Synthesis, Crystal Structure and Properties of a 1D Heteronuclear Cobalt-sodium Polymer with Bridging Ligand 2-(2-Hydroxy-3-methoxybenzylidene)Hydrazinecarbothioamide①
2018-11-22LIYuLinLIWeiLIChngHongTANXiongWenDeprtmentofChemistryndMterilsSieneHengyngNormlUniversityHengyng421008ChinDeprtmentofChemilEngineeringHunnInstituteofTehnologyHengyng421002ChinKeyLortoryofFuntionlMetlorgniCompoundso
LI Yu-Lin LI Wei, LI Chng-Hong TAN Xiong-Wen (Deprtment of Chemistry nd Mterils Siene,Hengyng Norml University, Hengyng 421008, Chin) (Deprtment of Chemil Engineering, Hunn Institute of Tehnology, Hengyng 421002, Chin) (Key Lortory of Funtionl Metl-orgni Compounds of Hunn Provine, Hengyng 421008, Chin)

A new cobalt-sodium coordination polymer [CoNa(C9H9N3O2S)2·H2O]2·C2H3N·H2O with cobalt chloride, 2-(2-hydroxy-3-methoxybenzylidene)hydrazinecarbothioamide and sodium hydroxide, has been synthesized and characterized, and its electrochemical and fluorescent properties were also studied.
1 INTRODUCTION
Schiff base and related metal complexes have experienced long standing applications in biology and medicine[1,2], as well as in the catalysis of chemical and petrochemical processes[3,4].Thiosemicarbazone is an important class of Schiff base compounds derived from pyridine[5–7].Thiosemicarbazones and related metal complexes with sulfur and nitrogen functions arise from their significant antifungal, antiprotozoal, antibacterial, and anticancer activity[8,9].Researches based on this ligand are extremely more on the dioxovanadate(V) complexes[10,11],but less investigated on the cocalt(III)-sodium complex[12,13].In this contest, we have designed and synthesized a new cobalt-sodium coordination polymer [CoNa(C9H9N3O2S)2·H2O]2·C2H3N·H2O (1) with 2-(2-hydroxy-3-methoxybenzylidene)hydrazinecarboth ioamide (L).Preliminary results of the electrochemical and fluorescent properties of1are also reported.
2 EXPERIMENTAL
2.1 Materials and instruments
All reagents were of analytical grade and used as obtained from commercial sources and used without further purification.2-(2-Hydroxy-3-methoxybenzylidene)hydrazinecarbothioamide (L) was prepared previously[14].Crystal structure determination was carried out on a Bruker SMART APEX CCD singlecrystal diffractometer.Elemental analyses were performed on a Perkin-Elmer 2400 elemental analyzer.The IR spectra were recorded on a Bruker Vector22 FT-IR spectrophotometer using KBr discs.The luminescence spectra for the powdered samples were measured on an RF-5301PC spectrofluorometer with a xenon arc lamp as the light source.The electrochemical properties were measured on an EC550 electrochemical workstation from Wuhan.
2.2 Synthesis of 1
0.1 mmol of Co(NO3)2·6H2O (29.11 mg) dissolved in 0.6 mL methanol, 0.2 mmol ofL(45.054 mg)dissolve in mixed solvents with 0.4 mL DMF and 5 mL acetonitrile and 0.3 mmol of NaOH (12.0 mg)were dissolved in 15 mL reaction flask and covered with cap, and reacted for 4 hours at 80 ℃.Finally,red block crystals suitable for X-ray diffraction were obtained, which were washed with distilled water.Yield: 43.60%, m.p.: 291.0~293.0 ℃.Anal.and Calcd.(%) for C38H45Co2N13Na2O11S4: C, 39.62; H,3.94; N, 15.81.Found (%): C, 39.50; H, 3.93; N,15.87.Selected IR data (KBr pellet, cm-1): 3443(m),3280(m), 2922(w), 2824(w), 1579(vs), 1488(vs),1436(vs), 1292(s), 1217(s), 1139(m), 1104(m),967(m), 855(w), 735(m), 598(w), 540(w), 479(w),428(w).
