Ionothermal Synthesis, Structure and Luminescent Properties of a New 2-D Bismuth(III) Coordination Polymer with (6,5)-Connected Topological Sheet①
2018-11-22FENGYuQuanCHENShuYangWANGLuXINGZhengZheng
FENG Yu-Quan CHEN Shu-Yang WANG Lu XING Zheng-Zheng
(College of Chemistry and Pharmacy Engineering,Nanyang Normal University, Nanyang 473061, China)

A new 2-D Bi3+coordination polymer {Bi4(μ3-O)2(μ4-O)2(C7H3NO4)2}n(1, C7H3NO4= pyridine-2,6-dicarboxylate) containing (6,5)-connected topological sheet with the Schläfli symbol of (44·63·84·124)(45·64·8) has been synthesized by an ionothermal method using the ionic liquid 1-ethyl-3-methylimidazolium bromide ([Emim]Br) as solvent, and characterized by C, H and N elemental analyses, energy-dispersive X-ray spectroscopy (EDS), infrared spectrum (IR spectrum) and single-crystal X-ray diffraction.The molecular structural units [Bi4(μ3-O)2(μ4-O)2(C7H3NO4)2] are connected to each other via bridging O atoms, extending into an interesting 2-D layered structure.These resulting layers are further assembled by weak C–H··O hydrogen bonds into a 3-D supramolecular architecture.Moreover, its thermal stability and the luminescent properties have been also studied.
1 INTRODUCTION
In recent years, the use of bismuth(III) coordination polymers in biological and clinical applications has been the object of an increased interest[1-3].These applications have led to many efforts toward the rational design and synthesis of bismuth(III)coordination polymers[4,5].Due to the fact that the large radii, hydrolysis, variable coordination numbers and fl exible coordination environments of Bi3+cations make it dif fi cult to control the reactions and obtain desired structures, which results in a limited number of bismuth(III) coordination polymers with novel structural features.Therefore, the design and assembly of novel bismuth(III) polymers are meaningful work.During the synthesis process, the judicious choice of organic ligands plays a crucial role.In view of their various coordination modes, the dicarboxylic acids are often used as bridging ligands to construct coordination polymers[6].For example,pyridine-2,6-dicarboxylic acid shows a large variation in its coordination behavior[7,8], in addition,engaging in O,N,O΄-chelation can also serve as a linker between the adjacent metal cations.As our continual studies[9-14], under the above considerations, employing pyridine-2,6-dicarboxylate (pdc2-)as ligand, our group has obtained a novel twodimensional Bi(III) coordination polymer {Bi4(μ3-O)2(μ4-O)2(C7H3NO4)2}n(1) containing (6,5)-connected topological net with the Schläfli symbol of(44·63·84·124)(45·64·8) under ionothermal conditions using the ionic liquid 1-ethyl-3-methylimidazolium bromide ([Emim]Br) as solvent.
2 EXPERIMENTAL
2.1 Instruments and reagents
Elemental analysis of C, H and N was performed with a Perkin Elmer 240 analyzer.Energy dispersive spectroscopy (EDS) analysis was performed on a FEI-Quanta 200 scanning electron microscope.IR spectrum of compound1was recorded on a Nicolet 5700 FT-IR spectrometer using KBr pellets in a range of 4000~400 cm-1.The thermal stabilities were performed on an STA449F3 differential thermal analyzer.The luminescent properties were measured on a FLS980 fluorescence spectrometer.Crystal diffraction data were collected on a Bruker SMART APEX-II CCD diffractometer equipped with a graphite-monochromated Mo-Kα radiation (l =0.71073 Å) at 296(2) K using an ω-φ scan mode.All chemicals were of reagent grade obtained from commercial sources and were used without further purification.
