Synthesis and Characterization of a Palladium Complex Supported by Bidentate Ligand and Catalysis of the Vinyl Polymerization of Norbornene①
2018-11-22LIHuiPingMAOMinJieYUJinZHOUXinBoJIANLiJuZHANGDnDnLINMeiJunCHENJinXinZHANGZhiChunCollegeofChemistryndMterilsScienceFujinNormlUniversityFuzhou350007ChinStteKeyLortoryofStructurlChemistryFujinInstituteofReserch
LI Hui-Ping MAO Min-Jie YU Jin-F ZHOU Xin-Bo JIAN Li-Ju ZHANG Dn-Dn LIN Mei-Jun CHEN Jin-Xin, ZHANG Zhi-Chun (College of Chemistry nd Mterils Science,Fujin Norml University, Fuzhou 350007, Chin) (Stte Key Lortory of Structurl Chemistry, Fujin Institute of Reserch on the Structure of Mtter, Chinese Acdemy of Sciences, Fuzhou 350002, Chin)
The complex N,O-bis{[2-[(2,4-dimethylphenyl)imino]-5-methoxyl]-phenol}palladium(Ⅱ ) (C32H32N2O4Pd) has been synthesized by the reaction of 2-[(2,4-dimethylphenyl)imino]-5-methoxyl-phenol with Pd(CH3COO)2, and showed excellent catalytic activity up to 1.183 × 107g of PNB (mol of Pd)−1h−1for the addition polymerization of norbornene by using methylaluminoxane (MAO) as a cocatalyst.
1 INTRODUCTION
The most common way to form the polymerization of norbornene is vinyl addition polymerization[1-3].This synthetic polynorbornene (PNB) with a large volume has many excellent properties[4,5], such as high thermal stability and corrosion resistance, and has high use value in production and life.The catalysis is an indispensable material in the norbornene (NB) of polymerization.From the earliest Ziegler-Natta[6]catalyst to the late-transition metal catalysts[7,8], the variety of catalysts have been increasing, and their performance have been continuously improved.Now the late-transition metal catalysts have been widely used in the polymerization of norbornene[9-12].The late-transition metal catalysts have advantages in many aspects, such as high catalytic activity, low cost, high yield and so on[13].
Among these, palladium complexes have drawn great attention due to their comparatively high catalytic activity for the polymerization of nor-bornene[14].In order to find a catalyst with high activity in palladium complexes, we could research for new ligands.At the same time, we can also use methylaluminoxane (MAO) as the co-catalyst in the palladium complex catalysis[15,16].In this paper, we synthesized and characterized the palladium metal complex (N,O-bis{[2-[(2,4-dimethylphenyl)imino]-5-methoxyl]-phenol}palladium(II)).After the activation of methylaluminoxane (MAO), the catalytic activity reached up to 1.183 × 107g of PNB (mol of Pd)−1h−1.
2 EXPERIMENTAL
2.1 Materials and measurements
All operations were sensitive to air and moisture,which were in rigid with the Schlenk technology standard[17].Ethanol was added into the molecular sieve seal preservation.Methylaluminoxane (MAO)and norbornene (NB) were bought from Aldrich firm,and norbornene (NB) was dried and purified by sodium, which was steamed out without water and oxygen.The stock solution of NB was prepared in dichloromethane.The rest reagents are of pure analysis grade and used without any special treatment.
Element analysis (EA) was tested by Vario MICRO microanalyzer.Fourier transform infrared(FT-IR) spectrum was used to collect infrared spectra of 500~4000 cm-1range on a Nicolet-360 infrared spectrometer using traditional KBr pellet technique.The single-crystal X-ray diffraction was performed by SCX min MERCURY2 diffractometer.Thermogravimetric (TG) instrument was used for a METTLER TGA/SDTA851 thermogravimetric analyzer, and N2was piped in as the shielding gas,with the determination temperature ranging from 30 to 600 °C at a heating rate of 10 °C/min.The wideangle X-ray diffraction (WAXD) spectrum was characterized by using Phlips X’pert Pro X-ray diffractometer with monochromatic radiation at the wavelength of 1.54 Å.
