Downregulation of signal transduction and STAT3 expression exacerbates oxidative stress mediated by NLRP3 inflammasome
2018-10-22HuaBaiQiFangZhangJuanJuanDuanDeJunYuLiJieLiu
Hua Bai , Qi-Fang Zhang , Juan-Juan Duan De-Jun Yu Li-Jie Liu
1 Medical Laboratory Center, Third Affiliated Hospital, Guizhou Medical University, Duyun, Guizhou Province, China
2 Department of Neurology, Third Affiliated Hospital, Guizhou Medical University, Duyun, Guizhou Province, China
3 Key Laboratory of Endemic and Ethnic Diseases of Ministry of Education, and Key Laboratory of Medical Molecular Biology, Guizhou Medical University, Guiyang, Guizhou Province, China
Abstract Activated nucleotide binding to the oligonucleotide receptor protein 3 (NLRP3) inflammasome is possibly involved in the pathogenesis of Alzheimer’s disease through oxidative stress and neurogenic inflammation. Low expression of the signal transducer and activator of transcription 3 (STAT3) gene may promote the occurrence of neurodegenerative diseases to some extent. To clarify the roles of the NLRP3 inflammasome and STAT3 expression in oxidative stress, (1) SHSY5Y cells were incubated with 1 mM H2O2 to induce oxidative stress injury, and the expression of human-cell-specific signal transduction, STAT3-shRNA silencing signal transduction and STAT3 were detected. Cells were pretreated with Ca2+ chelator BAPATA-AM (0.1 mM) for 30 minutes as a control. (2) Western blot assay was used to analyze the expression of caspase-1, NLRP3, signal transduction and STAT3. Enzyme-linked immunosorbent assay was used to analyze interleukin-1β levels. Flow cytometry was carried out to calculate the number of apoptotic cells. We found that H2O2 treatment activated NLRP3 inflammasomes and decreased phosphorylation of signal transduction and STAT3 serine 727. BAPTA-AM pretreatment abolished the H2O2-induced activation of NLRP3 inflammasomes, caspase-1 expression, interleukin-1β expression and apoptosis in SHSY5Y cells, and had no effect in cells with downregulated STAT3 expression by RNAi. Thefindings suggest that downregulation of signal transduction and STAT3 expression may enhance the oxidative stress mediated by NLRP3, which may not depend on the Ca2+ signaling pathway.
Key Words: nerve regeneration; signal transducer and activator of transcription 3; calcium; caspase-1; nucleotide binding to the oligonucleotide receptor protein 3; inflammasome; hydrogen peroxide; Alzheimer’s disease; shRNA; SHSY5Y cells; neural regeneration
Introduction
Oxidative stress plays an important role in the mechanism of Alzheimer’s disease (AD) and other neurodegenerative diseases (Puangmalai et al., 2017). The influence of the amyloid-beta peptide (Aβ) on the pathogenesis of AD may be mediated by its effects on oxidative homeostasis. Some Aβ deposits are phagocytosed by microglia and enter lysosomes in the brain,leading to lysosomal instability manifesting as AD progression(Lin et al., 2013; Zhang et al., 2016). Hydrogen peroxide (H2O2)is one of the primary mediators of oxidative stress and can rapidly induce higher order chromatin degradation. The ability for Aβ to generate H2O2provides a potential mechanism for the oxidative stress related to AD (Ahn et al., 2010). In PC12 cells pretreated with olanzapine, the depression in cell viability induced by H2O2is attenuated (Khan et al., 2015). We have previously reported that rapid high-order chromatin degra-dation in SHSY5Y cells is not a result of substantial oxidative stress but rather is triggered by signaling cascades initiated specifically by H2O2(Bai and Konat, 2003).
