing of 0.25 and 0.27 nm are attributed to the {200} and {131} facets (Fig.2d).These results suggest that more {130} facet is exposed in Sb2O3–S–2, consistent with the XRD results.

Fig.1.XRD patterns of Sb2O3 and Sb2O3–S–n (n = 2, 3, 4, 5 and 6)

Table 1.Peak Areas as Estimated from XRD Patterns of Different Face Sb2O3–S–n (n = 2, 3, 4, 5 and 6),where A1 and A2 Represent the Peak Area of {130} and the Total Peak Area of {121} and {040} Facets, respectively

Fig.2.TEM images of (a) Sb2O3–S–2, (b) Sb2O3–S–4, and HRTEM images of (c) Sb2O3–S–2, (d) Sb2O3–S–4
To investigate the photochromatic properties of S-doped Sb2O3nanocrystals, the UV-vis DRS of Sb2O3and Sb2O3–S–n (n = 2, 3, 4, 5 and 6) were measured.As shown in Fig.3, Sb2O3exhibits the absorption edge at 380 nm, indicating that it can only be excited by UV light.Compared with that of pure Sb2O3, the absorption band edge of Sb2O3–S–n(n = 2, 3, 4, 5 and 6) is red-shifted dramatically to the visible light region.It can be explained that the S 3p orbital is hybridized with the valence band of Sb2O3which broadens the valence band, and thus decreases the bandgap, causing the ref-shift of absorption edge[26,27].The absorption intensity of the S-doped Sb2O3in visible light region increases with the increment of TAA dosage because the doping amount of S is increased by more S2-formed at higher TAA dosage.
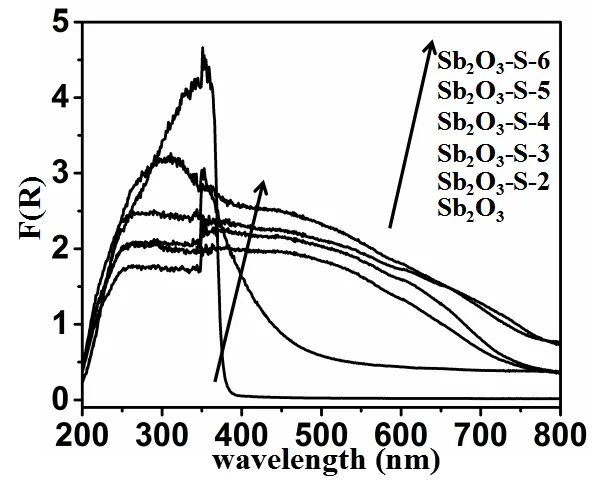
Fig.3.Diffuse reflectance absorption spectra of Sb2O3 and Sb2O3–S–n (n = 2, 3, 4, 5 and 6)