2.2 Preparation of the ligand and the complex
The main synthetic route for the title complex is shown in Scheme 1.According to the modification a reported procedure[18,19], palladium acetate (0.115g,0.51mmol) and 2-hydroxy-4-methoxyl-benzaldehyde (0.152 g, 1 mmol) were heated in ethanol (25 mL)along with 2,4-dimethyl-phenylamine (0.121 g, 1 mmol)[20].The resulting mixture was stirred and heated to 50 ℃ for 6 h.The solution was cooled slowly to room temperature and then removed to give an orange solid.The palladium complex was purified by crystallization from the mixture of dichloromethane and ethanol absolute[21].Anal.Calcd.(%) for C32H32N2O4Pd: C, 62.49; H, 5.24; N, 4.55.Found: C, 62.51; H, 5.27; N, 4.59% IR (KBr, cm-1):3447 (m), 2919 (m), 1605 (s), 1517 (s), 1425 (s),1373 (s), 1309 (s), 1254 (m), 1204 (m), 1168 (s),1143 (s), 1027(s), 975(s), 846 (m), 819(m), 791 (m),624 (m), 518 (m).
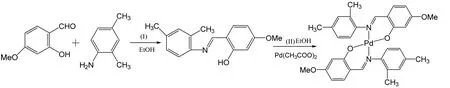
Scheme 1.Synthesis route of the title compound
2.3 Structure determination
An orange single crystal of the title complex was selected for single-crystal X-ray diffraction and the data were collected on a SCX min MERCURY2 diffractometer equipped with a graphite-monochromatic MoKα radiation (λ = 0.71073 Å) at 293(2) K using an ω-2θ scan mode in the range of 3.01≤θ≤27.48°.A total of 14044 reflections were obtained with 3221 unique ones (Rint= 0.0555) and used in the succeeding refinements.The structure of the title compound was solved by direct methods with SHELXS-97[22]program and refined with SHELX- 97[23]by full-matrix least-squares techniques on F2.All hydrogen atoms were added in their calculated positions and all non-hydrogen atoms were refined with anisotropic temperature factors.R = 0.0514, wR= 0.1356, GOOF = 1.040, (Δρ)max= 0.729 and(Δρ)min= -0.845 e/Å3.

Table 1.Selected Bond Lengths (Å) and Bond Angles (°)
3 RESULTS AND DISCUSSION
3.1 Description of the structure
It is seen from Fig.1 that the infrared absorption peak of C=N of the complex appears at 1605 cm-1,and the telescopic vibration and bending vibration of C–H at about 3000 and around 1400 cm-1, respectively.However, the infrared characteristic absorption peak at about 910~670 cm-1is due to the stretching vibration of the benzene ring.

Fig.1.IR spectra of the title compound
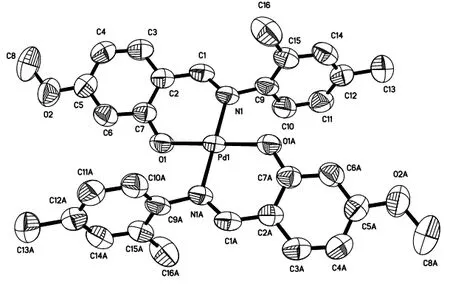
The molecular structure of the title complex is shown in Fig.2.Selected bond lengths and bond angles are listed in Table 1.The N(1)–C(1) bond in 1.307(5) Å belongs to the C=N (1.33 Å), which demonstrates that the reaction of Schiff base has happened.The central palladium atom is coordinated with two molecule ligands.And the structure of the complex shows an expected parallelogram environment around the Pd center.Additionally, the bond lengths of Pd(1)–O(1) and Pd(1)–N(1) are 1.974(3)and 2.020(3) Å.The O(1)–Pd(1)–N(1) and O(1A)–Pd(1)–N(1) bond angles are 91.83(13) and 88.17(13)°, respectively.The analysis results further prove the molecular structure.

Fig.2.Molecular structure of the palladium compound
3.2 Homopolymerization of NB
Under nitrogen atmosphere, the palladium complex, norbornene and dry CH2Cl2were added into a polymerization bottle with strong stirring[24].After the mixture was stirred for one minute, MAO as a co-catalyst was injected into the reaction system by means of an injection syringe, and the reaction was initiated.Five minutes later, acidic ethanol(vethanol:vHCl= 9:1) was added to terminate the reaction.The solid PNB was filtered, washed with ethanol, and dried in vacuum at 60 ℃ for 24 h.Unless otherwise stated, the total reaction volume was 15 mL.