2.2 Synthesis of {Bi4(μ3-O)2(μ4-O)2(C7H3NO4)2}n 1
Compound1was prepared by means of an ionothermal method using the ionic liquid 1-ethyl-3-methylimidazolium bromide ([Emim]Br) as solvent.A mixture of Bi(NO3)3·5H2O (0.36 g, 0.75 mmol),NaN3(0.26 g, 4.0 mmol), pyridine-2,6-dicarboxylic acid (0.24 g, 1.5 mmol) and [Emim]Br (C6H11N2Br)(1.0 g, 5.23 mmol) was stirred for half an hour,transferred into a 50 mL Teflon-lined stainless-steel autoclave and heated at 413 K for 6 days.Then the mixture was cooled to room temperature at a rate of 10 k/hour, obtaining colorless needle-like crystals of1.The product was filtered off and dried in a vacuum desiccator at room temperature.Interestingly, compound1could not be prepared using 5.23 mmol H2O(0.094 g) as solvent instead of the ionic liquid[Emim]Br (1.0 g, 5.23 mmol).However, it is worth noting that1can be synthesized by using 5.23 mmol(1.15 g)1-butyl-3-methylimidazolium bromide(C8H15BrN2) as ionic liquid instead of 1-ethyl-3-methylimidazolium bromide under the same reaction conditions of 413 K and 6 days.It indicates that ionothermal synthesis is a promising technique for preparing metal coordination compound with new structural features.
2.3 X-ray crystallographic determination
A suitable single crystal of1(0.29mm × 0.24mm× 0.17mm) was used for structural analysis.Intensity data were collected on a Bruker APEX-II CCD detector at 296(2) K using an ω-φ scan mode and Mo-Kα radiation (λ = 0.71073 Å) in a range of 2.10≤q≤25.00ºwith –15≤h≤16, –4≤k≤4 and –19≤l≤14.A total of 4257 reflections (1607 independent reflections, Rint= 0.0420) were measured.Absorption correction was applied by using the SADABS[15].The structure was solved by direct methods and refined by full-matrix least-squares on F2using the SHELXTL-97 software[16].All of the non-hydrogen atoms were refined anisotropically.The organic hydrogen atoms were generated geometrically.The selected bond lengths and bond angles of1are listed in Table 1.
3 RESULTS AND DISCUSSION
3.1 EDS and elemental analyses
The energy-dispersive X-ray spectroscopy (EDS)results for a single crystal of1reveal the presence of the elements Bi, C, N and O.Elemental analyses (C,H and N) for C14H6N2O12Bi4, calculated: C, 13.66; H,0.49; N, 2.28%.Found: C, 13.95; H, 0.64; N, 1.97%.The results of EDS and elemental analyses are well in agreement with those from the single-crystal X-ray structure analysis.
3.2 Infrared spectroscopy
In the IR spectrum (400~4000 cm-1) of1, the peaks at 1665, 1262 and 913 cm-1can be attributed to the -COO-groups belonging to the pdc2-ligands.In the low-frequency region, a series of absorptions in the range of 1580~1010 cm-1(1580, 1416, 1371,1329, 1262, 1164, 1070 and 1010 cm-1) can be assigned to the pdc2-ligands[9,12].

Table 1.Selected Bond Lengths (Å) and Bond Angles (°) for 1
3.3 Crystal structure
Single-crystal X-ray diffraction analysis reveals that compound1crystallizes in the monoclinic space group P21/n.Fig.1-left shows its molecular structural unit.Within the structural unit, Bi(1)atom is seven-coordinated by one nitrogen and six oxygen atoms: one N and two O atoms from one tridentate pdc2-ligand, one O atom from the other tridentate pdc2-ligand, two μ3-O and one μ4-O atoms, leading to a distorted pentagonal-bipyramidal coordination geometry (BiNO6), where the two μ3-O atoms are located in the axial positions and other atoms are located in the equatorial plane,while Bi(2) atom is coordinated to two oxygen atoms belonging to two different pdc2-ligands, one μ3-O atom and three μ4-O atoms to form a distorted square-bipyramidal geometry, where one μ4-O atom and one bridging carboxylate O atom are located in the axial positions.The Bi(1)–O and Bi(2)–O bond lengths vary in the ranges of 2.066(8)~2.675(8)and 2.123(8)~2.704(8) Å, respectively.These bond lengths fall in their normal ranges and they agree with those known complexes[5,6].On the basis of bond-strength calculations[17], the bond-valence sums (BVS) for Bi(1) and Bi(2) are +3.248 (1.607,0.587, 0.346, 0.339, 0.433, 0.270 and 0.206) and+3.169 (0.144, 0.915, 0.709, 0.691, 0.520 and 0.190), respectively, which illustrates that the valence of Bi element present in1is +3.In the molecular structural unit, the Bi(1) and Bi(2) atoms are linked by two bridging O atoms (one μ3-O and one μ4-O atoms), resulting in the formation of a four-membered ring {Bi(1)–O(5)–Bi(2)–O(6)}.Meanwhile, two Bi(1) atoms (Bi(1) and Bi(1)i, i:1–x, 1–y, 2–z) are connected with each other by bridging two carboxylate O atoms, generating a four-membered ring {Bi(1)–O(2)–Bi(1)i–O(2)i} (i:1–x, 1–y, 2–z).The Bi··Bi distances are 3.8216(15)and 4.3056(19) Å, respectively.