Nucleotide binding to the oligonucleotide receptor protein 3 (NLRP3) inflammasomes are important players in inflammation and are associated with neurodegenerative diseases (Baroja-Mazo et al., 2014). NLRP3 is an important component of NLRP3 inflammasomes, which mediate the maturation of interleukin (IL)-1β by activating caspase-1.The generation of reactive oxygen species (ROS), potassium(K) efflux and lysosomal destabilization are three classic approaches for inflammasome activation (Stehlik and Dorfl eutner, 2007; Feng and Liu, 2016). Ten years ago, studies of inflammasome activation focused particularly on the K ion.K+efflux activates NLRP3 inflammasomes in a specific manner, but modulating the ionic milieu in other ways also affects inflammasome activation. A later study focused on the role of calcium mobilization in activation of the inflammasome(Bigford et al., 2013). It has been shown that Ca2+signaling is required for activation of the NLRP3 inflammasome,and that mitochondrial damage during ATP stimulation is strongly associated with calcium mobilization (Elliott et al.,2018). Expression of specific proteins can regulate NLRP3 inflammasome activity through sequestering inflammasome components by homotypic interactions with caspase recruitment domains (CARDs), or through directly inhibiting the function of caspase-1 (Clapham, 2007). Calcium mobilization regulates diverse life processes, including gene transcription and expression, cellular proliferation and differentiation, and cellular metabolism and death (Aminzadeh et al., 2018).
Other factors not dependent on calcium mobilization may be involved in NLRP3 activation during oxidative stress and neuronal degradation. NLRP3 is an integral functional component in ischemic preconditioning of the isolated heart through a STAT3-dependent mechanism that does not involve the NLRP3 inflammasome (Zuurbier et al., 2012). Defi ciency of the innate immune NLRP3 receptor is associated with a reduction of STAT3 signaling. Mizushina et al. (2015)have shown that NLRP3 can control STAT3 signaling in alveolar epithelial cells and can independently affect the function of IL-1β production for macrophages and neutrophils.In the study, which used NLRP3−/−mice, reducing activation of STAT3 and increasing numbers of apoptotic cell numbers in alveolar epithelial cells regulated matrix metalloproteinase-9 and Bcl-2. On the basis of the above information, we assumed that a change of STAT3 signaling is likely to have a direct impact on the activation of NLRP3 inflammasomes in oxidative stress and neurodegeneration.
In this study, we used H2O2to induce oxidative stress as a classical model of AD, and attempted to explore the relationship between the NLRP3 inflammasome and oxidative stress.
Material and Methods
Cell culture and treatment
Human neuroblastoma SH-SY5Y cells (Shanghai Sixin Biotech Co., Ltd., Shanghai, China) were cultured in DMEM/F12 medium (Gibco/Thermo Fisher Scientific, Carlsbad,CA, USA) containing 10% fetal bovine serum (Sigma, St.Louis, MO, USA). An antibiotic-antimycotic mixture was added and incubated in 5% CO2at 37°C. The SH-SY5Y cells (5 × 106viable cells) were plated in a 60-mm Petri dish for 3 hours to facilitate sticking to the substratum before experiments. Subsequently, the cells were transferred to serum-free medium and grown overnight. The cells were washed in Hank’s balanced salt solution (Sigma) without phenol red. In some cell groups, calcium-free Hank’s solution was used and cells were treated with 1 mM H2O2(Sigma). Additionally, some cells were pre-treated with 0.1 mM 1,2-bis(o-aminophenoxy)ethane-N′,N′,N′,N′-tetraacetic acid tetra(acetoxymethyl)ester (BAPTA-AM; Boston Biochem,Cambridge, MA, USA), a Ca2+chelator, and incubated in the growth medium 30 minutes before the treatment with 1 mM H2O2. The control cells were supplemented with the same medium without H2O2. All cells were treated with the appropriate volume of dimethyl sulfoxide as the control.