2.3 Determination of the crystal structure
A single crystal with dimensions of 0.25mm ×0.22mm × 0.20mm was put on a Bruker SMART APEX CCD diffraction equipped with a graphitemonochromatic MoKα radiation (l = 0.71073 Å)using a φ-ω scan mode at 296(2) K.A total of 18921 reflections were collected in the range of 2.95≤θ≤26.50°, of which 3964 were independent (Rint=0.0683) and 5577 were observed (I > 2s (I)).All data were corrected by Lp factors and empirical absorption.The crystal structure was solved directly by program SHELXS-97, and refined by program SHELXL-97[15].The hydrogen and non-hydrogen atoms were corrected by isotropic and anisotropic temperature factors respectively through full-matrix least-squares method.The final R = 0.1091, wR =0.2412 (w = 1/[σ2(Fo2) + (0.0670P)2+ 14.1660P],where P = (Fo2+ 2Fc2)/3), (∆/σ)max= 0.000, S = 1.115,(∆ρ)max= 1.23 and (∆ρ)min= –0.80 e·Å-3.
3 RESULTS AND DISCUSSION
3.1 Crystal structure of 1
The molecular structure of1is revealed in Fig.1,and the 1D chain structure of1is shown in Fig.2.The selected bond lengths and bond angles are shown in Table 1.
[CoNa(C9H9N3O2S)2·H2O]2·C2H3N·H2O (1) is a heteronuclear molecular complex with space group.As illustrated in Fig.1, the unit structure is inclusive of one cobalt ion, one sodium ion, twoLions, one acetonitrile molecule and two water molecules, forming a heteronuclear complex.Each Co(III) is six-coordinated by two oxygen atoms, two nitrogen atoms and two sulfur atoms from twoLmolecules.In the CoN2S2O2octahedron, N(1), S(1),N(4) and O(2) locate at the equator plane, but O(4)and S(2) occupy the axial positions.Bond angles N(1)–Co(1)–S(1), S(1)–Co(1)–N(4), N(4)–Co(1)–O(2)) and O(2)–Co(1)–N(1) are 86.9(2), 91.5(2),88.8(2) and 93.1(2)º, respectively, with their sum to be 360.3º (close to 360º), suggesting a planar nature of S(1), O(2), N(1), N(4) and Co(1) (Plane equation:–6.824x + 4.582y + 6.766z = 0.1331, mean deviation from the plane is 0.0720 Å, and the Co··Co distance is 7.036 Å).Each cobalt center has ONS donor sets from the ligand forming two six-membered rings(Co(1), N(1), C(8), C(6), C(7), O(4) and Co(1), N(4),C(16), C(15), and C(17) and O(4)) and two five-membered rings (Co(1), N(1), N(2), C(9), S(1)and Co(1), N(4), N(5), C(18), S(2)) The mean deviations from the planes of six- and five-membered rings are 0.1124 and 0.0376 Å, which increased the stability of the cobalt complex[16].The average Co–O bond length is 1.951 Å, while Co–N is 1.908 Å,which is slightly longer than that of the similar Co/Na complex [CoNa(C23H28N2O4)(NCS)2(H2O)(Co–O = 1.8957, Co–N = 1.90705 Å)][17].Each Na(I)is five-coordinated by four oxygen atoms from twoLand one water molecule respectively, and one nitrogen atom fromL.For Na(1), τ = 0.32(θ1– θ2)/60,where θ1(O(5)–Na(1)–O(1) = 152.1(2)º) is the largest angle and θ2(O(5)–Na(1)–O(2) = 132.9(3)º)is the second largest angle in the coordination sphere.τ = 0 for ideal square pyramids, τ = 1 for ideal trigonal pyramids[18], and the Na··Na distance is 5.803 Å.TheLcan be described as tridentate (N(1),S(1), O(2) or N(4), S(2), O(4)) to each cobalt, and monodentate and bidentate to sodium (N(5), O(1),O(2))[19,20].As illustrated in Fig.2, a one-dimensional chain coordination polymer was formed through bridging the N(5) atom.

