Interaction of β-Cyclodextrin Catalyst with p-Chlorobenzonitrile for the Synthesis of 5-Substituted 1H-tetrazoles in n,n-Dimethylformamide: a DFT Study①
2018-11-22YIShanFengWANYaLiWANGXueYe
YI Shan-Feng WAN Ya-Li WANG Xue-Ye
(Key Laboratory for Green Organic Synthesis and Application of Hunan Province,Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan 411105, China)

The synthesis of 5-substituted 1H-tetrazoles in n,n-dimethylformamide (DMF) withb-cyclodextrin (b-CD) as catalyst can get an excellent yield in short reaction time, so the complex of b-CD with p-chlorobenzonitrile has been investigated using density functional theory (DFT) method.
1 INTRODUCTION
Cyclodextrins (CDs) are a class of macrocyclic oligosaccharides connected together by α-1,4 glycosidic bonds[1].a-, b- and g-CD, composed of six, seven and eight glucose units (Fig.1),respectively are commonly used in recent years[2].They have hydrophilic external and hydrophobic internal cavity, and thus a wide range of guest molecules can be embraced in their cavities[3,4].The ability of CDs to bind guests in their internal hydrophobic cavities plays an important role in changing the physicochemical properties of the guests[5].Due to their particular abilities, they have been extensively employed in facilitating organic reaction by the way of host-guest complexes[6-12].Currently, in view of efficiency, economy and environment, the CD catalyst has been demonstrated by numerous researchers.
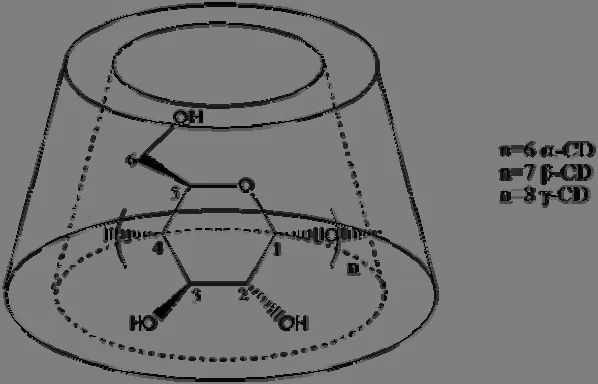
Fig.1.Structure of cyclodextrin
1H-Tetrazoles are a family of nitrogen-rich heterocycles with chemical instability and potential as precursors of alkylidenecarbenes[13,14].They have a wide range of applications, such as pharmaceutics[15],biomedicine and materials science[16,17].Therefore,the synthesis of 1H-tetrazoles has an attractive prospect.Much work has reported the methods for the synthesis of 1H-tetrazoles[18-22], but they still suffer from drawbacks such as long time or environmental unfriendliness.Thus, an efficient and environmentally friendly catalyst is useful.The 5-substituted 1H-tetrazoles can be synthesized with β-CD as the catalyst[23].Host-guest interaction of β-CD must play an important role in this process.The highly efficient β-CD as catalyst for the synthesis of 5-substituted 1H-tetrazoles has been studied experimentally, while the interaction of β-CD as catalyst with guest has not been reported.
In this work, the computational method is used to describe the host-guest interaction of β-CD catalyst with p-chlorobenzonitrile for the synthesis of 5-substituted 1H-tetrazoles in n,n-dimethylformamide(DMF).More attention is focused on the interaction of β-CD and p-chlorobenzonitrile using density functional theory (DFT) method.The minimum energy structure is investigated in water, DMF and DMSO.To know more details, the natural bonding orbital (NBO), nuclear magnetic resonance (NMR)spectra, frontier molecular orbitals and Mulliken charge are discussed.
2 COMPUTATIONAL METHODS
The initial geometry of β-CD is constructed in Chem3D Ultra (Version8.0) according to the crystal structure.And the structure of p-chlorobenzonitrile is built using Chemdraw and Chem3D Ultra.The host-guest complexes of β-CD and p-chlorobenzonitrile are constructed by manually in Gauss view making the p-chlorobenzonitrile into β-CD cavity through two possible ways (Fig.2).The glycosidic oxygen atoms of β-CD are placed on the xy plane and their center is defined as the same with the coordinate system.Meanwhile, the center of the guest is placed on the x-axis[3].The relative position between host and guest is measured by the distance between the centers of p-chlorobenzonitrile and β-CD.The guest molecule passes through the host cavity along the z-axis from 8 to –8 Å with a stepwise 1 Å and revolves from 0° to 360° around the z-axis at an interval of 30°.