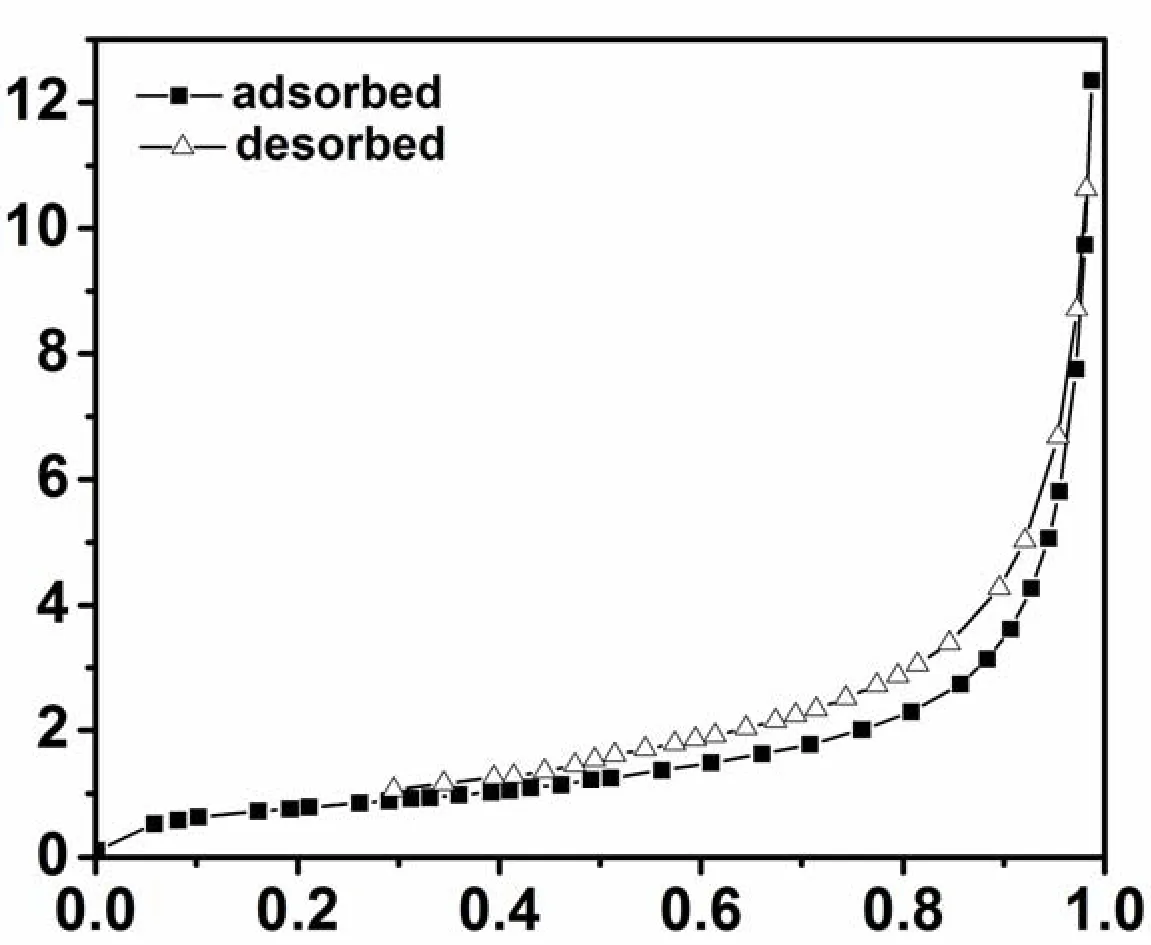
Fig.4.Nitrogen adsorption-desorption isotherm for Sb2O3–S–4
O3consists of the Sb 3d 3/2 binding energy peak at 539.8 eV and the overlapped peak of Sb 3d 5/2 and O 1s at 530.5 eV, indicating that the oxidation state of Sb in Sb2O3is +3 (Fig.5)[28,29].The S doping shifts both peaks to the lower energy region,and the shifts increase with the increase of TAA dosage.It can be explained that S is more electronically negative than O.The S doping increases the electron density around Sb, and thus decreases its binding energy.A higher amount of TAA results in a higher amount of S doped in Sb2O3, which increases the electron density around Sb, and reduces its bonding energy more significantly.However, the S doping exhibits no effects on the oxidation state of Sb.The binding energies of S in Sb2O3–S–2 are 161.3 and 162.5 eV, which is similar to that in Sb2O3–S–4 (161.1 and 162.3 eV), as shown in their high-resolution S 2p XPS spectra (Fig.5b),indicating that S is in the form of S2-in Sb2O3[30,31].However, the S 2p peak shifts towards the low binding energy region with the increase of TAA dosage, which is also attributed to the higher amount of S doped in Sb2O3at higher TAA dosages.Since the radius of S atom (180 pm) is larger than that of O atom (140 pm), the direct substitution of O with S is extremely difficult.Therefore, it can be deduced that S2-may be doped in the lattice space of Sb2O3[32].

