Synthesis, Structure and Photochromism of a New Diarylethene and Its Ag(I) Complex①
2018-11-22HANJingHANJiCiYUZhongZANGYnJINBingDeprtmentofMterilsPhysisndChemistryXiUniversityofTehnologyXi710048ChinDeprtmentofAppliedChemistryXiUniversityofTehnologyXi710048ChinShoolofAeronutisNorthwesternPolytehnilUniversity
HAN Jing HAN Ji-Ci YU Zhong ZANG Yn JIN Bing (Deprtment of Mterils Physis nd Chemistry,Xi’n University of Tehnology, Xi’n 710048, Chin) (Deprtment of Applied Chemistry, Xi’n University of Tehnology, Xi’n 710048, Chin) (Shool of Aeronutis, Northwestern Polytehnil University, Xi’n 710072, Chin)

A new symmetric diarylethene (BM-3-CP-3-TP) was designed, synthesized and structurally characterized successfully.BM-3-CP-3-TP showed color conversion between colorlessness and blue in different solvents and PMMA film in response to the alternative photo-stimulation of 254 nm light and ≥550 nm visible light.In addition,the reaction of AgBF4with BM-3-CP-3-TP generated a new complex 1 which exhibits normal photochromism in both solution and PMMA film.Integrating of Ag(I) ions into BM-3-CP-3-TP slightly modified the λmaxof the resulted complex 1.
1 INTRODUCTION
Photochromic diarylethenes are referred to as the most promising materials for their potential applications as versatile components of optical memory media, photonic switch devices, and photo-drive actuators attributable to their excellent thermal stabilities of both isomers (open and closed),sensitive photo-response, and high fatigue resistance[1-4].Photo-switches can be toggled back and forth between the two structurally and electronically different isomers via 6π electrocyclic reactions of the 1,3,5-hexatriene moieties in response to appropriate optical stimulations.After thirty years of developments since they were firstly synthesized by M.Irie in 1988[5], various diarylethenes with specific structural features had been designed and prepared to meet the requirements for future application as high density data storage including the high conversion rate of both photocyclization and photoreversion[6], non-destructive readout[7]and excellent thermal stabilities[8].
On the other hand, incorporation of metal ions into diarylethenes to construct multi-functional metal-organic frameworks (MOFs) was proved to be a rational strategy to realize the above-mentioned requirements.Based on the consideration, we have synthesized a series of coordination compounds by complexation of metal ions with diarylethenes to achieve photo-regulated magnetism[9], low energy readout[10], flurosence[11], and suppression of competitive side photoreaction[10].Although, theoretically, metal coordination will lead to the restraint of the rotation of thiophene group from the twisted ring-opening isomer to the coplanar ring-closed isomer, our results showed that most of the complexes show normal photochromism with only few exceptions[11,12].
As an extension of our work, herein we present the syntheses, structural characterization and photochromic properties of a new diarylethene and its Ag(I) coordination compound.1,2-Bis-[2΄-methyl-5΄-(3΄΄-cyanophenyl)-3΄-thienyl]perfluorocyolopentene (BM-3-CP-3-TP, Scheme 1) having two cyano groups was designed with the consideration of its potential coordination tendency to metal ions.Its crystal structure was analyzed by X-ray singlecrystal diffractions coupled with spectroscopic analysis.The photochromism of free ligand and its Ag(I) complex were further investigated both in solution and PMMA films.