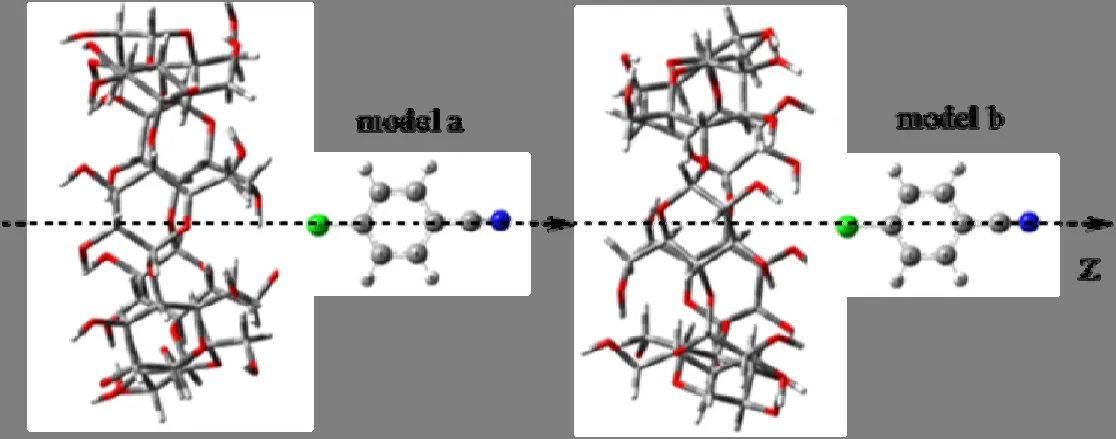
Fig.2.Two possible complexation models of p-chlorobenzonitrile into β-CD
All the calculations are performed using the Gaussian03 software package[24].The method of B3LYP is employed[25].The isolated β-CD and guest are optimized using 6-31G (d, p) basis set firstly in gas phase, then the solvent effect of water (ε =78.390), DMF (ε = 37.219) and dimethyl sulfoxide DMSO (ε = 46.700) are considered using the polarizable continuum model (PCM)[26].The inclusion complexes of each step are optimized by PM3 without any restriction.The precise optimization of inclusion complex is calculated at the B3LYP/6-31G(d, p) level in gas phase which is based on the results of the preliminary PM3 calculation and then in solvent.In addition, the same level is employed to obtain NBO, NMR, frontier molecular orbital and Mulliken charge in DMF.The NMR is investigated with gauge-including atomic orbital (GIAO) method and the tetra methyl silane (TMS) as internal reference for13C and1H, and NH3for15N[27,28].
3 RESULTS AND DISCUSSION
3.1 Energy analysis
The energy of the preliminary optimization is calculated at the PM3 level to find the minimum.Fig.3 shows the relationship between the energy of inclusion complex and the position for two possible complexation models.As shown in Fig.3, multiple local minima can be found for the entire process of inclusion in models a and b.Only the global minima of the two models are compared to a minimum.It is easy to find that the global minimum in model b is more negative than in model a.Thus, this inclusion complex is further refined and named as complex b(CB).

Fig.3.Graphic diagram of the energy for the inclusion complex of p-chlorobenzonitrile into β-CD at different positions
To know the influence of solvent in this reaction,β-CD, p-chlorobenzonitrile and CB are further investigated in water, DMF and DMSO.The parameters are recorded in Table 1.The energies of p-chlorobenzonitrile, β-CD and CB have some differences in water, DMF and DMSO.Thus, there must be some interactions between the solvent and the molecule in solvent, and the interaction has an influence on the occurrence of cycloaddition reaction.From the information of Table 1, it is easy to note that the value of interaction energy (ΔE) is positive in water and DMSO, while it is negative in DMF.The change of ΔE is an important sign of the driving force towards complexation and the negative value means favorable inclusion.Therefore, the p-chlorobenzonitrile can bind with β-CD efficiently in nonaqueous DMF, which is in agreement with the literature survey[23].