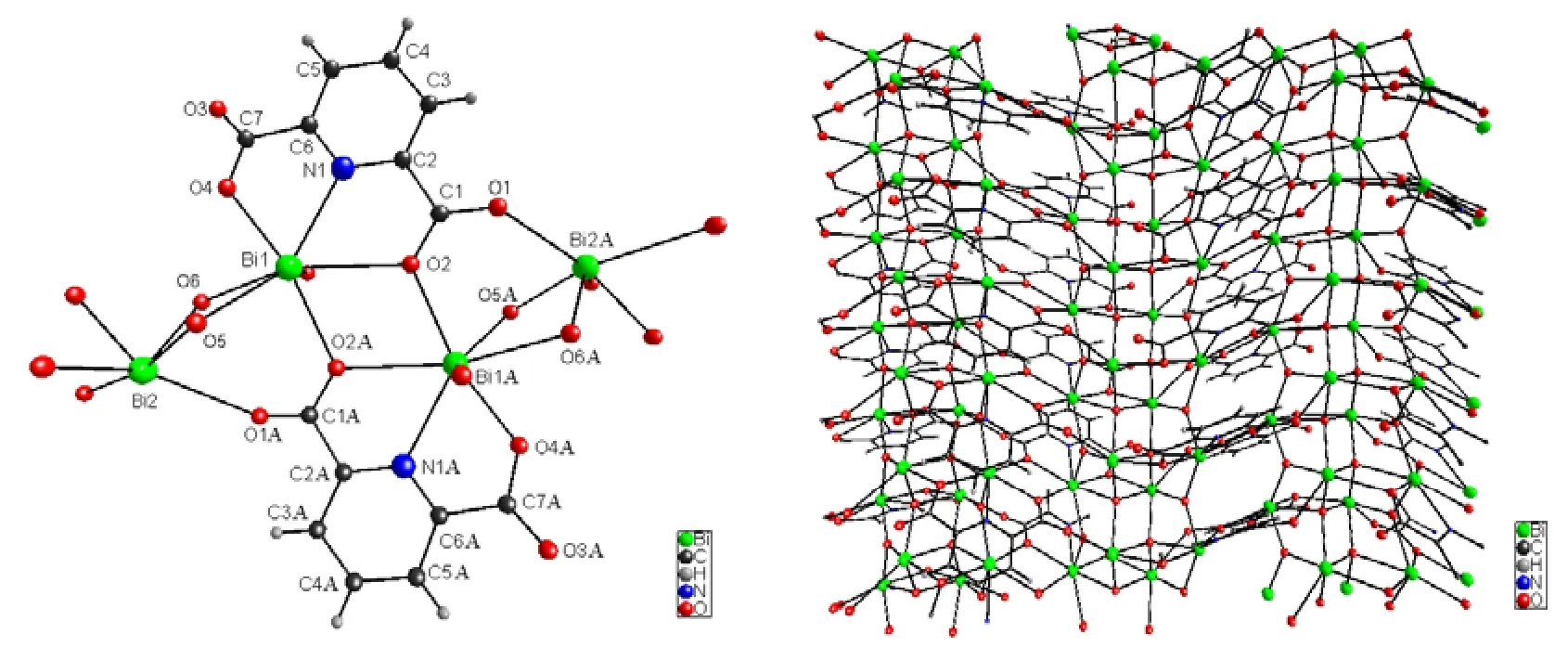
Fig.1.(left): View of the molecular structural unit of compound 1 (Symmetry code: A: 1–x, 1–y, 2–z);(right): the two-dimensional layered structure along the b axis
These molecular structural units are connected to each other via bridging O atoms, extending into a two-dimensional layered structure which is arranged in such a way that each pdc2-ligand coordinates to four surrounding Bi3+cations through one Bi–N and five Bi–O bonds.Fig.1-right shows its two-dimensional layered structure along the b axis.It is worth noting that the pdc2-ligand adopts a tetra-dentate coordination mode: one N atom ligates to one Bi3+centre, one carboxylate group, exhibiting a bridging-monodentate coordination mode,coordinates to two adjacent Bi3+cations, while the other carboxylate group exhibits a bridging-bidentate coordination mode connecting two different Bi3+cations.In order to better understand the structure of {Bi–O} layer, the topological approach is applied to simplify such a two-dimensional architecture[11,13](Here, the C, H and N atoms of pdc2-ligands were omitted for clarify).By considering the Bi(1) cations as 6-connected nodes and Bi(2) cations as 5-connected nodes, the structure of two-dimensional {Bi–O} layer can be simplified as a (6,5)-connected topological sheet with the interesting Schläfli symbol of(44·63·84·124)(45·64·8), as shown in Fig.2-left.To the best of our knowledge, it is a new kind of topological structure and represents the first(6,5)-connected topological sheet with such a Schläfli symbol.
Moreover, the weak C–H··O hydrogen bonds are observed in the structure of1.The formed {Bi–O}sheets are assembled by weak C–H··O hydrogen bonds (C(5)–H(5)··O(3), 0.93 Å, 2.45 Å, 3.097(16)Å, 126°, 2–x, 2–y, 2–z) into a 3-D supramolecular architecture (Fig.2-right).These hydrogen-bonds further stabilize the crystal structure of the polymer.