The activities of catalysts in norbornene polymerrization have been proved to be influenced by several parameters, and the reaction temperature, the dosage of MAO and the monomer concentration have been extensively investigated[25].
The effect of reaction temperature on the catalytic activity of the palladium complex is shown in Table 2.With an increase of reaction temperature from 0 to 45 ℃ , the catalytic activity and monomer conversion rate first increase and reach the maximum at about 30℃, and then decrease.The optimum polymeri- zation temperature is 30 ℃ .
The ratio variation of MAO/palladium complex has a great influence on the polymer yields and catalytic activities.As shown in Table 3, with the increase of Al/Pd ratio from 1500 to 3500, the polymer yields and catalytic activities first increase rapidly, and then tend to be steady when reaching 3000.Similar results are observed with other palladium catalysts reported previously.
As shown in Table 4, an increase in the molar ratio of NB/Pd caused a great increase of the catalytic activity.With increasing the ratio of NB/Pd, the catalytic activity and monomer conversion rate first increase and then decrease.A maximum catalytic activity value 11.83 × 106g of PNB (mol of Pd)−1h−1was obtained as the NB/Pd molar ratio is 40000.

Table 2.Influence of the Reaction Temperature on the Activity of the Complex
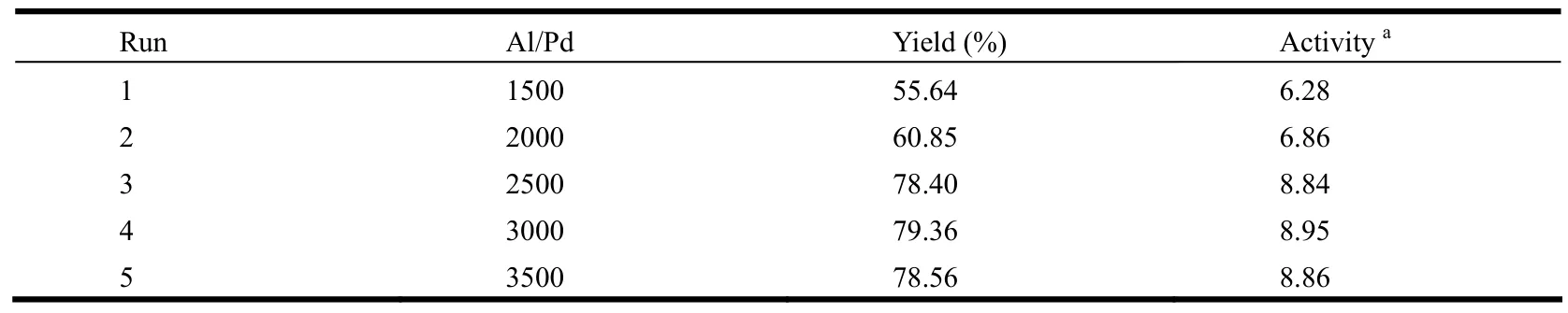
Table 3.Influence of the MAO Concentration on the Activity of the Complex
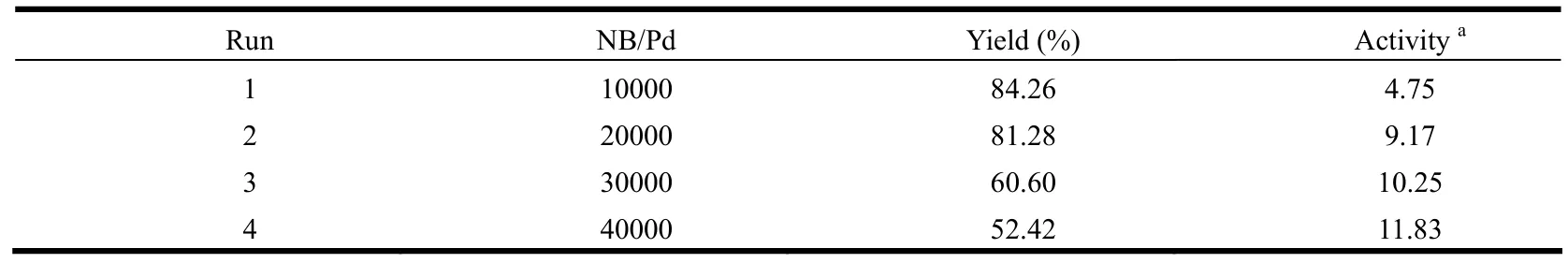
Table 4.Influence of the Monomer Concentration on the Activity of the Complex
3.3 XRD and TG analysis
As shown in Fig.3, wide-angle X-ray diffraction(WAXD) spectra show two intense diffraction peaks at 2θ of 10.23° and 18.74°, and the high intensity diffraction signal is attributed to the interaction of the interchain or intersegment distance of PNBs, while the low intensity diffraction signal belongs to the interaction of the intrachain distance[26,27].XRD analysis results show that PNBs exhibit higher stacking density, which is consistent with the results reported in literature.