Table 1.Calculated Interaction Energy (kJ·mol-1) of CB for Different Solvents
3.2 Hydrogen bond interactions
To know the interaction between host and guest,the hydrogen bond is investigated by the natural bond orbital (NBO) analysis[29].The results are summarized in Table 2.

Table 2.Bond Lengths (Å), Bond Angles (°) and Stabilization Energy E(2) (kcal·mol-1) of Hydrogen Bonds for CB
The hydrogen bond is defined as an X–H··Y interaction in which the distance of H··Y is less than 3.0 Å but the angle is larger than 90°[30].The strength of hydrogen bond is measured by the second-order Moller-Plesset perturbation stabilization energy E(2)which is in relation to the donor-acceptor delocalization[31].The value of E(2)in CB reveals that more hydrogen bonds form guest to β-CD.That is to say, the lone pairs are mainly delocalized to the anti-bonds of β-CD, especially for the lone pairs on the nitrogen atom (N148) of cyanogroup.They are delocalized to O105–H134and C20–H67which result in strong stabilization energy.Additionally, the value of E(2)lower than 2.0 kcal·mol-1indicates typical weak hydrogen bond[32].Therefore, the calculated data indicate two classical hydrogen bonds and one weak hydrogen bond in CB.Thus, it can imply that the hydrogen bonds play a significant role in CB.And the hydrogen bonds between nitrogen atom (N148)and secondary hydroxyl make great contribution to driving the p-chlorobenzonitrile molecule into the cavity of b-CD and keeping the stability of CB.
3.3 NMR study
To confirm the relative position between p-chlorobenzonitrile and β-CD in DMF, the1H NMR of β-CD in the presence and absence of p-chlorobenzonitrile in DMF is calculated.The isotropic chemical shift (δ) and chemical shift displacement (Δδ) are calculated according to:

where σ is the isotropic shielding value.The spectra data are shown in Table 3.There is an obvious upfield shift for H3and H5in CB compared to free β-CD, which indicates that the internal H3and H5protons are more sensitive to the complexation effect than the other protons located outside.A relatively high association appears between p-chlorobenzonitrile and the internal protons of β-CD.

Table 3.1HNMR Chemical Shift (ppm) of the b-CD for Free and CB
The chemical shift ranges of13C and15N spectra are large.Thus, they are sensitive to reflect alteration in the chemical environment.The guest13C NMR and15N NMR in the presence and absence of β-CD are recorded in Table 4.The C1and C5are on the meta, C2and C4on the ortho, while C6on the para of benzene ring in p-chlorobenzonitrile.Owing to the deformation of the guest, there are some differences about the values of carbon atoms in the same position of benzene ring upon complexation.As is known, low shielded electrons correspond to large isotropic chemical shift and vice versa.From the information in Table 4, it is easy to note that the chemical shifts of p-chlorobenzonitrile have some changes.All atoms except for C3and N12in CB exhibit downfield shifts in contrast to the free, and the remarkable downfield shift is for C11.It represents that most carbon atoms of p-chlorobenzonitrile possess more positive charges and are high electropositive in the presence of β-CD and the carbon atom of cyanogroup (C11) is more obvious than others.Obviously, the N12moves to upfield significantly.It indicates that more positive charges focus on the carbon atom of cyanogroup (C11) and more negative charges concentrate on the nitrogen atom of cyanogroup (N12) upon complexation.

Table 4.13CNMR Chemical Shift (ppm) of p-Chlorobenzonitrile for the Free and CB
3.4 Frontier molecular orbital
The frontier molecular orbitals which can reflect the electronic structures are investigated.The highest occupied molecular orbital (HOMO) and the lowest virtual molecular orbital (LUMO) of CB are shown in Fig.4.It should be noted that the HOMO is mainly spread around β-CD, and the LUMO almost focuses on p-chlorobenzonitrile.The HOMO represents the ability to donate electron while the LUMO as an electron acceptor represents the ability to obtain electron[33].Thus, the p-chlorobenzonitrile in CB is more likely to accept electrons than isolated p-chlorobenzonitrile.