Fig.5.XPS spectra of Sb2O3 and Sb2O3–S–n (n = 2 and 4): a Sb 3d, b S 2p
To further confirm that S is doped in the lattice space of Sb2O3crystal, Sb2O3and Sb2O3–S–4 were characterized with Raman spectroscope.As shown in Fig.6, Sb2O3exhibits Raman peaks at 216, 257,293, 442, 498, 593 and 680 cm-1.In addition to these characteristic peaks of Sb2O3, a new peak appears at 1440 cm-1in the Raman spectrum of Sb2O3–S–4 due to the S doping.Compared with those of Sb2O3, the full widths at half maximum of the Raman peaks of Sb2O3–S–4 are broader and the peak symmetry deteriorates, suggesting that S2-enters into the lattice space of Sb2O3and enhances the crystallographic defects of Sb2O3crystal[33].
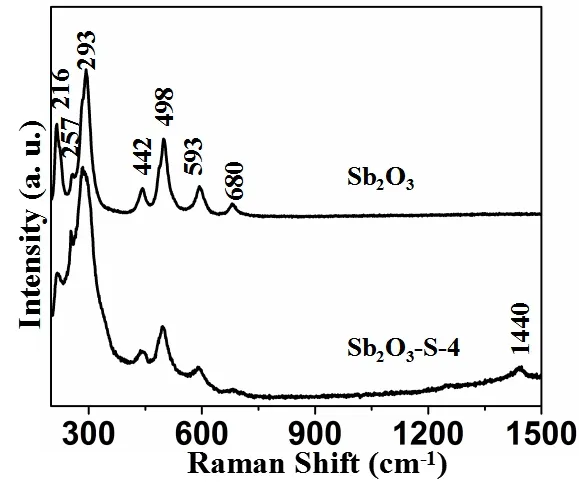
Fig.6.Raman spectra of Sb2O3 and Sb2O3–S–4
The visible-light-driven photocatalytic activities of Sb2O3–S–n (n = 2, 3, 4, 5 and 6) were explored using the degradation of MO as a model.The temporal concentration changes of MO over Sb2O3and Sb2O3–S–n (n = 2, 3, 4, 5 and 6) were monitored by measuring the UV-vis absorption of MO solution at 464 nm to determine the catalytic activities (Fig.7).No degradation of MO was observed in the absence of catalyst.Since the Sb2O3could not be excited under visible light, the MO degradation was negligible in the presence of Sb2O3.All Sb2O3–S–n (n = 2, 3, 4, 5 and 6) samples exhibit obvious catalytic activities for the MO degradation under visible light illumination and Sb2O3–S–4 shows the best catalytic performance with the degradation efficiency up to 99.2% in 40 min.It has been well accepted that large specific areas are conducive to catalytic activity because of the large contact areas between catalyst and reactants.However, the specific surface area of Sb2O3–S–4 is the smallest, compared with those of other S-doped Sb2O3samples, yet exhibits the best catalytic performance, suggesting that S doping is the major contributor to the photocatalytic activity and it is not affected by the specific surface area[34].As demonstrated by Raman analysis, the S doping can enhance the crystallographic defects of Sb2O3,which is conducive to the separation of photogenerated electrons and holes of Sb2O3, and thus improves its visible light photocatalytic performance.However, excessive S doping can cause too many defect sites that may become the recombination centers of photogenerated electrons and holes,and thus affect the catalytic activity.These explain that the highest photocatalytic activity was achieved with Sb2O3–S–4.It is worth noting that the area ratio between the diffraction peak of {130} and that of {121} and {040} facets (overlapped peak) of Sb2O3–S–n (n = 2, 3, 4, 5, and 6) (A1/A2) is directly related to its photocatalytic activity.Smaller A1/A2ratios result in higher photocatalytic activities,indicating that the preferential growth of {130}crystal is unconducive to the photocatalytic activity.The absorption of MO at 464 nm is attributed to its–N=N–.The absorption decreases as the reaction proceeded over Sb2O3–S–4 (Fig.7b), suggesting that –N=N is cleaved by the photocatalytic reaction[35].