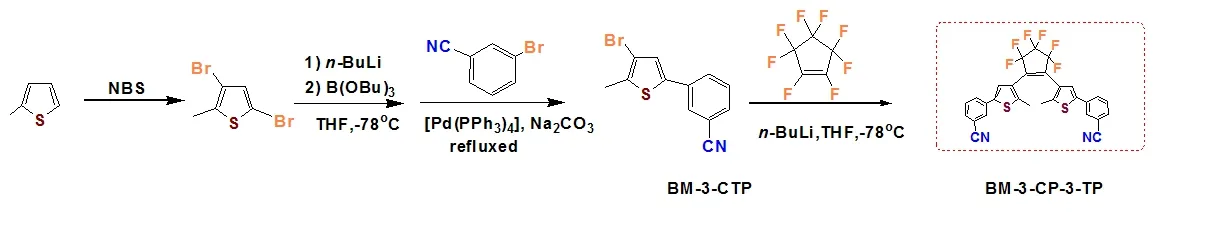
Scheme 1.Synthesis of BM-3-CP-3-TP
2 EXPERIMENTAL
2.1 Synthesis of BM-3-CP-3-TP (L)
BM-3-CP-3-TP was prepared according to a modified literature[15]by employing m-bromobenzonitrile in the place of p-bromobenzonitrile, as shown in Scheme 1.Anal.Calcd.for C29H16F6N2S2:C, 61.05; H, 2.83; N, 4.91%.Found: C, 61.30; H,2.66; N, 4.75%.IR (KBr pellet): 2958 (w), 2920(w),2584(w), 2226 (m), 1272 (s).1H NMR (CDCl3, 400 MHz), 7.804 (s, 1H, benzonitrile-H), 7.76 (d, 1H, J= 7.886, benzonitrile-H), 7.589 (d, 1H, J = 7.603,benzonitrile-H), 7.51(t, J = 7.788, benzonitrile-H),7.332(s, 1H, thiophene-H), 2.000(s, 3H, -CH3).Single crystals suitable for X-ray measurements were obtained by recrystallization from THF at room temperature.
2.2 Synthesis of [AgL]BF4∙2H2O (1)
Reaction of AgBF4(0.0290 g, 0.15 mmol) and BM-3-CP-3-TP (0.0571 g, 0.1 mmol) in benzene(10 mL) resulted in the formation of complex1as pale yellow solid (yield: 70.58 wt%).Crystals of1were recrystallized from its benzene solution by slow evaporation for 1 week.Anal.Calcd.for AgC29H20F10N2S2BO2: C, 43.47; H, 2.52; N, 3.50; S,8.00%.Found: C, 43.60; H, 2.37; N, 3.41; S, 7.62%.IR (KBr pellet): 2925 (w), 2258(m), 1272 (s),1061(s).ESI-MS (MeOH, positive mode): [Ag-CN]+H2O (m/z 147.9292), [Ag-(C6H5- CN)2]+H2O(m/z 331.0096), [AgL]+(m/z 678.9702), [Ag2L]2+H2O (m/z 802.8738), [Ag L2]+(m/z 1249.0289).
2.3 Structure determination
Single crystals of the colorless ligand (0.36mm ×0.30mm × 0.24mm) were used for data collection using Bruker Smart APEXIICCD with graphite-monochromated MoKα radiation (λ = 0.71073 Å, μ= 0.262 mm-1) at 296(2) K.A total of 2391 reflections were collected in the range of 2.20≤θ≤25.10º.The structure was solved by direct methods followed by subsequent Fourier calculations.The non-hydrogen atoms were refined anisotropically.The final cycle of full-matrix least-squares refinement was based on 1612 reflections.All calculations were performed using the SHELXL-97(Sheldrick, 2008)[14].The final R = 0.0808 and wR =0.2107 for 1612 observed reflections with I > 2σ(I),and R = 0.1114, wR = 0.2322 (R =Σ||Fo| – |Fc||/|Fo|and Rw = [Σw(Fo2– Fc2)2/w(Fo2)2]1/2) for all data.(Δρ)max= 0.799, (Δρ)min= –0.723 e·Å-3and S =1.059.Selected bond lengths and bond angles of the ligand are shown in Table 1.

Table 1.Selected Bond Lengths (Å) and Bond Angles (°)
2.4 Materials and instruments
All reagents and solvents were purchased from commercial sources and used as received without further purification.All reactions and manipulations during synthesis were carried out under a nitrogen atmosphere.Solvents were dried using standard procedures and distilled under a nitrogen atmosphere prior to use.
FT-IR spectra in KBr (4000~450 cm-1) were recorded using an IR Prestige-21 spectrophotometer.1H NMR spectra were measured on a Bruker INOVA-400MHz spectrometer in CDCl3solution at room temperature (with tetramethylsilane as internal reference).Absorption spectra in solution and PMMA film were measured on the Hitachi U-3900H spectrophotometer.Solvents were used as purchased and not degassed.The concentration of solution is ca.4 ×10-5mol/L.Photoirradiation was carried out using a 100 W Xe lamp in atmosphere,and light with appropriate wavelength was isolated by passing it through the RANYAN cut-off filters.The PMMA films of BM-3-CP-3-TP or1were prepared by dissolving them in THF at 70 ℃ (0.5 wt%) and draining on a quartz glass sheet.