Table 1.Selected Bond Lengths (Å) and Bond Angles (°) of the Complex
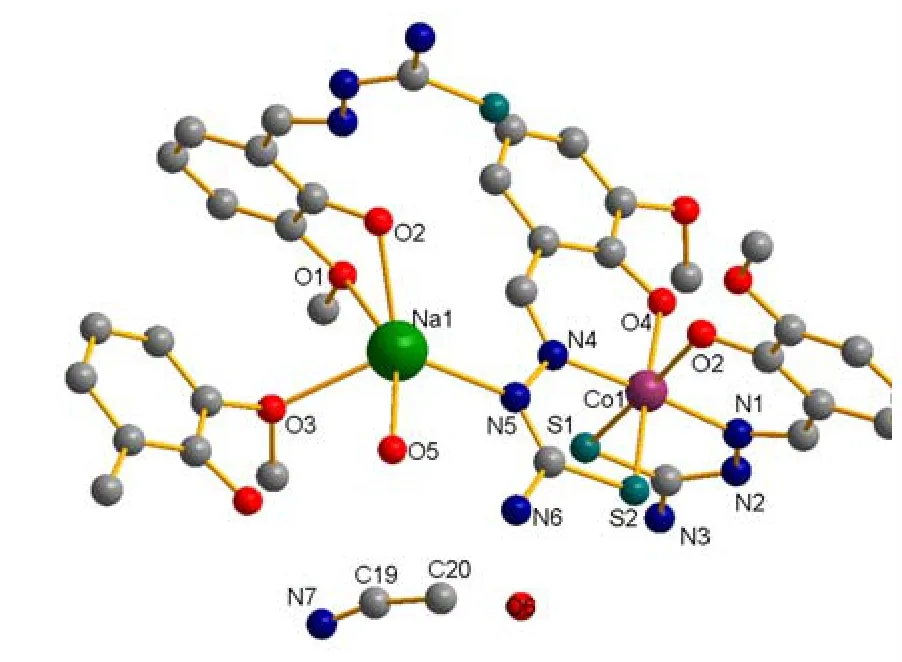
Fig.1.Molecule structure of complex 1

Fig.2.1D chain structure of complex 1
3.2 Electrochemical properties
We employed a conventional three-electrode system for the cyclic voltammetric measurement(CV), where an Ag/AgCl electrode, a glassy carbon electrode and a platinum electrode as the reference electrode, the working electrode and the counter electrode, respectively, were chosen.1was dissolved in THF, and the resulting solution has a concentration of 4 × 10-5mol/L.A NaClO4solution of 3 × 10-3mol/L is used as the supporting electrolyte.The scan range was –0.60~1.20 V.The cyclic voltammograms of1and theligand at a potential scan rate of 0.05 V·s-1are shown in Fig.3.There exists an oxidation peak with the oxidation potential of 0.555 V of the complex, and the ligand is not, which corresponds to the CoII/CoIIIoxidation process[21,22].
3.3 Fluorescent property
Coordination polymers have been reported to have the ability to adjust the emission wavelength of organic materials through the incorporation of metal centers.It is of great significance to investigate the fluorescent properties of coordination polymers in view of their potential applications as light-emitting diodes (LEDs).The emission spectra of Co(III)complex1and the ligandwere studied in the solid state at room temperature and depicted in Fig.4.There are emission bands at 466 nm (λex= 376 nm)for1.Such fluorescence emissions may be assigned to intra-ligand π-π* transitions, since the free L ligand exhibits a similar broad emission at 389 nm under 300 nm excitation.The emission band of complex1is 77 nm red-shifted, compared with the L ligand, which is attributed to the coordination interactions between the metal atom and the ligand,and such emission bands may be tentatively assigned to ligand-to-metal charge transfer (LMCT)[23].

Fig.3.Cyclic voltammogram of 1 and the ligand in THF (scan rate: 0.05 V·s-1)

Fig.4.Spectra of complex 1 and the ligand
REFERENCES
(1) (a) Fouad, D.A.; Bayoumi, A.; ElGahami, M.A.; Ibrahim,S.A.; Hammam, A.M.Synthesis and thermal studies of mixed ligand complexes of Cu(II),Co(II), Ni(II) and Cd(II) with mercaptotriazoles and dehydroacetic acid.Coord.Chem.Rev.1974, 13, 101–132.(b) Wu, L.M.; Li, Q.; Jin, L.F.;Zhou, Z.Q.Synthesis and structure of the acylhydrazone Schiff base.Chin.J.Struct.Chem.2010, 9, 1399–1403.