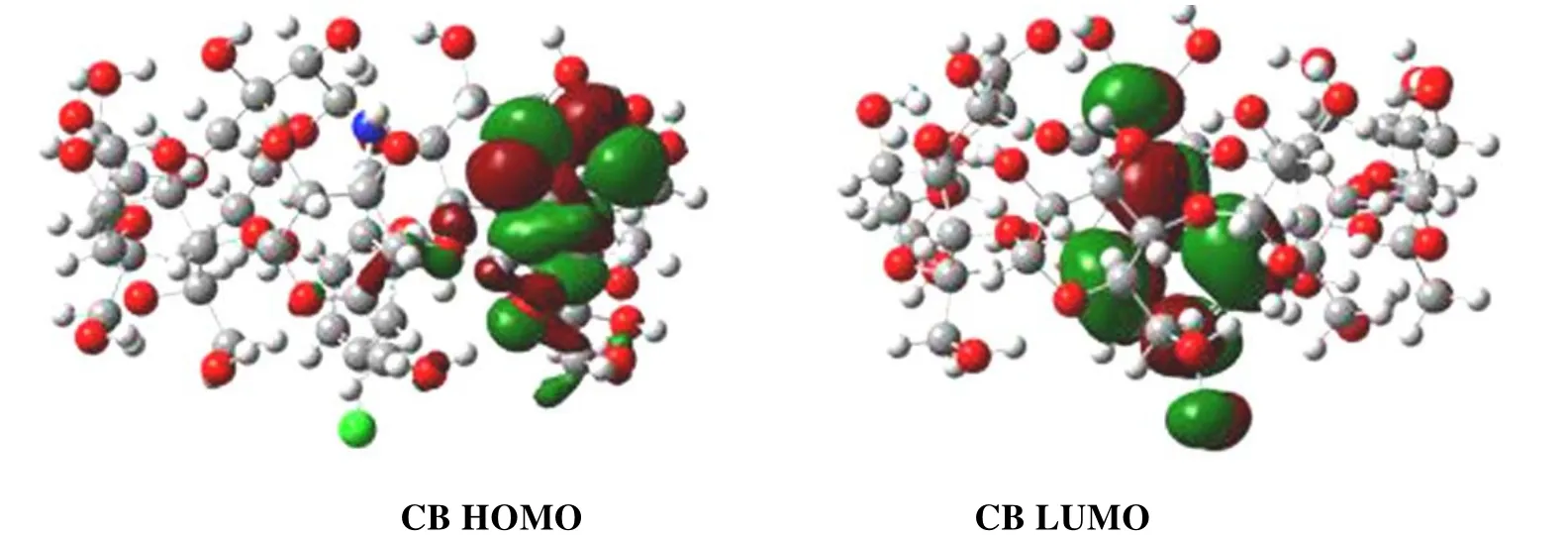
Fig.4.HOMO and LUMO orbitals of CB
The HOMO energy can be used to measure the ionization potential (IP ≈ –EHOMO)[34,35]and the LUMO energy has association with the electron affinity (EA ≈ –ELUMO)[35].In addition, the energy gap between HOMO and LUMO is a significant sign for chemical activity and smaller value of energy gap means more activity[36].In this case, the energy gaps of reactants are investigated to know more details about β-CD in catalyzing p-chlorobenzonitrile, as shown in Fig.5.The energy gaps of HOMO-LUMO between p-chlorobenzonitrile and NaN3with β-CD as catalyst are smaller than that without β-CD, and the gap between the HOMO of p-chlorobenzonitrile and the LUMO of NaN3in the presence of β-CD is the smallest.Thus, the chemical activity of p-chlorobenzonitrile and NaN3with β-CD as catalyst is improved and the major match is the HOMO of p-chlorobenzonitrile with the LUMO of NaN3.The conclusion is supported by the result from Fig.4 that the LUMO is mainly focused on p-chlorobenzonitrile in CB.The electrophilicity (ω) of free p-chlorobenzonitrile and CB is calculated[37]:

The values in the presence and absence of β-CD are 3.8060 and 3.4771 eV, respectively.The electrophilicity of p-chlorobenzonitrile is more obvious with β-CD catalyst.The energy of HOMO is larger in complex than in free, while the LUMO is smaller.Therefore, the IP of guest is decreased and EA is enhanced upon complexation.The chemical activity and electrophilicity of p-chlorobenzonitrile are greater after the formation of inclusion complex.This suggests that the p-chlorobenzonitrile in the cavity of β-CD is more active and electrophilic than free.