3 RESULTS AND DISCUSSIONS
3.1 Crystal structure characterization of BM-3-CP-3-TP
The FT-IR spectrum of BM-3-CP-3-TP is illustrated in Fig.1.The vibration peaks of 2958,2920 and 2854 cm-1are assigned to the C–H bond vibrations.The typical C≡N stretching vibration peak appears at 2226 cm-1.The 1272 cm-1peak is assigned to the C–F stretching vibration.This information indicated that the BM-3-CP-3-TP is the same structure as designed.
Crystallization from THF led to the isolation of ligand as a non-solvated form.Fig.2 depicts the crystal structure of BM-3-CP-3-TP determined by X-ray crystallographic analysis.In the crystal,BM-3-CP-3-TP molecule is fixed in an anti-parallel conformation with the dihedral angel of 59.69ºbetween two thienyl rings.The two methyl groups on two thiophene rings are presented as transdirection relative to the thiophene ring plane,indicating a photo-reactive configuration.The distance between reactive carbon atoms, C(11) and C(11A), is 3.57 Å, which is short enough for the photocyclization reaction to take place in the crys-talline phase[16].The CºN distance is 1.144(7) Å.
The hexafluorocyclopentene ring is found to be planar.The dihedral angle between the mean plane of cyclopentene ring and the two thiophene rings is 48.87º.The dihedral angle between the mean plane of benzonirile ring and these two thiophene rings is 24.44º.The mean torsion angles between thiophene and hexafluorocyclopentene rings (C(13A)–C(13)–C(10)–C(11) and C(13)–C(13A)–C(10A)–C(11A))are the same to be –47.44º.
In the crystal of BM-3-CP-3-TP, independent molecules are arranged next to each other through intermolecular C–H…F hydrogen bonds, i.e.the contacts between F atoms of hexafluorocyclopentene and H atoms from benzonirile as well as methyl,forming a network as shown in Fig.3.In addition,intramolecular hydrogen bonding between F and thienyl H atoms also exists in1.The detailed D–H…A interactions and related angles are summarized in Table 2.These hydrogen bonds lead to a close packing of molecules.

Fig.1.IR spectra of BM-3-CP-3-TP and 1
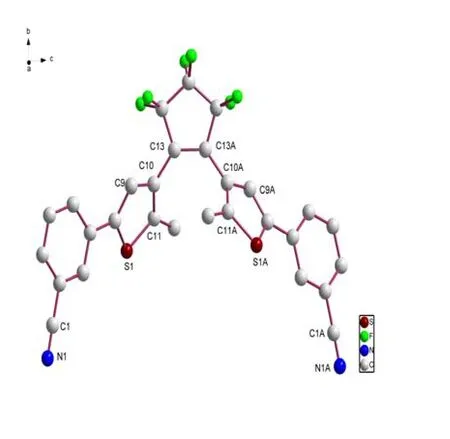
Fig.2.Crystal structure of BM-3-CP-3-TP.H atoms are omitted for clarity.A: 1–x, y, 0.5–z F
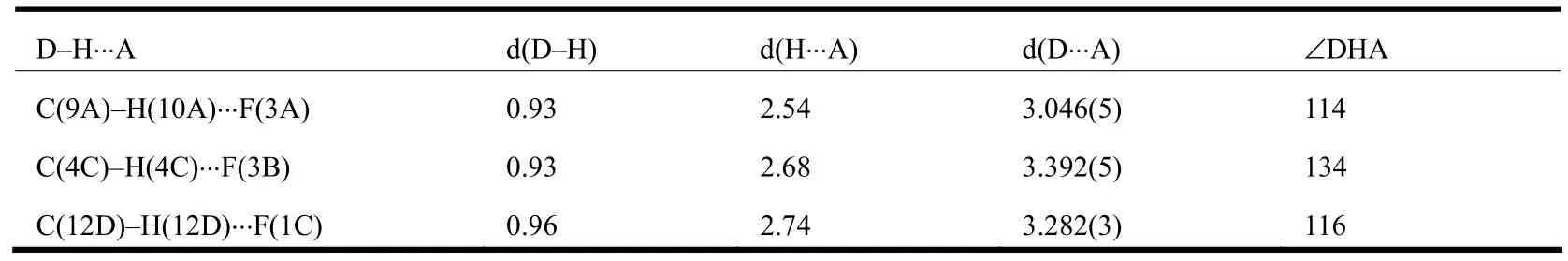
Table 2.Hydrogen Bond Lengths (Å) and Bond Angles (°)
3.2 Structural characterization of complex 1
The structure of complex1was determined by elemental analysis, FT-IR, MS and TG analysis.In the FT-IR spectrum of1(Fig.1), the typical peak of BF4¯ is observed at 1061 cm-1, suggesting the presence of the anion.The characteristic C≡N stretching vibration peak of ligand was found at 2258 cm-1for1, indicating the ligand is included into1.The significant shift of C≡N stretching is ca.+32 cm-1relative to the corresponding band in the free ligand (2226 cm-1), confirming a considerable effect from metal complexation[17-20].These observations proved the formation of complex1.