Fig.3.WAXD diagram of polynorbornene
Thermal analysis curves (TG) of PNBs, which were catalyzed by the Pd complex, are show in Fig.4.TG curves show that PNBs are thermally stable up to 410.25 ℃ and then begin to decompose at higher temperature of 477.65 ℃ . The results indicate that PNBs possess excellent thermal stability in the N2atmosphere[28].
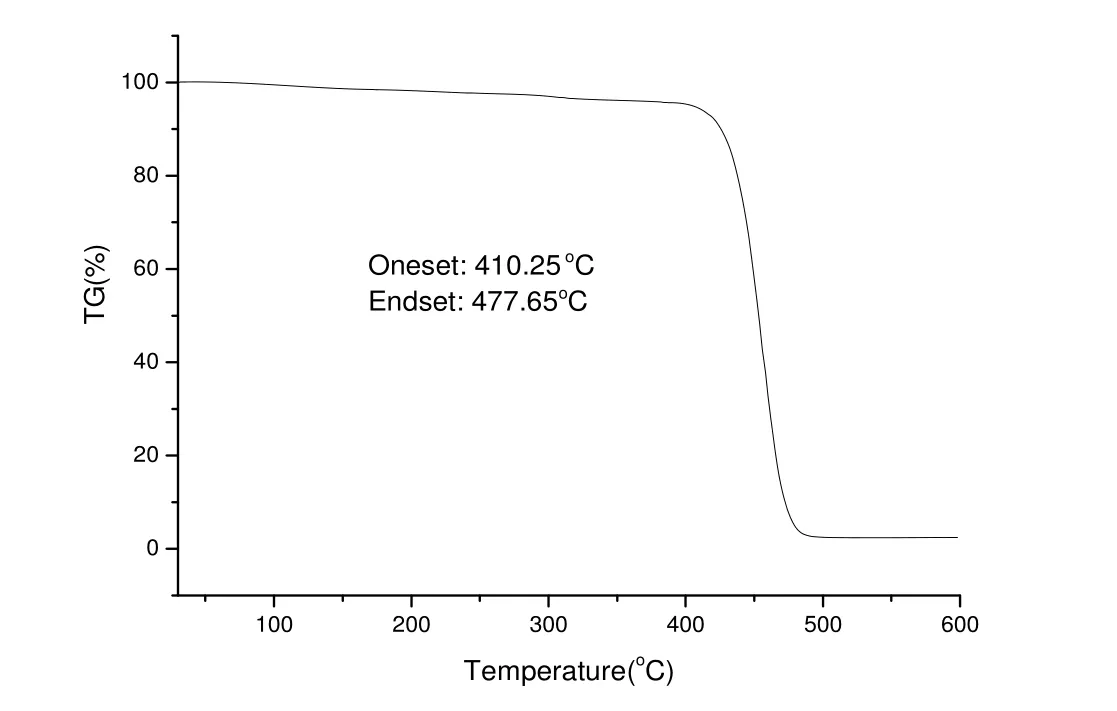
Fig.4.TG of polynorbornene
4 CONCLUSION
The palladium complex has been synthesized and characterized.The elemental analysis, IR spectra and single-crystal X-ray diffraction studies revealed that the central palladium atom is coordinated with the two molecule ligands.We explored the effects of catalyst on the polymerization of norbornene in different conditions of MAO dosage, reaction temperature, and monomer concentration.Significantly,the catalytic activity is up to 1.183 × 107g of PNB(mol of Pd)−1h−1.
REFERENCES
(1) Ivin, K.; Mol, C.Olefin metathesis and metathesis polymerization .Academic Pr.Inc.1997, 11, 461–472.
(2) Gui, G.Q.; Bao, F.; Gao, H.Y.Vinyl polymerization of norbornene catalyzed by a new bis(β-ketoamino)nickel(II) complex-methylaluminoxane system.Appl.Organometal.Chem.2005, 19, 627–632.