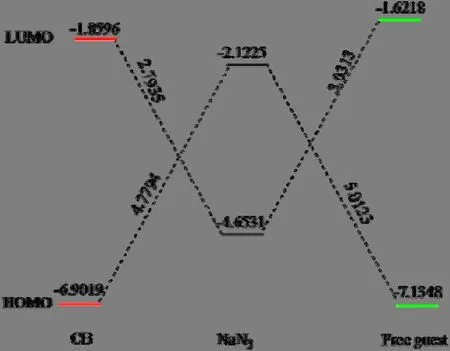
Fig.5.HOMO-LUMO energy gap (eV) for reactants in the presence and absence of β-CD
3.5 Mulliken charge
In order to confirm the results about electronic distribution, the Mulliken charge analyses for isolated guest and CB are researched and recorded in Table 5.The total charges of p-chlorobenzonitrile change from 0 to 0.0267 upon complexation.This indicates the p-chlorobenzonitrile with positive charges in the complex.In other words, the p-chlorobenzonitrile donates electron upon complexation and the total charges of all atoms are certainly zero, sob-CD in CB must accept electron and vice versa.Therefore, p-chlorobenzonitrile is electron-deficient with electrophilicity in complex, which is in good agreement with the conclusion from frontier molecular orbitals analysis.

Table 5.Mulliken Charges (e) for Isolated Guest and Guest in Complex
The obvious charge transfer can be found on the atoms in functional group (-CN).They are more sensitive to be affected by β-CD.The C11possesses more positive charges, while N12has more negative charges in CB, which is in accordance with the result from13CNMR.However, more positive charges are distributed on cyanogroup and p-chlorobenzonitrile than negative charges.Thus, the functional group is easier to be attacked by azide ions in the presence of β-CD as catalyst.
3.6 Catalytic mechanism
Based on the above conclusions, a plausible reaction pathway is proposed and shown in Fig.6.In the first step, the p-chlorobenzonitrile enters into the cavity forming hydrogen bonds between nitrogen atom (N148) and the secondary hydroxyl, then the electron density redistributes between b-CD and p-chlorobenzonitrile.The hydrogen bonds ofb-CD with p-chlorobenzonitrile help p-chlorobenzonitrile enter the cavity of b-CD, and fix p-chlorobenzonitrile in the cavity.The formation of hydrogen bonds changes the charge distribution of the p-chlorobenzonitrile, and benefits to the [2+3]cycloaddition.The p-chlorobenzonitrile is electrondeficient with electrophilicity and more positive charges focus on the carbon atom of cyanogroup (C11)and more negative charges concentrate on the nitrogen atom of cyanogroup (N12).The NaN3can produce azide ions to attack the atoms in functional group (-CN) more easily.Thus, the activity of p-chlorobenzonitrile reacting with the azide ions is increased in the presence of b-CD.Thus, the b-CD catalyst can activate the p-chlorobenzonitrile and facilitate the [2+3] cycloaddition reaction for the synthesis of 5-substituted 1H-tetrazoles by the way of molecular complex.