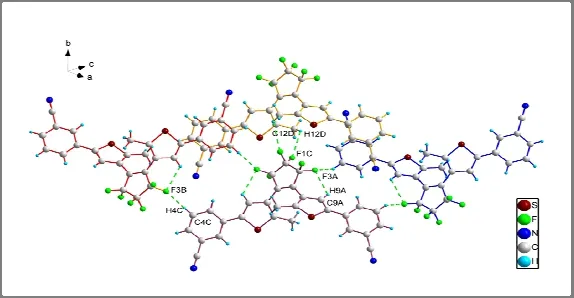
Fig.3.Packing structure of BM-3-CP-3-TP showing H bonds.(A: 1–x, y, 0.5–z; B: 0.5–x, 1.5–y, 1–z; C: x, y, 1 + z; D: x, 1 + y, 1 + z)
The mass spectrum of1indicates a peak (m/z =678.9702) corresponding to [AgL]+(Fig.4).The appearance of [Ag2L(H2O)]2+at m/z = 802.9738 and[Ag(CN)(H2O)]+at m/z = 147.9292 further confirmed the presence of water molecule in complex1.The detailed assignment is summarized in Table 3.
The thermal gravimetric analysis (TGA) of complex1revealed that the first-step weight loss of 4.56% (calcd.4.51%) from r.t.to 115 °C corresponds to the release of two H2O molecules per formula unit (Fig.5).Since the liberation of coordinated water usually occurs in relative higher temperature[21], the two H2O molecules in complex1are determined as crystallization water.Further heating reveals a continuous weight loss, corresponding to the release of ligand to give the residue.