(2) (a) Cristina, T.; Ciriano, T.A.Catalysis and organometallic chemistry of rhodium and iridium in the oxidation of organic substrates.Organometallic Oxidation Catalysis2006, 97–124.(b) Jin, R.Y.; Sun, X.H.; Liu, Y.F.; Chen, B.; Shen, S.Q.; Ma, H.X.Synthesis, structure and biological activity of 5-benzyl-4-amino-1,2,4-triazole-3-thione Schiff base.Chin.J.Struct.Chem.2014, 2, 253–257.(c) Johnson, D.K.; Murphy, T.B.; Rose, N.J.;Goodwin, W.H.; Pickart, L.Cytotoxic chelators and chelates1.Inhibition of DNA synthesis in cultured rodent and human cells by aroylhydrazones and by a copper(II) complex of salicylaldehyde benzoyl hydazone.Inorg.Chim.Acta1982, 67, 159–16.
(3) (a) Tembe, G.L.; Ganeshpure, P.A.Catalytic oxyfunctionalization of alkanes by manganese and ruthenium clusters.Reaction Kinetics and Catalysis Letters1999, 67, 83–88.(b) Li, F.R.; Zhang, X.; Wang, D.D.; Xue, Z.N.; Ma, Y.K.Syntheses, crystal structures and antibacterial activities of Schiff base ligand and its nickel(II) complex.Chin.J.Struct.Chem.2014, 9, 1367–1374.
(4) (a) Dutta, S.K.; Samanta, S.; Mukhopadhyay, S.; Burckel, P.; Pinkerton, A.; Chaudhury, M.Spontaneous assembly of a polymeric helicate of sodium with LVO2units forming the strand: photoinduced transformation into a mixed-valence product.Inorg.Chem.2002, 41, 2946–2952.(b) Hu, J.F.;Qin, Y.R.; Li, Y.; Liu, W.; Wang, Y.; Li, Y.H.Syntheses, structures, and luminescent properties of new Zn(II)–Y(III) and Zn(II)–Ln(III) Schiff base complexes.Chin.J.Struct.Chem.2015, 8, 1273–1280.
(5) Mendes, I.C.; Botion, L.M.; Ferreira, A.V.M.; Castellano, E.E.; Beraldo, H.Vanadium complexes with 2-pyridineformamide thiosemicarbazones:in vitro studies of insulin-like activity.Inorg Chim.Acta2009, 362, 414–420.
(6) Majid, R.; Hassan, K.Schiff base and non-Schiff base macrocyclic ligands and complexes incorporating the pyridine moiety- the first 50 years.Coord.Chem.Rev.2014, 280, 203–253.
(7) Lian, Q.Y.; Hu, H.N.; Li, C.H.; Li, D.P.; Jiao, X.Y.; Li, Y.X.A new tetranuclear cubane-like Ni(II) complex based on Schiff-base ligand: synthesis,crystal structure and magnetic properties.Chin.J.Struct.Chem.2017, 36, 273–279.
(8) Yuan, S.; Zhang, Y.J.; Hou, J.Y.; Liu, Q.L.; Li, D.P.; Li, Y.X.Syntheses, crystal structures and luminescent properties of two Schiff base complexes.Chin.J.Struct.Chem.2016, 35, 965–972.
(9) Maurya, M.R.; Khurana, S.; Shailendra, A.A.; Zhang, W.J.; Rehder, D.Synthesis, characterization and antiamoebic studies of dioxovanadium(V)complexes containing ONS donor ligands derived from S-benzyldithiocarbazate.Eur.J.Inorg.Chem.2003, 1966–1973.
(10) Wang, S.Y.; Wang, W.M.; Zhang, H.X.; Shen, H.Y.; Jiang, L.; Cui, J.Z.; Gao, H.L.Seven phenoxido-bridged complexes encapsulated by 8-hydroxyquinoline Schiff base derivatives and beta-diketone ligands: single-molecule magnet, magnetic refrigeration and luminescence properties.Dolton Trans.2016, 45, 3362–3371.
(11) Koo, B.K.; Jang, Y.J.; Lee, U.Vanadium(IV) complexes with N,N,S-donor systems.Bull.Korean Chem.Soc.2003, 24, 1014–1016.
(12) Anson, C.W.; Ghosh, S.; Hammes-Schiffer, S.; Stahl, S.S.Co(salophen)-catalyzed aerobic oxidation of p-hydroquinone: mechanism and implications for aerobic oxidation catalysis.J.Am.Chem.Soc.2016, 138, 4186–4193.