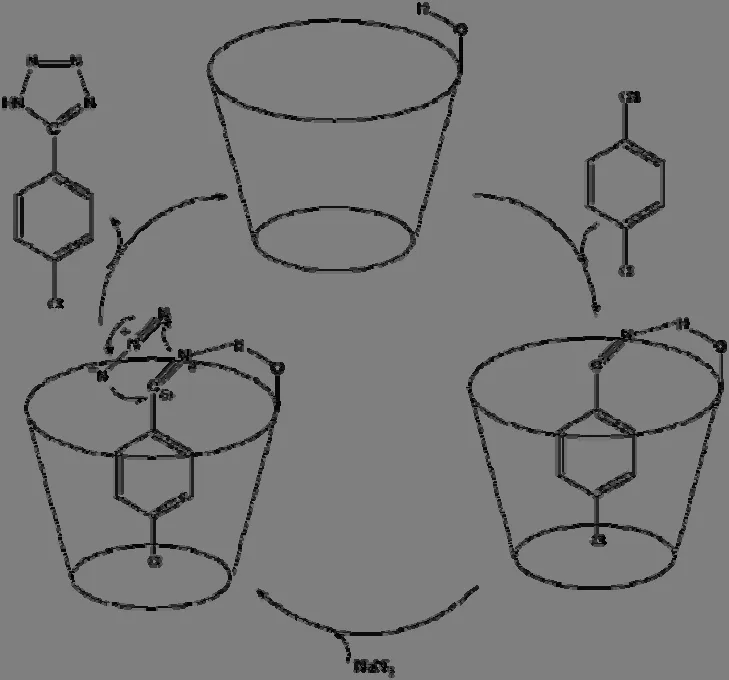
Fig.6.Proposed mechanism for β-CD catalyst synthesis of 5-substituted 1H-tetrazoles
4 CONCLUSION
The energy results show that the p-chlorobenzonitrile can bind with β-CD efficiently in nonaqueous DMF.The NBO analysis suggests that the hydrogen bonds between nitrogen atom (N148) and the secondary hydroxyl make great contribution to driving the guest into the cavity of b-CD and keeping stability of the complex.The1H NMR of b-CD shows that the p-chlorobenzonitrile is the main association with internal protons of b-CD, and the13C and15N spectra reflect more positive charges focus on the carbon atom of cyanogroup (C11) and more negative charges concentrate on the nitrogen atom of cyanogroup (N12) upon complexation.The p-chlorobenzonitrile in the cavity of β-CD is more active and electrophilic than the free.The functional group of p-chlorobenzonitrile is easier to be attacked by azide ions in the presence of β-CD as catalyst.
REFERENCES
(1) Zhang, J.; Ma, P.X.Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective.Adv.Drug.Delivery Rev.2013, 65, 1215-1233.
(2) Fleischmann, C.; Ritter, H.Color indicator for supramolecular polymer chemistry: phenolphthalein-containing thermo- and pH-sensitive N-(isopropyl) acrylamide copolymers and β-cyclodextrin complexation.Macromol.Rapid Comm.2013, 34, 1085-1089.
(3) Li, Z.; Couzijn, E.P.A.; Zhang, X.Intrinsic properties of α-cyclodextrin complexes with benzoate derivatives in the gas phase: an experimental and theoretical study.J.Phys.Chem.B2012, 116, 943-950.
(4) López, C.A.; de Vries, A.H.; Marrink, S.J.Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes.Sci.Rep.-UK.2013, 3, 2071-2076.
阿里偏着头,想了想,觉得阿东说得有理。于是他不等阿东开口教他怎么磕头,便使劲磕了起来。墓穴尚未封口,水泥边毛毛糙糙。等阿东制止他时,他的额头已经磕出了血,水泥边沾上他的血印。
(5) Nakamura, A.; Inoue, Y.Electrostatic manipulation of enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate within γ-cyclodextrin cavity through chemical modification inverted product distribution and enhanced enantioselectivity.J.Am.Chem.Soc.2005, 127,5338-5339.
(6) Breslow, R.; Dong, S.D.Biomimetic reactions catalyzed by cyclodextrins and their derivatives.Chem.Rev.1998, 98, 1997-2011.
(7) Strimbu, L.; Liu, J.; Kaifer, A.E.Cyclodextrin-capped palladium nanoparticles as catalysts for the Suzuki reaction.Langmuir.2003, 19, 483-485.
(8) Surendra, K.; Krishnaveni, N.S.; Mahesh, A.; Rao, K.R.Supramolecular catalysis of Strecker reaction in water under neutral conditions in the presence of β-cyclodextrin.J.Org.Chem.2006, 71, 2532-2534.