Based on the above data and elemental analysis results, it can be concluded that: (1) BF4-is involved in complex1; (2) two H2O molecules present as crystallization solvents; and (3) complex1is probably a binuclear complex and its formula is determined as [AgL]BF4·2H2O.
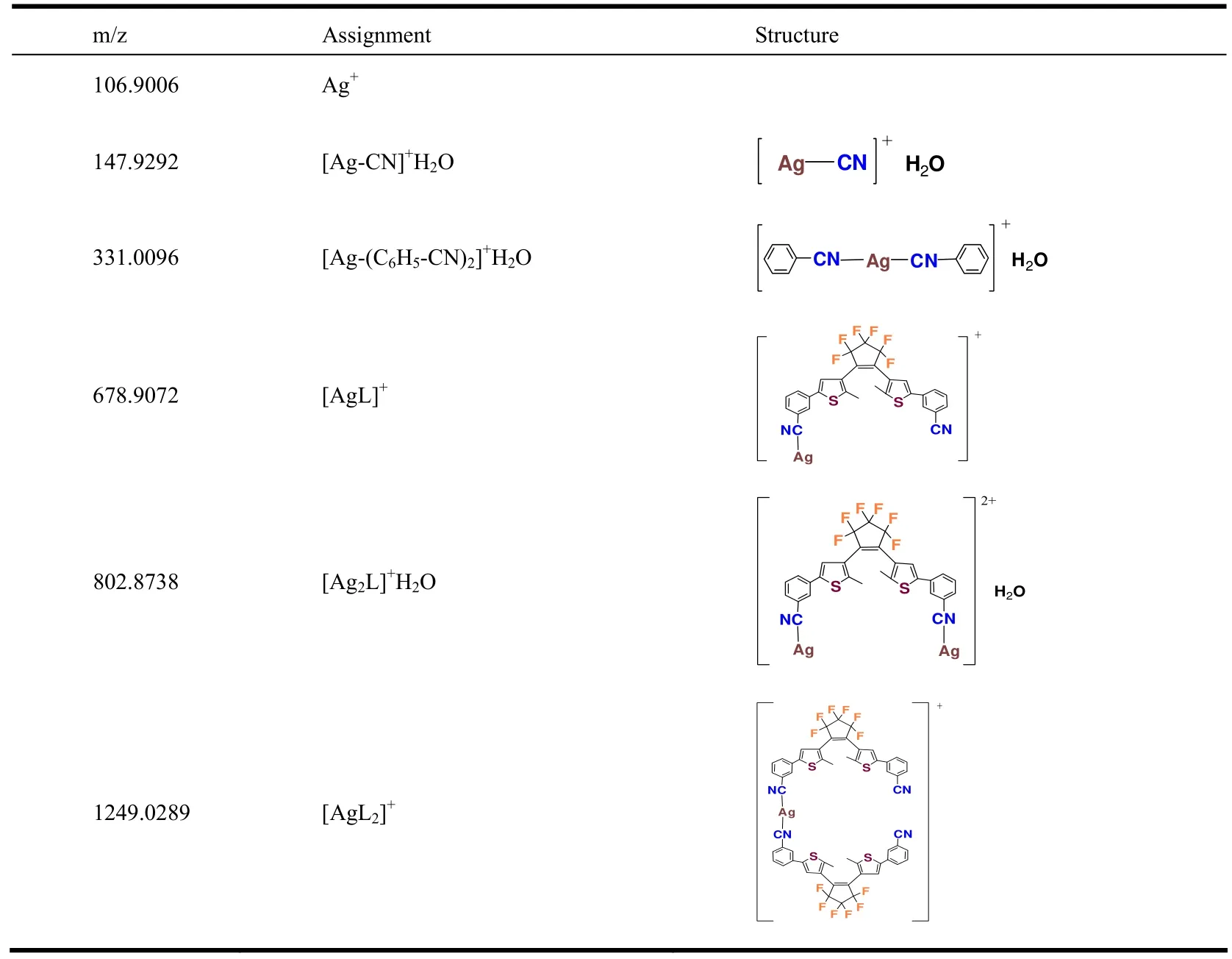
Table 3.ESI-MS Data of Complex 1

Fig.4.ESI-MS of complex 1 in MeOH

Fig.5.TG curve of complex 1
3.3 Photochromism of BM-3-CP-3-TP
3.3.1 Photochromism in solutions
The photochromism of ligand is firstly examined in THF and its UV-vis spectra are shown in Fig.6(a).Before photo-irradiation, the maximum absorption of the pale yellow solutionis 380 nm, which is ascribed to the intraligand π→π* and n→π* transitions of the thiophene and benzonirile rings.Upon UV irradiation with 254 nm light, new band at 585 nm in visible region emerged in the absorption spectrum.The intensity of the new band increased along with prolonged light irradiation until reaching PSS after 20 min photo-irradiation and the solution turned blue.The appearance of 585 nm band indicated the photocyclization of open-form to closedform.The e is calculated to be 6319 L∙mol-1∙cm-1.