STAT3 silencing and overexpression
Human-cell-specific STAT3-shRNA (shRNA) or sh scramble (shScr) were cloned into lentivirus vector pFLU-EGFP,according to the modified method from Yang et al. (2009),using shRNA sequence: 5′-GAT CCG CAT CTG CCT AGA TCG GCT ATT CAA GAG ATA GCC GAT CTA GGC AGA TGT TTT TTG-3′, or shScr sequence: 5′-GAT CCG TCG AGC TAA TGC GAG TAG CGT TGC TGT GCT ATC GGT TCA GAG TAG ATG TTT TTT G-3′. Resulting recombinant vectors were verified by sequencing, and then were transfected in 293T cells (American Type Culture Collection, Manassas, VA, USA) to produce lentiviruses with packaging vectors.Lentiviruses were collected and centrifuged to remove cell debris, thenfiltered by 0.45-mm cellulose acetatefilters. Cells were infected with lentiviruses carrying pFLU-EGFP STAT3 shRNA, and were later sorted with EGFP-positive cells byflow cytometry (BD Biosciences, San Jose, CA, USA). Human STAT3 cDNA in pCMV was purchased from Sino Biological (Beijing, China) and transfected into cells to overexpress STAT3. Cells overexpressing STAT3 were selected by hygromycin. The efficiency of STAT3 silencing or overexpression was determined by western blot assay. Adenovirus gene vector pFLU-EGFP was constructed by Shanghai GeneChem Co., Ltd. (Shanghai, China).
Reverse transcription-polymerase chain reaction(RT-PCR)
Total RNA was extracted from various SH-SY5Y cells through an RNeasy Plus Mini Kit (Beijing Qiagen Technology Biology Co., Ltd., Beijing, China) according to the manufacturer’s protocol. The AMV Reverse Transcriptase kit (Applied Biosystems Foster, CA, USA) was used for reverse transcription,and each random primer was approximately 1 μg. Primers for human NLRP3 were: up, 5′-GAT CTT CGC TGC GAT CAA CAG-3′; and down, 5′-CGT GCA TTA TCT GAA CCC CAC-3′. The primers for β-actin were: up, 5′-GCC TCA GGT AGT GCT G-3′; and down, 5′-GTC GGA AGG TGG ACA GCG A-3′. The total RNA (2 μg) was used for synthesis of the first strand cDNA at 42°C with 20 μL reaction mixture containing 0.5 μg oligo (dT) 15, and 0.25 mM of each dNTP,15 U AMV reverse transcriptase, and 20 U RNase inhibitor.The incubation time for the reaction was 5 minutes at 95°C.Each PCR cycle was set up for denaturation at 94°C for 30 seconds, primer annealing at 56°C for 40 seconds, extension at 72°C for 50 seconds, and afinal extension step at 72°C for 8 minutes. PCR amplification was performed for 30 cycles. All samples were assayed in triplicate, and relative gene expression was quantified using Image-ProPlus 6.0 software (Media Cybernetics, Washington, DC, USA).
Western blot assay
The SH-SY5Y cells were treated and divided into groups as follows: mock group (blank control) and shScr group (cells were transfected with an empty vector), H2O2group and shScr + H2O2group (normal cells treated solely with H2O2),shRNA group (cells transfected by lentiviral pFLU-EGFPsh-STAT3 plasmid to construct a stable cell line that can inhibit STAT3 protein), and H2O2+ shRNA group (cells infected by lentiviral pFLU-EGFPshSTAT3 plasmid, then treated with H2O2). The cells were lysed and the protein level was measured by using a bicinchoninic acid protein assay kit (Pierce,Rockford, IL, USA). The lysate protein mixture with 40 μg lysis buffer was subjected to analysis with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Subsequently,the protein was transferred to a nitrocellulose membrane.The membrane was blocked with 1× tris-buffered saline with 0.1% Tween-20 (TBS-Tween) and 5% skim milk at 4°C overnight. Subsequently, the membrane was washed three times,then was soaked in buffer containing the primary antibody for 2 hours at room temperature. The following primary antibodies were used: rabbit anti-human caspase-1 (1:400;Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-human NLRP3 (1:500; Santa Cruz Biotechnology), rabbit anti-human STAT3 (1:100; Cell Signaling Technology, Danvers, MA, USA) and rabbit anti-p-STAT3 antibody (1:100;Cell Signaling Technology), or rabbit anti-human β-actin monoclonal antibody (1:1000; Cell Signaling Technology),rabbit anti-human tubulin monoclonal antibody (1:1000;Cell Signaling Technology) and rabbit anti-human GAPDH monoclonal antibody (1:1000; Cell Signaling Technology).After washing three times with TBS-Tween buffer, horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2000;Thermo-Pierce, Rockford, IL, USA) was added with an enhanced chemiluminescence kit (Merck Millipore Corporation, Billerica, MA, USA), according to the manufacturer’s instructions. Optical density densitometry was performed on scanned immunoblot images by the Quantity One soft-ware from Bio-Rad Laboratories (Shanghai, China).