3.4 Thermal stability
The thermal stability of1was investigated between 25 and 800 °C at a heating rate of 10 K·min-1in a dynamic N2atmosphere (gas flow = 0.1 L·min-1), and the resulting curve is shown in Fig.3.The results indicate a one-step mass loss of 27.22%between 275 and 670 °C (Δmcalcd/m = 26.83%),which corresponds to the release of organic pdc2-ligands.

Fig.2.(left): View of the (6,5)-connected topological Bi-O sheet with the Schläfli symbol of (44·63·84·124)(45·64·8); (right): Three-dimensional supramolecular architecture of 1 (The dashed lines indicate weak C–H··O hydrogen bonds)

Fig.3.TG curve of compound 1
3.5 Luminescent properties
It is well known that metal coordination polymer containing aromatic systems can exhibit luminescence.This promotes us to investigate its luminescent properties.Thus, the fluorescence properties of1in the solid state were measured at room temperature.One emission peak is observed at about 428 nm under the excitation of 381 nm (Fig.4).The emission peak can be assigned to a π*-π emission of the pdc2-ligand[18-22].Meanwhile, the CIE chromaticity coordinate (CIE 1931) of1was provided according to its emission spectrum.The result illustrates that compound1displays a blue emission(Fig.5).Moreover, in order to better learn its luminescent properties, its decay lifetime was also studied.The luminescent decay curve of1is shown in Fig.6.Its ln(Intensity) versus decay time can be well fitted to the single exponential function (I =Aexp(–t/τ)) (where A is the constant) and the lifetime τ is 1.094 ns (λem: 428 nm and λex: 381 nm).