Fig.6.UV-Vis spectra of BM-3-CP-3-TP in THF (c = 4 × 10-5 mol/L)
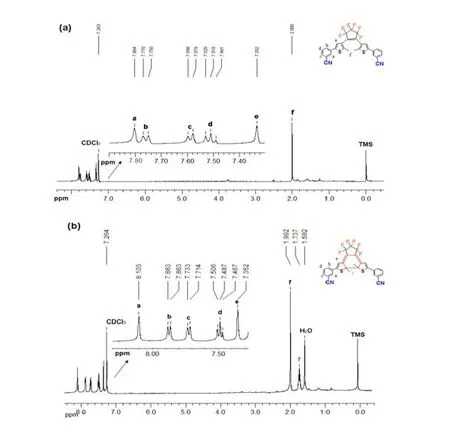
Fig.7.1H NMR spectrum of BM-3-CP-3-TP in open-form (a) and PSS (b)
To confirm the above ring-closure reaction,1H NMR investigations were further applied in both open-form and PSS (Fig.7).The CDCl3solution1H NMR spectrum of BM-3-CP-3-TPin open-form showed four resonances in the range from 7.332 to 7.804 ppm, characteristic of the benzonitrile H atoms, one resonance assigned to thienyl H atoms(7.332 ppm) and one strong methyl H signal (2.000 ppm).These signals also further proved the structure of BM-3-CP-3-TP.While in the1HNMR spectrum of BM-3-CP-3-TP in PSS, in addition to the minute shifts of previous six proton signals, the new signal at 1.737 ppm assigned to H signal of the closed-form was observed[22], indicating that the open-form was converted to the closed-form.
Upon excitation into the PSS with l > 550 nm light, absorptions due to the closed-form weakened gradually and the solution recovered to the original pale yellow indicative of the regeneration of the open form as a result of photo-cycloreversion (Fig.6(b)).Thus, BM-3-CP-3-TP absorbs UV light (254 nm)and undergoes efficient conversion to its ring-closed counterpart with the visual color change of a THF solution from pale yellow to blue.Visible light (>550 nm) triggers the reverse reaction and regenerates the original species.
The photochromism of BM-3-CP-3-TP in solutions with different polarity was further investigated.The reversible photochromism was observed successsfully in n-hexane, petroleum ether and methanol, respectively.The maximum absorption due to close-ring form is 575, 580, and 595 nm for nhexane, petroleum ether and methanol, respec- tively.The order of polarity of solvents is the same as that of λmaxof the close-form, suggesting a positive effect of solvents on photochromism[23].
3.3.2 Photochromism in PMMA film
In order to realize practical applications, photochromic materials must be easily shaped into coatings, monoliths or any other desired forms by various techniques.Therefore, a polymeric material is preferred and the photochromism of BM-3-CP-3-TP was examined in PMMA films.As anticipated, BM-3-CP-3-TP underwent reversible ring-closure and ring-opening reactions in PMMA films, accompanying the films color converting between initial colorless and photo-induced blue.The lmaxof photo-excited closed-ring isomer is centered at 580 nm (Fig.8).
In addition, good reversibility and reproducibility were observed for BM-3-CP-3-TP after 10 cycles of coloration and decoloration.The excellent fatigue resistance indicated a potential application as practical material.
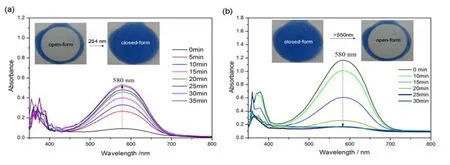
Fig.8.UV-Vis spectra of BM-3-CP-3-TP in PMMA film.The pattern of the PMMA image is developed by photo-irradiating the open-form film with 254 nm light followed by irradiation upon visible light with a circular mask
3.4 Photochromism of complex 1
3.4.1 Photochromism in THF
The photoreactions of complex1were performed in THF and monitored by UV-vis spectrometer (Fig.9).The initial solution is colorless and it has a maximum absorption at ca.370 nm in UV-vis spectrum attributable to the intraligand π→π* and n→π* transitions.Ring closing of1o(where “o” refers to the open-ring form) can be triggered by direct photo-excitation with 254 nm light, accompanying the color of1oturning blue.Cyclization of1oto1c(where “c”refers to the closed-ring form)was observed by the appearance of a new strong band of λmax= 590 nm in the UV-vis absorption spectrum.The newly appeared absorptions are characteristic of the ring-closed ligand isomer (585 nm).The blue color is ascribed to the photogenerated closed-ring form1c.Irradiation of1cwith visible light (> 550 nm)regenerated1oand the blue turned back to colorless.These phenomenon and spectral changes clearly suggested that the metal complexation doesn’t prohibit the photochromism of ligand.Ag(I)coordination modifies slightly the photochromic behavior of ligand and causes a small red shift in the spectroscopic properties of the closed-ring form(590 nm) compared to the free ligand in the same solvent (585 nm, THF).Besides, it took longer time for1to complete the ring-closure but shorter time for ring-opening reaction in solution.And the evalue of 3490 L∙mol-1∙cm-1for complex1is smaller than that of free ligand.These evidences probably indicate the photochromism of1was constrained to some extent.