Fig.7.(a) Temporal changes of concentration of MO monitored by the UV-vis absorption spectra at 464 nm on Sb2O3 and Sb2O3–S–n (n = 2, 3, 4, 5 and 6); (b) Temporal absorption spectral patterns of MO during the photodegradation process over Sb2O3–S–4
4 CONCLUSION
In the present work, S-doped Sb2O3nanocrystals were prepared with SbCl3and thioacetamide (TAA)by a hydrothermal method and characterized.Its photocatalytic activity was explored using the degradation of MO as a model reaction.It is found that the dosage of TAA can affect the preferential growth direction of Sb2O3and S enters the lattice space of Sb2O3in the form of S2-and enhances the crystallographic defect.The S doping extends the light absorption edge of Sb2O3to the visible light region, which endows its photocatalytic activities under visible light illumination.Among the S-doped Sb2O3sample prepared with different TAA dosages,Sb2O3–S–4 exhibits the best photocatalytic performance with 99.2% MO degraded in 40 min under optimal conditions.Based on these results, it can be concluded that the preferential growth of{130} facet is unfavorable for the photocatalytic activity of Sb2O3.
REFERENCES
(1) Fresno, F.; Portela, R.; Suarez, S.; Coronado, J.M.Photocatalytic materials: recent achievements and near future trends.J.Mater.Chem.A2014, 2,2863-2884.
(2) Seo, H.K.; Shin, H.S.Study on photocatalytic activity of ZnO nanodisks for the degradation of rhodamine B dye.Mater.Lett.2015, 159,265-268.
(3) Zhu, L.J.; Xue.H; Xiao, L.R.; Chen, Q.H.Characterizations and photocatalytic activities of nanocrystalline MTiO3(M = Sr, Pb, Co) prepared via a general self-propagating combustion method.Chin.J.Struct.Chem.2012, 31, 1852-1860.
(4) Lin, Y.H.; Hsueh, H.T.; Chang, C.W.; Chu, H.The visible light-driven photodegradation of dimethyl sulfide on S-doped TiO2: characterization,kinetics, and reaction pathways.Appl.Catal.B- Environ2016, 199, 1-10.
(5) Poongodi, G.; Mohan, K.R.; Jayavel, R.Influence of S doping on structural, optical and visible light photocatalytic activity of ZnO thin films.Ceram.Int.2014, 40, 14733-14740.
(6) Lin, Y.H.; Chou, S.H.; Chu, H.A kinetic study for the degradation of 1,2-dichloroethane by S-doped TiO2under visible light.J.Nanopart.Res.2014, 16, 1-12.
(7) Lin, Y.X.; Lin, S.; Luo, M.H.; Liu, J.H.Enhanced visible light photocatalytic activity of Zn2SnO4via sulfur anion-doping.Mater.Lett.2009, 63,1169-1171.
(8) Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y.Visible-light photocatalysis in nitrogen-doped titanium oxides.Science2001, 293,269-271.
(9) Wei, M.; Wan, J.M.; Hu, Z.W.; Wang, B.; Peng, Z.Q.Synthesis, electron transfer and photocatalytic activity of TiO2nanotubes sensitized by meso-tetra (4-carboxyphenyl) porphyrin under visible-light irradiation.Rsc Adv.2015, 5, 58184-58190.
(10) Cai, J.B.; Wu, X.Q.; Li, S.X.; Zheng, F.Y.; Zhu, L.C.; Lai, Z.H.Synergistic effect of double-shelled and sandwiched TiO2@Au@C hollow spheres with enhanced visible-light-driven photocatalytic activity.Acs Appl.Mat.Interfaces2015, 7, 3764-3772.
(11) Virkutyte, J.; Jegatheesan, V.; Varma, R.S.Visible light activated TiO2/microcrystalline cellulose nanocatalyst to destroy organic contaminants in water.Bioresour.Technol.2012,113, 288-293.
(12) Dai, G.P.; Yu, J.G.; Liu, G.Synthesis and enhanced visible-light photoelectrocatalytic activity of p−n junction BiOI/TiO2nanotube arrays.J.Phys.Chem.C2011, 115, 7339-7346.