(3) Kennedy, J.P.; Makowski, H.S.Carbonium ion polymerization of norbornene and its derivatives.J Macromol.Sci.A.1967, 1, 345–370.
(4) Liu, F.S.; Gao, H.Y.; Song, K.M.; Zhao, Y.; Long, J.M.; Zhang, L.; Zhu, M.; Wu, Q.Neutral nickel complexes chelating monoamidinate ligands:syntheses, characterizations and catalytic properties toward ethylene oligomerization and norbornene polymerization.Polyhedron.2009, 28,673–678.
(5) Gibson, V.C.; Spitzmesser, S.K.Advances in non-metallocene olefin polymerization catalysis.Chem.Rev.2003, 103, 283–315.
(6) Huang, J.; Rempel, G.L.Ziegler-Natta catalysts for olefin polymerization: mechanistic insights from metallocene systems.Prog.Polym.Sci.1955,20, 459–526.
(7) Zhang, W.J.; Wang, Y.H.; Redshaw, C.; Hao, X.; Sun, W.H.2-Aldiminophenoxytitanium chloride complexes: synthesis, characterization, and ethylene (co-)polymerization behavior.J.Org.Chem.2012, 715, 119–128.
(8) Zhang, J.; Wang, X.; Jin, G.X.Polymerized metallocene catalysts and late transition metal catalysts for ethylene polymerization.Coordin.Chem.Rev.2006, 250, 95–109.
(9) Johnson, L.K.; Killian, C.M.; Brookhart, M.New Pd(Ⅱ) and Ni(Ⅱ)-based catalysts for polymerization of ethylene and α-O lefins.J.Am.Chem.Soc.1995, 117, 6414–6415.
(10) Small, B.L.; Brookhart, M.; Bennett, A.M.A.Highly activity iron and cobalt catalysts for the polymerization of ethylene.J.Am.Chem.Soc.1998,120, 4049–4050.
(11) Britovsek, G.J.P.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; McTavish, S.J.; Solan, G.A.; White, A.J.P.; Williams, D.J.Novel olefin polymerization catalysts based on iron and cobalt.Chem.Commun.1998, 7, 849–850.
(12) Desjardins, S.Y.; Cavell, K.J.; Hoare, J.L.; Skelton, B.W.; Sobolev, A.N.; White, A.H.; Keim, W.Single component N–O chelated arylnickel(II)complexes as ethene polymerisation and CO/ethene copolymerisation catalysts.Examples of ligand induced changes to the reaction pathway.J.Organomet.Chem.1997, 544, 163–174.
(13) Yu, Y.H.; Chen, J.X.; Meng, S.Q.; Li, C.; Lan, M.Y.; Zhang, Z.C.Nickel(II) and copper(II) complexes as catalysts for norbornene vinyl polymerization bearing teradentate β-ketoiminato ligands.J.Mol.Catal.A: Chem.2013, 380, 104–111.
(14) Peruch, F.; Cramail, H.; Deffieux, A.Homopolymerization and copolymerization of styrene and norbornene with Ni-based/MAO catalysts.Macromol.Chem.Phys.1998, 199, 2221–2227.
(15) Younkin, T.R.; Conner, E.F.; Henderson, J.I.; Friedrich, S.K.; Grubbs, R.H.; Bansleben, D.A.Neutral, single-component nickel(II) polyolefin catalysts that tolerate heteroatoms.Science.2000, 287, 460–462.
(16) Wang, X.; Jin, G.X.Preparation, structure, and ethylene polymerization behavior of half-sandwich picolyl-functionalized carborane iridium,ruthenium, and rhodium complexes.Chem.-Eur.J.2005, 11, 5758–5764.
(17) Chang, F.; Zhang, D.; Xu, G.; Yang, H.; Li, J.; Song, H.; Sun, W.Synthesis and characterization of new bis(1-arylimino methylenyl naphthalen-2-oxy)nickel complexes and their catalytic behavior for vinyl polymerization of norbornene.J.Organomet.Chem.2004, 689, 936–946.
(18) Hu, Y.J.; Zou, H.H.; Zeng, M.H.; Weng, N.S.Two bis-[1-(aryliminomethyleny)-2-oxy-naphthalen] nickel catalysts for the polymerization of methyl methacrylate.J.Org.Chem.2009, 694, 366–372.