Fig.9.UV-Vis spectra of complex 1 in THF (c = 3 × 10-5 mol/L)
3.4.2 Photochromism in PMMA film
Complex1also underwent the expected reversible photoisomerization in PMMA film, with keeping the same lmaxof photo-induced closed isomer in THF (Fig.10).Its photo-fatigue resistance was further examined and the absorbance of photo-induced isomer retained almost unchangeable after 10 cycles of coloration and decoloration,indicating a stable photo-fatigue resistance.
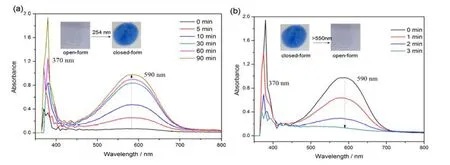
Fig.10.UV-Vis spectra of complex 1 in the PMMA film
4 CONCLUSION
A new symmetric diarylethene (BM-3-CP-3-TP)bearing two cyano groups was designed, synthesized and structurally characterized successfully.X-ray single-crystal diffraction analysis reveals the two thienyl rings present as photo-reactive anti-parallel configuration and the carbon distance of photocyclization is 3.57 Å.BM-3-CP-3-TP showed color conversion between colorless and blue in different solvents and PMMA film in response to the alternative photo-stimulation of 254 nm light and ≥550 nm visible light.In addition,reaction of AgBF4with BM-3-CP-3-TP generated a new complex1which exhibits normal photochromism in both solution and PMMA film,demonstrating the complexation doesn’t prevent the photochromism of ligand.Integrating of Ag(I) ions into BM-3-CP-3-TP slightly modified the λmaxof resulted complex1.Both BM-3-CP-3-TP and complex1displayed good photo-fatigue resistance.
REFERENCES
(1) Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S.Photochromism of diarylethene molecules and crystals: memories, switches, and actuators.Chem.Rev.2014, 114, 12174–12277.
(2) Kobatake, S.; Yamada, M.; Yamada, T.; Irie, M.Photochromism of 1,2-bis(2-methyl-6-nitro-1-benzothiophen-3-yl)perfluorocyclopentene in a single-crystalline phase: dichroism of the closed-ring form isomer.J.Am.Chem.Soc.1999,121, 8450–8456.
(3) Irie, M.Diarylethenes for memories and switches.Chem.Rev.2000, 100, 1685–1716.
(4) Lucas, L.N.; Esch, J.V.; Kellogg, R.M.; Feringa, B.L.Photocontrolled self-assembly of molecular switches.Chem.Commun.2001, 37,759–760.
(5) Irie, M.; Mohri, M.Thermally irreversible photochromic systems.Reversible photocyclization of diarylethene derivatives.J.Org.Chem.1998, 53,803–808.
(6) Matsuda, K.; Shinkai, Y.; Yamaguchi, T.; Nomiyama, K.; Isayama, M.; Irie, M.Very high cyclization quantum yields of diarylethene having two N-methylpyridinium ions.Chem.Lett.2003, 32, 1178–1179.
(7) Nakagawa, T.; Hasegawa, Y.; Kawai, T.Nondestructive luminescence intensity readout of a photochromic lanthanide(III) complex.Chem.Commun.2009, 45, 5630–5632.
(8) Morimitsu, K.; Shibata, K.; Kobatake, S.; Irie, M.Dithienylethenes with a novel photochromic performance.J.Org.Chem.2002, 67, 4574-4578.
(9) Han, J.; Maekawa, M.; Suenaga, Y.; Ebisu, H.; Nabei, A.; Kuroda-Sowa, T.; Munakata, M.Photochromism of novel metal coordination polymers with 1,2-bis(2΄-methyl-5΄-(carboxylic acid)-3΄-thienyl)perfluorocyclopentene in the crystalline phase.Inorg.Chem.2007, 46, 3313–3321.
(10) Munakata, M.; Han, J.; Maekawa, M.; Suenaga, Y.; Kuroda-Sowa, T.; Nabei, A.; Ebisu, H.Reversible MLCT switching of a copper(II)coordination polymer with 1,2-bis(2΄-methyl-5΄-(4΄΄-pyridyl)-3΄-thienyl)perfluorocyclopentene in crystalline phase.Inorg.Chim.Acta2007, 360,2792–2796.