Enzyme-linked immunosorbent assay (ELISA)
The total protein concentration was measured by the DC protein assay kit from Bio-Rad Laboratories (Hercules,CA, USA) following the manufacturer’s protocol. Interleukin-1beta (IL-1β) levels were measured with SH-SY5Y cell culture medium using IL-1β Quantikine ELISA kits from R&D Systems (Minneapolis, MN, USA) according to manufacturer’s instructions. Optical density values were measured at 450 mm with a microplate reader (Bio-Rad Laboratories)in each well.
Flow cytometry
Approximately 5 × 106cells were collected in 5 mL tubes,then centrifuged and re-suspended in 500 μL binding buffer(140 mM NaCl, 2.5 mM CaCl2, 10 mM HEPES/NaOH, pH 7.4) and stained with 5 μl Annexin-FITC (BD Pharmingen,San Diego, CA, USA) and 10 μL propidium iodide (PI; BD Pharmingen) for 5 minutes at room temperature in the dark.An excess of 1× binding buffer was added to afinal volume of 500 μL after incubation for 15 minutes at 20–25°C in the dark. The stained apoptotic cells were counted using a FACScanflow cytometer (BD FACS Aria, CA, USA).
Statistical analysis
Data, presented as the mean ± SD, were analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Significant differences between the groups were analyzed by one-way analysis of variance and Tukey’s post hoc test. The experimental manipulations were repeated separately, and the control group and treatment group had equal numbers per group. A value of P < 0.05 was considered statistically significant.
Results
Effects of STAT3 downregulation on NLRP3 mRNA expression induced by H2O2
The human SH-SY5Y cells were transfected by using a lentiviral pFLU-EGFPshSTAT3 plasmid to construct a stable cell line that can inhibit STAT3 protein, referred to as the shRNA group. A blank control (mock) group was transfected with an empty vector. H2O2was used to treat SH-SY5Y cells to produce the oxidative stress model in the H2O2group, or in transfected cells for the modified group (shRNA + H2O2). The cells in all four groups were treated with the calcium chelating agent, BAPTA-AM. NLRP3 inflammasome activation in the oxidative stress model, and the effect of STAT3 downregulation on NLRP3 mRNA (141 bp) expression, was detected by RT-PCR 24 hours after intervention. NLRP3 mRNA expression was significantly increased in cells of the H2O2group compared with those of the mock group (P < 0.05). NLRP3 mRNA expression was also significantly increased in the cells of the modified group (H2O2+ shRNA) compared with those of the mock group (P < 0.01). There was a small increase in NLRP3 mRNA expression in the cells of the shRNA group,but it was not significantly different from that of the mock group (P > 0.05; Figure 1B). NLRP3 mRNA expression showed no significant difference between the shRNA group and the mock group (untreated group in Figure 1A and shScr group in Figure 1B) after adding BAPTA-AM. NLRP3 mRNA expression was still significantly increased in the H2O2+ shRNA group compared with the mock group (P < 0.01)after adding BAPTA-AM. However, adding BAPTA-AM significantly decreased expression in the H2O2group to a level similar to the shScr group (P < 0.01; Figure 1).