(13) Ziegenbalg, S.; Horning, D.; Gorls, H.; Plass, W.Cobalt(II)-based single-ion magnets with distorted pseudotetrahedral[N2O2] coordination:experimental and theoretical investigations.Inorg.Chem.2016, 55, 4047–4058.
(14) Vrdoljak, V.; Cindric, M.; Milic, D.; Matkovic, D.; Novak, P.; Kamenar, B.Synthesis of five new molybdenum(VI) thiosemicarbazonato complexes.Crystal structures of salicylaldehyde and 3-methoxy-salicylaldehyde 4-methylthiosemicarbazones and their molybdenum(VI) complexes.Polyhedron2005, 24, 1717–1726.
(15) (a) Sheldrick, G.M.Program for Bruker Area Detector Absorption Correction.University of Göttingen: Göttingen, Germany1997.(b) Sheldrick, G.M.SHELXS-97, Program for Crystal Structure Solution.University of Göttingen: Göttingen, Germany1997.
(16) Armstrong, J.E.; Crossland, P.M.; Frank, M.A.; Van Dongen, M.J.; McNamara, W.C.Hydrogen evolution catalyzed by a cobalt complex containing an asymmetric Schiff-base ligand.Dolton Trans.2016, 45, 5430–5433.
(17) Kousik, G.; Harms, K.; Bauza, A.Heteronuclear cobalt(III)/sodium complexes with salen type compartmental Schiff base ligands: methylene spacer regulated variation in nuclearity.Dalton Transactions.A2018, 47, 331–347.
(18) Lu, Z.L.; Fan, T.; Guo, W.W.; Lu, J.; Fan, C.H.Synthesis, structure and magnetism of three cuban Cu(II) and Ni(II) complexes based on flexible Schiff-base ligand.Inorg.Chim.Acta2013, 400, 191–196.
(19) Yang, X.X.; Leng, J.D.; Liu, J.L.; Jia, J.H.; Tong, M.L.Synthesis, structures and magnetic properties of two tetranuclear and decanuclear mangan.clusters bearing the multidentate Schiff-base ligands.Chin.J.Inorg.Chem.2015, 31, 1831–1838.
(20) Niu, M.J.; Li, H.H.; Li, Z.; Li, X.; Dou, J.M.; Wang, S.DNA/protein interaction, cytotoxic activity and magnetic properties of amino-alcohol Schiff base derived Cu(II)/Ni(II) metal complexes: influence of the nuclearity and metal ions.RSC Adv.2015,5, 37085–37095.
(21) Xu, J.S.; Yuan, Y.L.; Li, W.; Deng, P.; H.; Deng, J.Carbon paste electrode modified with a binuclear mangan.Complex as a sensitive voltammetric sensor for tryptophan.Microchim.Acta2011, 174, 239–245.
(22) Li, W.; Li, C.H.; Kuang, Y.F.; Deng, P.H.; Zhang, S.; H.; Xu, J.S.A carbon paste electrode modified with a cobalt(II) coordination polymer for the direct voltammetric determination of tryptophan.Microchim.Acta2012, 176, 455–461.
(23) Liu, Q.; Li, C.H.; Li, D.P.; Li, J.; Li, Y.X.A Cd(II) coordination polymer constructed by 5-(4-(1H-tetrazol-5-yl)phenoxy)isophthalic acid: synthesis,crystal structure and luminescent properties.Chin.J.Inorg.Chem.2014, 30, 1367–1372.
杂志排行
结构化学的其它文章
- Synthesis and Characterization of a Palladium Complex Supported by Bidentate Ligand and Catalysis of the Vinyl Polymerization of Norbornene①
- Structural, Electronic, Optical and Thermodynamic Properties of Nanolaminated Boride Cr4AlB6①
- Synthesis, Structure and Photochromism of a New Diarylethene and Its Ag(I) Complex①
- Ionothermal Synthesis, Structure and Luminescent Properties of a New 2-D Bismuth(III) Coordination Polymer with (6,5)-Connected Topological Sheet①
- Synthesis, Crystal Structure and Photoluminescence of a Dinulear Copper Complex①
- Iodoplumbate(II)-based Hybrid Templated by 1,4-Diazabicyclo[2.2.2]octane Derivative: Structure,Photocurrent Response Behavior and Photocatalytic Activity for the Degradation of Organic Dye①