(9) Murthy, S.N.; Madhav, B.; Kumar, A.V.; Rao, K.R.Nageswar, Y.V.D.Facile and efficient synthesis of 3,4,5-substituted furan-2(5H)-ones by using β-cyclodextrin as reusable catalyst.Tetrahedron2009, 65, 5251-5256.
(10) Hapiot, F.; Tilloy, S.; Monflier, E.Cyclodextrins as supramolecular hosts for organometallic complexes.Chem.Rev.2006, 106, 767-781.
(11) Surendra, K.; Krishnaveni, N.S.; Sridhar, R.; Rao, K.R.Synthesis of β-hydroxysulfides from alkenes under supramolecular catalysis in the presence of β-cyclodextrin in water.J.Org.Chem.2006, 71, 5819-5821.
(12) Kumar, A.; Tripathi, V.D.; Kumar, P.β-Cyclodextrin catalysed synthesis of tryptanthrin in water.Green Chem.2011, 13, 51-54.
(14) Wardrop, D.J.; Komenda, J.P.Dehydrative fragmentation of 5-hydroxyalkyl-1H-tetrazoles: a mild route to alkylidenecarbenes.Org.Lett.2012, 14,1548-1551.
(15) Herr, R.J.5-Substituted-1H-tetrazoles as carboxylic acid isosteres: medicinal chemistry and synthetic methods.Bioorg.Med.Chem.2002, 10,3379-3393.
(16) Truica-Marasescu, F.; Wertheimer, M.R.Nitrogen-rich plasma-polymer films for biomedical applications.Plasma Process.Polym.2008, 5, 44-57.
(17) Wittenberger, S.J.; Donner, B.G.Dialkyltin oxide mediated addition of trimethylsilyl azide to nitriles: a novel preparation of 5-substituted tetrazols.J.Org.Chem.1993, 58, 4139-4141.
(18) Kantam, M.L.; Kumar, K.B.S.; Sridhar, C.Nanocrystalline ZnO as an efficient heterogeneous catalyst for the synthesis of 5-substituted 1H-tetrazoles.Adv.Synth.Catal.2005, 347, 1212-1214.
(19) Bonnamour, J.; Bolm, C.Iron salts in the catalyzed synthesis of 5-substituted 1H-tetrazoles.Chem.Eur.J.2009, 15, 4543-4545.
(20) Nasrollahzadeh, M.; Bayat, Y.; Habibi, D.; Moshaee, S.FeCl3-SiO2as a reusable heterogeneous catalyst for the synthesis of 5-substituted 1H-tetrazoles via [2+3] cycloaddition of nitriles and sodium azide.Tetra.Lett.2009, 50, 4435-4438.
(21) Kantam, M.L.; Kumar, K.B.S.; Raja, K.P.An efficient synthesis of 5-substituted 1H-tetrazoles using Zn/Al hydrotalcite catalyst.J.Mol.Catal.A-Chem.2006, 247, 186-188.
(22) Amantini, D.; Beleggia, R.; Fringuelli, F.; Pizzo, F.; Vaccaro, L.TBAF-catalyzed synthesis of 5-substituted 1H-tetrazoles under solventless conditions.J.Org.Chem.2004, 69, 2896-2898.
(23) Patil, D.R.; Wagh, Y.B.; Ingole, P.G.; Singh, K.; Dalal, D.S.β-Cyclodextrin-mediated highly efficient [2+3] cycloaddition reactions for the synthesis of 5-substituted 1H-tetrazoles.New J.Chem.2013, 37, 3261-3266.
(24) Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, Jr.J.A.; Vreven, T.; Kudin, K.N.;Burant, J.C.; Millam, J.M.; Iyengar, S.S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G.A.; Nakatsuji, H.;Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J.E.;Hratchian, H.P.; Cross, J.B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A.J.; Cammi, R.; Pomelli, C.; Ochterski,J.W.; Ayala, P.Y.; Morokuma, K.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Zakrzewski, V.G.; Dapprich, S.; Daniels, A.D.; Strain, M.C.; Farkas,O.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Ortiz, J.V.; Cui, Q.; Baboul, A.G.; Clifford, S.; Cioslowski, J.; Stefanov, B.B.;Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Challacombe,M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Gonzalez, C.; Pople, J.A.Gaussian, Inc., Pittsburgh PA2013, Gaussian 03, Revision B.05.