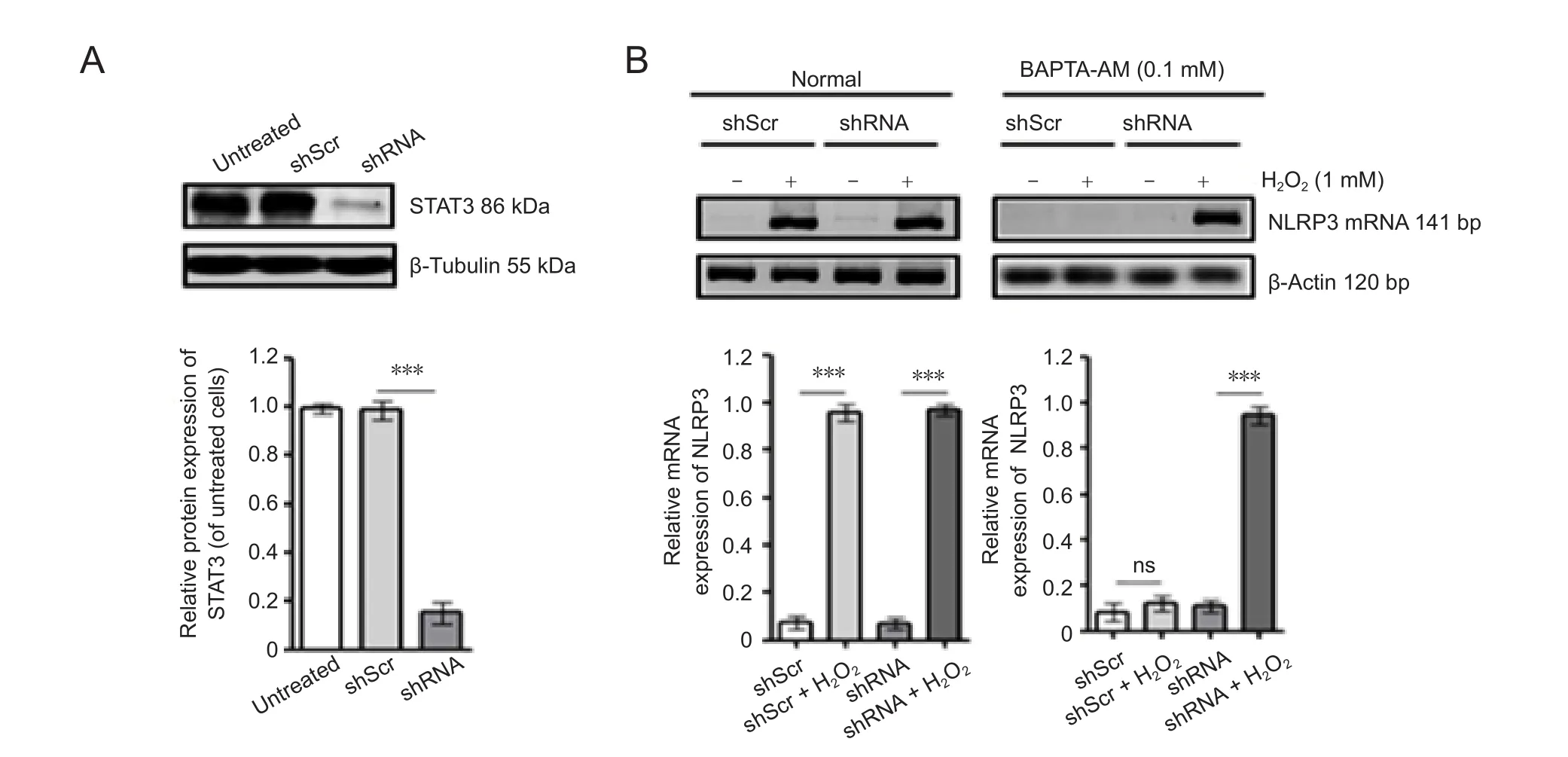
Figure 1 Effect of STAT3 silencing on NLRP3 expression in response to H2O2 in SH-SY5Y cells.
Effects of STAT3 silencing on caspase-1 expression induced by H2O2
We sought to further characterize the mechanism that activates the NLRP3 inflammasomes via STAT3 downregulation by investigating another component of the inflammasome,caspase-1. The four cell groups were treated as mentioned above. Caspase-1 protein expression was detected by western blot assay. The results showed that caspase-1 expression was significantly increased in the cells of the modified group compared with those of the mock group (P < 0.01).Caspase-1 expression was also significantly increased in the H2O2group compared with that of the mock group (P< 0.05), but there was no significant difference between the mock group and the shRNA group (P > 0.05; Figure 2).