(19) Zhang, D.D.; Zhou, X.B.; Jian, L.J.; Lin, M.J.; Li, H.P.; Chen, J.X.; Zhang, Z.C.Synthesis, structure and norbornene polymerization catalyzed by bis{(2-benzhydryl-4,6-dimethyl-phenyl)-(3,5-di-tert-butyl-2-methyl-benzylidene)-amine-N,O}nickel(Ⅱ).Chin.J.Struct.Chem.2017, 36,1479–1485.
(20) Meng, S.Q.; Li, C.; Yu, Y.H.; Lan, M.Y.; Chen, J.X.; Zhang, Z.C.Synthesis, structure and norbornene polymerization activity of a novel palladium(Ⅱ) complex with N,N-bis(2-benzoyl-3-oxobutane)-trans-1,2-diaminocyclohexane Ligand.Chin.J.Struct.Chem.2014, 33, 115–121.
(21) Lan, M.Y.; Liang, H.; Lu, X.C.; Deng, J.Q.; Cheng, X.; Chen, J.X.; Zhang, Z.C.Synthesis, structure and norbornene polymerization catalyzed by nickel(Ⅱ) complex bearing N,O-bis(1-(6-ethylpyridin-2-ylimino)-methylenyl)naphthalen-2-ol ligand.Chin.J.Struct.Chem.2015, 34,447–452.
(22) Sheldrick, G.M.SHELXS-97, Program for X-ray Crystal Structure Solution.University of Göttingen, Göttingen, Germany1997.
(23) Sheldrick, G.M.SHELXL-97, Program for X-ray Crystal Structure Refinement.University of Göttingen, Göttingen, Germany1997.
(24) Zhou, X.B.; Jian, L.J.; Zhang, D.D.; Lin, M.J.; Li, H.P.; Chen, J.X.; Zhang, Z.C.Synthesis, crystal structure and characterization of a nickel complex based on phenoxyimine ligand and catalysis of the vinyl polymerization of norbornene.Chin.J.Struct.Chem.2017, 36, 1889–1895.
(25) Yu, Y.H.; Chen, J.X.; Meng, S.Q.; Li, C.; Lan, M.Y.; Zhang, Z.C.Synthesis, structure and norbornene polymerization catalyzed by palladium complex bearing the 1,3-diphenyl-2-((quinolin-8-ylamino)-methylene)-propane-1,3-dione ligand.Chin.J.Struct.Chem.2013, 32, 620–624.
(26) Zhao, C.T.; do Rosário Ribeiro, M.; De Pinho, M.N.; Subrahmanyam, V.S.; Gil, C.L.; Lima, A.P.Structural characteristics and gas permeation properties of polynorbornenes with retained bicyclic structure.Polymer.2001, 42, 2455–2462.
(27) Haselwander, T.F.; Heitz, W.; Krügel, S.A.; Wendorff, J.H.Polynorbornene: synthesis, properties and simulations.Macromol.Chem.Phys.1996,197, 3435–3453.
(28) Hou, J.X.Sun, W.H.; Zhang, D.H.; Chen, L.Y.; Li, W.; Zhao, D.F.; Song, H.B.Preparation and characterization of acylhydrazone nickel(II)complexes and their catalytic behavior in vinyl polymerization of norbornene and oligomerization of ethylene.J.Mol.Catal.A: Chem.2005, 231,221–233.
杂志排行
结构化学的其它文章
- Structural, Electronic, Optical and Thermodynamic Properties of Nanolaminated Boride Cr4AlB6①
- Synthesis, Structure and Photochromism of a New Diarylethene and Its Ag(I) Complex①
- Ionothermal Synthesis, Structure and Luminescent Properties of a New 2-D Bismuth(III) Coordination Polymer with (6,5)-Connected Topological Sheet①
- Synthesis, Crystal Structure and Properties of a 1D Heteronuclear Cobalt-sodium Polymer with Bridging Ligand 2-(2-Hydroxy-3-methoxybenzylidene)Hydrazinecarbothioamide①
- Synthesis, Crystal Structure and Photoluminescence of a Dinulear Copper Complex①
- Iodoplumbate(II)-based Hybrid Templated by 1,4-Diazabicyclo[2.2.2]octane Derivative: Structure,Photocurrent Response Behavior and Photocatalytic Activity for the Degradation of Organic Dye①