(13) Jiang, Z.Y.; Kuang, Q.; Xie, Z.X.; Zheng, L.S.Syntheses and properties of micro/nanostructured crystallites with high‐ energy surfaces.Adv.Funct.Mater.2010, 20, 3634-3645.
(14) Yongfu, S.; Fengcai, L.; Shan, G.; Bicai, P.; Jingfang, Z.; Yi, X.Atomically thin tin dioxide sheets for efficient catalytic oxidation of carbon monoxide.Angew.Chem.Int.Ed.2013, 52, 10569-10572.
(15) Sun, Y.F.; Liu, Q.H.; Gao, S.; Cheng, H.; Lei, F.C.; Sun, Z.H.; Jiang, Y.; Su, H.B.; Wei, S.Q.; Xie, Y.Pits confined in ultrathin cerium(IV)oxide for studying catalytic centers in carbon monoxide oxidation.Nat.Commun.2012, 4, 2899-8.
(16) Long, J.L.; Chang, H.J.; Gu, Q.; Xu, J.; Fan, L.Z.; Wang, S.C.; Zhou, Y.G.; Wei, W.; Huang, L.; Wang, X.X.; Liu, P.; Huang, W.Gold-plasmon enhanced solar-to-hydrogen conversion on the {001} facets of anatase TiO2nanosheets.Energy Environ.Sci.2014, 7, 973-977.
(17) Ma, X.C.; Dai, Y.; Guo, M.; Huang, B.B.Relative photooxidation and photoreduction activities of the {100}, {101}, and {001} surfaces of anatase TiO2.Langmuir2013, 29, 13647-13654.
(18) Xing, M.Y.; Yang, B.X.; Yu, H.; Tian, B.Z.; Bagwasi, S.; Zhang, J.L.; Gong, X.Q.Enhanced photocatalysis by Au nanoparticle loading on TiO2single-crystal (001) and (110) facets.J.Phys.Chem.Lett.2013, 4, 3910-3917.
(19) Lei, C.X.; Jiang, X.L.; Huang, X.; Liu, X.; Zeng, D.Q.; Ma, Y.T.; Wang, L.S.; Peng, D.L.Improved liquid phase deposition of anatase TiO2hollow microspheres with exposed {001} facets and their photocatalytic activity.Appl.Surf.Sci.2015, 359, 860-867.
(20) Liu, H.; Dong, X.N.; Liu, T.T.; Lv, M.X; Yuan, Y.M.; Hao, Y.F.; Zhu, Z.F.Additive-free and novel synthesis of TiO2colloidal-assembled rice-like mesoporous structures: exposed {001} facets and enhanced photocatalytic activity.Mater.Lett.2015, 157, 222-224.
(21) Zhao, G.S.; Liu, W.; Li, J.Y.; Lv, Q.Y.; Li, W.X.; Liang, L.T.Facile synthesis of hierarchically structured BiVO4oriented along (010) facets with different morphologies and their photocatalytic properties.Appl.Surf.Sci.2016, 390, 531-539.
(22) Zhou, J.; Zheng, C.H.; Yang, Y.; Guo, L.Facile synthesis of novel nest-shaped Sb2O3micro/nanostructures and their optical properties.Rsc Adv.2016, 6, 89799-89802.
(23) Li, L.; Chai, S.H.,; Binder, A.; Brown, S.; Veith, G.M.; Dai, S.Catalytic CO oxidation over gold nanoparticles: support modification by monolayer-and submonolayer-dispersed Sb2O3.Catal.Lett.2014, 144, 912-919.
(24) Wang, J.J.; Feng, L.J.; Chao, X.L.Feng, Y.N.Performance of room temperature vulcanized (RTV) silicone rubber-based composites:DBDPO/RTV and DBDPE/Sb2O3/RTV.J.Macromol.Sci.2012, 51, 1245-1250.