Moreover, after adding BAPTA-AM, caspase-1 expression was still significantly increased in the modified group compared with that in the mock group (P < 0.05). Caspase-1 in the mock group had no significant differences when compared with the H2O2group or the shRNA group after adding BAPTA-AM (P > 0.05; Figure 2).
Effects of transfected lentiviral pFLU-EGFPshSTAT3 plasmid on the expression of IL-1β protein before or after BAPTA-AM treatment in the oxidative stress model ofthe human SH-SY5Y cells
We next characterized IL-1β, a downstream mediator in NLRP3 inflammasome activation (Yin et al., 2017). IL-1β protein in each group was detected by ELISA. IL-1β expression was significantly decreased in the mock group compared with that of the modified group (P < 0.01) and H2O2group(P < 0.05). IL-1β expression was not significantly different between the shRNA group and mock group (P > 0.05). IL-1β expression in SH-SY5Y cells was significantly increased in the modified group compared with the mock group after adding BAPTA-AM (P < 0.01). IL-1β expression was not significantly different between the mock group and H2O2group or shRNA group (P > 0.05 for each comparison; Figure 3).
Effects of transfected lentiviral pFLU-EGFPshSTAT3 plasmid on cell apoptosis in the oxidative stress model of the human SH-SY5Y cells
It is speculated that SH-SY5Y cell apoptosis and cellular morphology changes appear after downregulation of STAT3 expression and treatment with H2O2in NLRP3 inflammasome activation. Cell apoptosis was detected by Annexin V/PI staining. There was more cellular apoptosis in the H2O2group and the modified group than in the mock group (P <0.05). The ratio of cellular apoptosis was significantly lower in the H2O2group after adding BAPTA-AM (P < 0.05), but no significant difference in the ratio of cellular apoptosis before and after the addition of BAPTA-AM was found in the modified group or shRNA group (Figure 4).
STAT3 expression is critical for NLPR3 expression and NLRP3 inflammasome activation
To examine whether STAT3 expression is sufficient to influence NLRP3 expression, first, we expressed STAT3 or vehicle (empty vector) in the shRNA group to obtain two groups: shRNA + vehicle group (V-shRNA) and shRNA +STAT3 group (O-shRNA). STAT3 was overexpressed in the O-shRNA group compared with the V-shRNA group (Figure 5A). Second, cells in the O-shRNA group were pretreated with BAPTA-AM and then without or with H2O2. Unlike in the shRNA group, NLRP3 and caspase-1 expressions were partly inhibited by BAPTA-AM (Figure 5B). In a rescue test to return STAT3 gene expression after silencing, STAT3 was significantly increased in the O-shRNA group compared with that in the V-shRNA group or mock group (Figure 5A). Furthermore, NLRP3 protein expression was significantly increased in the H2O2+ O-shRNA group compared with that in the O-shRNA group (P < 0.05). Caspase-1 protein expression was also significantly increased in the H2O2+ O-shRNA group compared with that in the O-shRNA group (P < 0.05). These data suggest that STAT3 expression is important to activate NLRP3 expression.