(11) Han, J.; Zang, Y.; Yu, Z.; He, X.; Guo, P.Synthesis, photochromism and morphology changes of a new asymmetric fluorescent quaterthiophene.Tetra.Lett.2015, 56, 5213–5217.
(12) Han, J.; Li, S.; Yu, Z.; Chen, H.; Zang, Y.; Wang, Q.J.A photochromic AgBF4complex with cis-1,2-dicyano-1,2-bis(2΄,4΄,5΄-trimethyl-3΄-thienyl)ethene: synthesis, structure and photochromism.Inorg.Chim.Acta2017, 464, 11–17.
(13) Cromer, D.T.; Waer, J.T.International Tables for X-ray Crystallography, Vol IV, The Kynoch Press, Birmingham, England1974.
(14) Sheldrick, G.M.SHELXTL.Version 6.10, Bruker AXS Inc., Madison, Wisconsin, USA2008.
(15) Liu, G.; Tu, Q.D.; Zhang, Q.; Fan, C.B.; Yang, T.S.1,2-Bis[5-(4-cyanophenyl)-2-methyl-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene: a photochromic diarylethene compound.Acta Cryst.2008, E64, o938.
(16) Kobatake, S.; Uchida, K.; Tsuchida, E.; Irie, M.Single-crystalline photochromism of diarylethenes: reactivity-structure relationship.Chem.Commun.2002, 38, 2804–2805.
(17) Maxim, L.K.; Ekaterina, A.K.; Andrei, I.D.Theoretical study on reactivity of coordinated nitriles: structure, bonding and reactivity of[ReCl4(NCCH3)2].J.Mol.Struc-Theochem.2004, 671, 229–237.
(18) Fillaut, J.L.; Dua, N.N.; Geneste, F.; Toupet, L.; Sinbandhit, S.Nitrile ligands for controlled synthesis of alkynyl-ruthenium based homo and hetero bimetallic systems.J.Organomet.Chem.2006, 691, 5610–5618.
(19) Alexandra, L.G.; Uwe, R.Effect of counteranions in self-assembled silver(I)-coordination polymers of 4-aminobenzonitrile.Cryst.Growth Des.2012, 12, 854–861.
(20) Maxim, L.K.Is the charge on the nitrile carbon atom a driving force of the nucleophilic addition to coordinated nitriles? J.Mol.Struc-theochem.2004, 674, 33–42.
(21) Quan, C.Y.; Han.J.; Yu, Z.; Gao, Q.Synthesis and properties of photochromic cobalt(II) complex.J.Xian Univ.Tech.2014, 30, 331–335.
(22) Cao, D.K.; Feng, J.Q.; Ren, M.; Gu, Y.W.; Song, Y.A mononuclear cobalt(II)-dithienylethene complex showing slow magnetic relaxation and photochromic behavior.Chem.Commun.2013, 49, 8863–8865.
(23) Chen, Y.; Zeng, D.X.Study on photochromic diarylethene with phenolic Schiff base: preparation and photochromism of diarylethene with benzoxazole.J.Org.Chem.2004, 69, 5037–5040.
杂志排行
结构化学的其它文章
- Synthesis and Characterization of a Palladium Complex Supported by Bidentate Ligand and Catalysis of the Vinyl Polymerization of Norbornene①
- Structural, Electronic, Optical and Thermodynamic Properties of Nanolaminated Boride Cr4AlB6①
- Ionothermal Synthesis, Structure and Luminescent Properties of a New 2-D Bismuth(III) Coordination Polymer with (6,5)-Connected Topological Sheet①
- Synthesis, Crystal Structure and Properties of a 1D Heteronuclear Cobalt-sodium Polymer with Bridging Ligand 2-(2-Hydroxy-3-methoxybenzylidene)Hydrazinecarbothioamide①
- Synthesis, Crystal Structure and Photoluminescence of a Dinulear Copper Complex①
- Iodoplumbate(II)-based Hybrid Templated by 1,4-Diazabicyclo[2.2.2]octane Derivative: Structure,Photocurrent Response Behavior and Photocatalytic Activity for the Degradation of Organic Dye①