Fig.4.Emission spectrum of 1 in the solid state
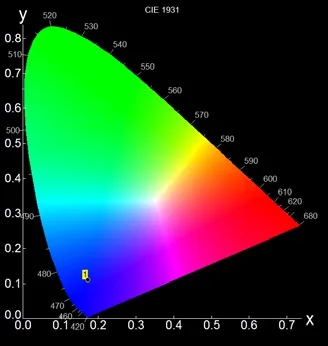
Fig.5.CIE (1931) chromaticity diagram for the emission spectrum of 1

Fig.6.Decay curve (black line) and the fitted curve (red line) for compound 1
4 CONCLUSION
In summary, a novel 2-D Bi3+coordination polymer based on the pdc2-ligand has been synthesized under ionothermal conditions.It exhibits a new layered structure containing interesting {Bi–O}sheets, and features the first 2-D Bi3+coordination polymer containing (6,5)-connected topological sheet with the interesting Schläfli symbol of(44·63·84·124)(45·64·8).Its luminescent properties reveal compound1owns a blue emission under the excitation wavelength of 381 nm and its value of luminescent decay is 1.094 ns.The compound enriches the family of known Bi3+coordination polymers.Its successful synthesis reveals that more Bi3+coordination polymers with different dimensions may be obtained, and further endeavours in the exploration of other metal polymers containing the pdc2-ligand are underway in our group.
REFERENCES
(1) Wang, X.; Zhang, X.; Lin, J.DNA-binding property and antitumor activity of bismuth complex with 1,4,7,10-tetrakis(2-pyridylmethyl)-1,4,7,10-tetraazacyclo dodecane.Dalton Trans.2003, 12, 2379−2380.
(2) Guo, Y.C.; Chen, S.Y.; Sun, R.Z.; Feng, Y.Q.Synthesis, crystal structure and antibacterial activity of dibenzyl dithiocarbamate bismuth complex.Chin.J.Struct.Chem.2014, 33, 1768−1772.
(3) Sun, H.; Zhang, L.; Szeto, K.Y.Bismuth in medicine.Met.Ions Biol.Syst.2004, 41, 333−378.
(4) Feng, Y.Q.; Hu, Y.L.; Wang, H.W.; Cao, F.P.A new linear bismuth coordination polymer based on 1,10-phenanthroline-2,9-dicarboxylic acid:ionothermal synthesis, crystal structure and fluorescence properties.Acta Cryst.C712015, 679–682.
(5) Sun, R.Z.; Guo, Y.C.; Liu, W.M.; Chen, S.Y.; Feng, Y.Q.Syntheses, crystal structures and antibacterial activities of complexes[(C9H18NS2)3M(III)] (M = Sb and Bi).Chin.J.Struct.Chem.2012, 31, 655-660.
(6) Zhang, T.; Xue, L.P.Ionothermal synthesis, crystal structure and photocatalytic property of a new cobalt coordination polymer.Chin.J.Struct.Chem.2015, 34, 417-422.
(7) Xu, J.; Su, W.P.; Hong, M.C.A series of lanthanide secondary building units based metal-organic frameworks constructed by organic pyridine-2,6-dicarboxylate and inorganic sulfate.Cryst.Growth Des.2011, 11, 337–346.
(8) Zhang, W.; Feng, Y.Q.A novel dinuclear bismuth(III) coordination compound: bis(μ-pyridine-2,6-dicarboxylato)-κ4O2,N,O6:O6′;κ4O2:O2′,N,O6-bis[(azido-κN)(1,10-phenanthroline-κ2N,N′)bismuth(III)] tetrahydrate.Acta Cryst.C702014, 562–565.
(9) Feng, Y.Q.; Qiu, D.F.; Fan, H.T.; Li, M.; Huang, Q.Z.; Shi, H.Z.A new 3-D open-framework cadmium borovanadate with plane-shaped channels and high catalytic activity for the oxidation of cyclohexanol.Dalton Trans.2015, 44, 8792–8796.
(10) Feng, Y.Q.; Zhong, Z.G.; Wang, H.W.; Fan, H.T.; Bi, D.Q.; Wang, L.; Xing, Z.Z.; Qiu, D.F.A novel open-framework copper borovanadate with enhanced catalytic performance for oxidation of benzylic C–H bond.Chem.Eur.J.2017, 23, 9962−9967.
(11) Feng, Y.Q.; Fan, H.T.; Zhong, Z.G.; Wang, H.W.; Qiu, D.F.Cd3(MoO4)(TeO3)2: a polar 3D compound containing d10-d0scalp effect cations.Inorg.Chem.2016, 55, 11987−11992.