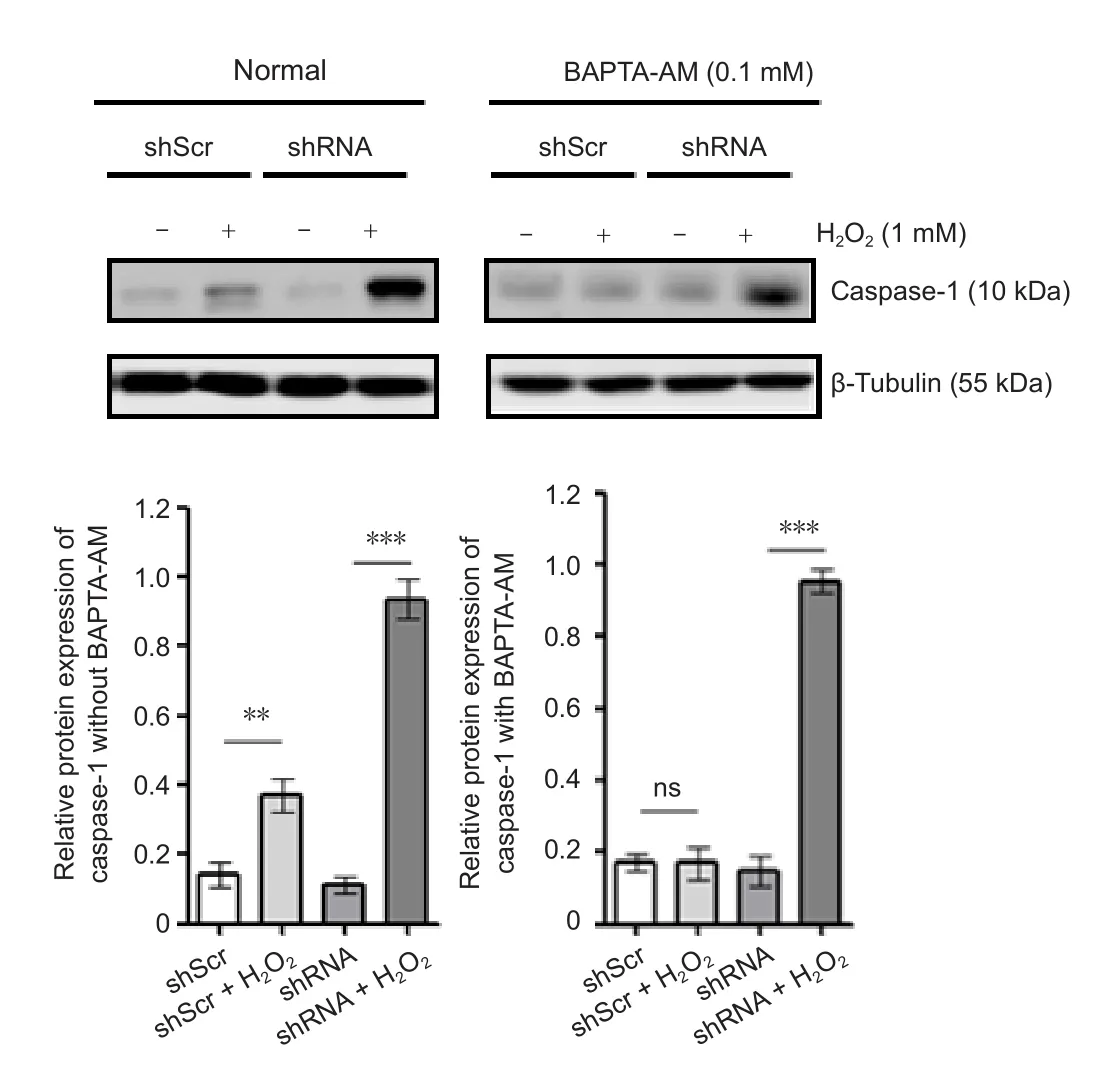
Figure 2 Effect of the transfected STAT3 plasmid for caspase-1 expression before (without) or after (with) BAPTA-AM in an oxidative stress model of SH-SY5Y cells.
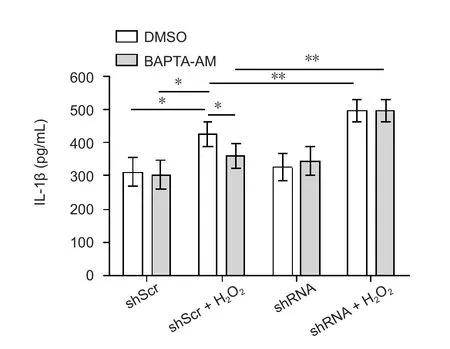
Figure 3 Effect of STAT3 downregulation on IL-1β production.