(25) Zhu, L.J.; Xue, H.; Xiao, L.R.Chen, Q.H.Preparation and photocatalytic performance of cubic Sb2O3nanocrystalline.Chin.J.Inorg.Chem.2012, 28, 2165-2169.
(26) Umebayashi, T.; Yamaki, T.; Yamamoto, S.; Miyashita, A.; Tanaka, S.; Sumita, T.; Asai, K.Sulfur-doping of rutile-titanium dioxide by ion implantation: Photocurrent spectroscopy and first-principles band calculation studies.J.Appl.Phys.2003, 93, 5156-5160.
(27) Li, X.H.; Lu, J.B.; Dai, Y.; Guo, M.; Huang, B.B.The synthetic effects of iron with sulfur and fluorine on photoabsorption and photocatalytic performance in codoped.Int.J.Photoenergy2012, 4, 1-7.
(28) Liu, D.N.; He, G.H.; Zhu, L.; Zhou, W.Y.; Xu, Y.H.Enhancement of photocatalytic activity of TiO2nanoparticles by coupling Sb2O3.Appl.Surf.Sci.2012, 258, 8055-8060.
(29) He, G.H.; Liang, C.J.; Ou, Y.D.; Liu, D.N.; Fang, Y.P.;Xu, Y.H.Preparation of novel Sb2O3/WO3photocatalysts and their activities under visible light irradiation.Mater.Res.Bull.2013, 48, 2244-2249.
(30) Yang, H.M.; L, Mei.; Fu, L.J.; Tang, A.D.; Stephen, M.Controlled assembly of Sb2S3nanoparticles on silica/polymer nanotubes: insights into the nature of hybrid interfaces.Sci.Rep-UK.2013, 3, 1-10.
(31) Chen, H.N.; Zhu, L.Q.; Liu, H.C.; Li, W.P.Efficient iron sulfide counter electrode for quantum dots-sensitized solar cells.J.Power.Sources.2014, 245, 406-410.
(32) Lin, Y.X.; Lin, S.; Luo, M.H.;Liu, J.H.Enhanced visible light photocatalytic activity of Zn2SnO4via sulfur anion-doping.Mater.Lett.2009, 63,1169-1171.
(33) Arcibarorozco, J.; Bandosz, T.Visible light enhanced removal of a sulfur mustard gas surrogate from a vapor phase on novel hydrous ferric oxide/graphite oxide composites.J.Mater.Chem.A2014, 3, 220-231.
(34) Xue, H.; Li, Z.H.; Wu, L.; Ding, Z.X.; Wang, X.X.; Fu, X.Z.Nanocrystalline ternary wide band gap p-block metal semiconductor Sr2Sb2O7:?hydrothermal syntheses and photocatalytic benzene degradation.J.Phys.Chem.C2008, 112, 5850-5855.
(35) Li, W.J.; Li, D.Z.; Zhang, W.J.; Hu, Y.; He, Y.H.; Fu, X.Z.Microwave synthesis of ZnxCd1−xS nanorods and their photocatalytic activity under visible light.J.Phys.Chem.C2011, 114, 2154-2159.
杂志排行
结构化学的其它文章
- Synthesis and Characterization of a Palladium Complex Supported by Bidentate Ligand and Catalysis of the Vinyl Polymerization of Norbornene①
- Structural, Electronic, Optical and Thermodynamic Properties of Nanolaminated Boride Cr4AlB6①
- Synthesis, Structure and Photochromism of a New Diarylethene and Its Ag(I) Complex①
- Ionothermal Synthesis, Structure and Luminescent Properties of a New 2-D Bismuth(III) Coordination Polymer with (6,5)-Connected Topological Sheet①
- Synthesis, Crystal Structure and Properties of a 1D Heteronuclear Cobalt-sodium Polymer with Bridging Ligand 2-(2-Hydroxy-3-methoxybenzylidene)Hydrazinecarbothioamide①
- Synthesis, Crystal Structure and Photoluminescence of a Dinulear Copper Complex①