(25) Lee, C.; Yang, W.; Parr, R.G.Development of the colle-salvetti correlation-energy formula into a functional of the electron density.Phys.Rev.B1998, 37, 785-789.
(26) Mennucci, B.Polarizable continuum model.Wires Comput.Mol.Sci.2012, 2, 386-404.
(27) Wang, T.; Wu, Y.; Wang, X.Molecular structure and vibrational bands and13C chemical shift assignments of both enmein-type diterpenoids by DFT study.Spectrochim.Acta A2014, 117, 449-458.
(28) Toušek, J.; Miert, S.V.; Pieters, L.; Baelen, G.V.; Hostyn, S.; Maes, B.U.W.; Lemière, G.; Dommisse, R.; Marek, R.Structural and solvent effects on the13C and15N NMR chemical shifts of indoloquinoline alkaloids: experimental and DFT study.Magn.Reson.Chem.2008, 46, 42-51.
(29) Glendening, E.D.; Landis, C.R.; Weinhold, F.Natural bond orbital methods.Wires Comput.Mol.Sci.2012, 2, 1-42.
(30) Desiraju, G.R.The C–H··O hydrogen bond: structural implications and supramolecular design.Acc.Chem.Res.1996, 29, 441-449.
(31) Chocholoušová, J.; Špirko, V.; Hobza, P.First local minimum of the formic acid dimer exhibits simultaneously red-shifted O–H··O and improper blue-shifted C–H··O hydrogen bonds.Phys.Chem.Chem.Phys.2004, 6, 37-41.
(32) Uccello-Barretta, G.; Balzano, F.; Sicoli, G.; Friglola, C.; Aldana, I.; Monge, A.; Paolino, D.; Guccione, S.Combining NMR and molecular modeling in a drug delivery context: investigation of the multi-mode inclusion of a new NPY-5 antagonist bromobenzene sulfonamide into β-cyclodextrin.Bioorg.Med.Chem.2004, 12, 447-458.
(33) Yan, Z.; Zuo, Z.; Lv, X.; Li, Z.; Li, Z.; Huang, W.Adsorption of NO on MoO3(010) surface with different location of terminal oxygen vacancy defects:a density functional theory study.Appl.Surf.Sci.2012, 258, 3163-3167.
(34) Politzer, P.; Abu-Awwad, F.A comparative analysis of Hartree-Fock and Kohn-Sham orbital energies.Theor.Chem.Acc.1998, 99, 83-87.
(35) Zhan, C.G.; Nichols, J.A.; Dixon, D.A.Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies.J.Phys.Chem.A2003, 107, 4184-4195.
(36) Prabhu, A.A.M.; Sankaranarayanan, R.K.; Venkatesh, G.; Rajendiran, N.Dual fluorescence of fast blue RR and fast violet B: effects of solvents and cyclodextrin complexation.J.Phys.Chem.B2012, 116, 9061-9074.
(37) Dinar, K.; Sahra, K.; Seridi, A.; Kadri, M.Inclusion complexes of N-sulfamoyloxazolidinones with β-cyclodextrin: a molecular modeling approach.Chem.Phys.Lett.2014, 595–596, 113-120.
猜你喜欢
杂志排行
结构化学的其它文章
- Synthesis and Characterization of a Palladium Complex Supported by Bidentate Ligand and Catalysis of the Vinyl Polymerization of Norbornene①
- Structural, Electronic, Optical and Thermodynamic Properties of Nanolaminated Boride Cr4AlB6①
- Synthesis, Structure and Photochromism of a New Diarylethene and Its Ag(I) Complex①
- Ionothermal Synthesis, Structure and Luminescent Properties of a New 2-D Bismuth(III) Coordination Polymer with (6,5)-Connected Topological Sheet①
- Synthesis, Crystal Structure and Properties of a 1D Heteronuclear Cobalt-sodium Polymer with Bridging Ligand 2-(2-Hydroxy-3-methoxybenzylidene)Hydrazinecarbothioamide①
- Synthesis, Crystal Structure and Photoluminescence of a Dinulear Copper Complex①