(12) Feng, Y.Q.; Bi, D.Q.; Hu, Y.L.; Zhong, Z.G.; Guo, Y.C.Ionothermal synthesis, crystal structure and antibacterial activities of a new 3d-4f hetero-metallic compound containing two kinds of ligands.Chin.J.Struct.Chem.2015, 34, 1598−1605.
(13) Feng, Y.Q.; Li, M.; Fan, H.T.; Huang, Q.Z.; Qiu, D.F.; Shi, H.Z.A novel open-framework copper borophosphate containing 1-D borophosphate anion with 10-MR windows and 12-MR channels.Dalton Trans.2015, 44, 894−897.
(14) Feng, Y.Q.; Ding, C.H.; Fan, H.T.; Zhong, Z.G.; Qiu, D.F.; Shi, H.Z.Employing a new 12-connected topological open-framework copper borovanadate as an effective heterogeneous catalyst for oxidation of benzyl-alkanes.Dalton Trans.2015, 44, 18731–18736.
(15) Sheldrick, G.M.SADABS.Program of Empirical Absorption Correction for Area Detector Data.University of Göttingen: Göttingen, Germany1996.
(16) Sheldrick, G.M.SHELX-97, Program for Crystal Structure Analysis.University of Göttingen: Göttingen, Germany1997.
(17) O’Keeffe, M.; Brese, N.E.Bond-valence parameters for anion-anion bonds in solids.Acta Cryst.B481992, 152–154.
(18) Wang, E.R.; Huang, J.H.; Gu, X.Y.; Cheng, J.W.Structural modulation of two luminescent bismuth-organic frameworks by the mixed-ligand synthetic strategy.Chin.J.Struct.Chem.2017, 36, 1100-1107.
(19) Xu, X.L.; Yao, P.F.; Hu, D.Y.; Li, H.Y.; Liu, H.F.; Huang, F.P.Cd/Mn(II) complexes with heterocyclic substituted 1,2,4-triazole ligands:syntheses, crystal structures, and luminescence properties.Chin.J.Struct.Chem.2017, 36, 958-964.
(20) Ma, P.T.; Wan, R.; Si, Y.N.; Hu, F.; Wang, Y.Y.; Niu, J.Y.; Wang, J.P.Double-malate bridging tri-lanthanoid cluster encapsulated arsenotungstates:syntheses, structures, luminescence and magnetic properties.Dalton.Trans.2015, 44 11514–11523.
(21) Feng, Y.Q.; Jiang, L.T.; Xing, Z.Z.; Wang, L.Mixed-solvothermal synthesis, crystal structure and luminescence of a new dinuclear yttrium(III)coordination polymer with 1-D wave-like infinite chains.Chin.J.Struct.Chem.2018, 37, 825–831.
(22) Feng, Y.Q.; Wang, L.; Xing, Z.Z.; Huang, Q.Z.; Ma, P.T.A new Cu(II) coordination polymer constructed from two kinds of ligands and rare[Ta2OF10]2−anion: synthesis, crystal structure and fluorescent properties.Inorg.Chem.Commun.2018, 93, 15–19.
杂志排行
结构化学的其它文章
- Synthesis and Characterization of a Palladium Complex Supported by Bidentate Ligand and Catalysis of the Vinyl Polymerization of Norbornene①
- Structural, Electronic, Optical and Thermodynamic Properties of Nanolaminated Boride Cr4AlB6①
- Synthesis, Structure and Photochromism of a New Diarylethene and Its Ag(I) Complex①
- Synthesis, Crystal Structure and Properties of a 1D Heteronuclear Cobalt-sodium Polymer with Bridging Ligand 2-(2-Hydroxy-3-methoxybenzylidene)Hydrazinecarbothioamide①
- Synthesis, Crystal Structure and Photoluminescence of a Dinulear Copper Complex①
- Iodoplumbate(II)-based Hybrid Templated by 1,4-Diazabicyclo[2.2.2]octane Derivative: Structure,Photocurrent Response Behavior and Photocatalytic Activity for the Degradation of Organic Dye①