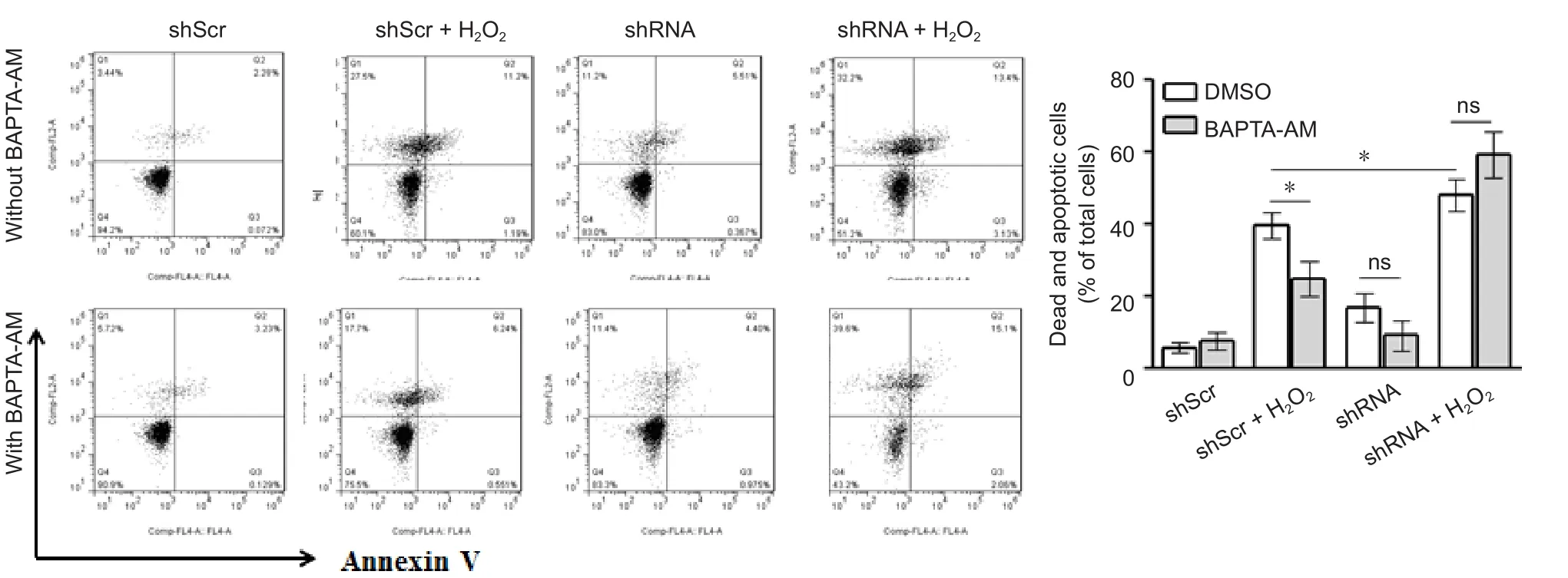
Figure 4 Effect of STAT3 silencing on H2O2-induced dead and apoptotic cells.
It has been shown that STAT3 serine727 phosphorylation in mitochondria is required to regulate mitochondrial ROS production and accumulation, and the serine 727 to alanine mutation of STAT3 augments cellular ROS (Zhang et al., 2013b). It is speculated that BAPTA-AM may be com-pletely consumed when blocking H2O2-induced cell death and apoptosis, while STAT3 silencing reduces the level of cellular STAT3 serine phosphorylation. This could trigger extra endogenous ROS production, which in turn would activate the NLRP3 inflammasome, cell death and apoptosis.Therefore, we examined if H2O2treatment increases STAT3 serine727 phosphorylation. H2O2treatment significantly increased ser727 phosphorylation of STAT3 (Figure 6). Protein expression of p-STAT3 was significantly increased in the modified group compared with that of the shRNA group(P < 0.01), and was significantly decreased in the modified group compared with that of the H2O2+ O-shRNA group (P< 0.05). In addition, the levels of STAT3 serine phosphorylation were obviously increased in response to oxidative stresses in a variety of cell lines and primary cells (unpublished data).
Discussion
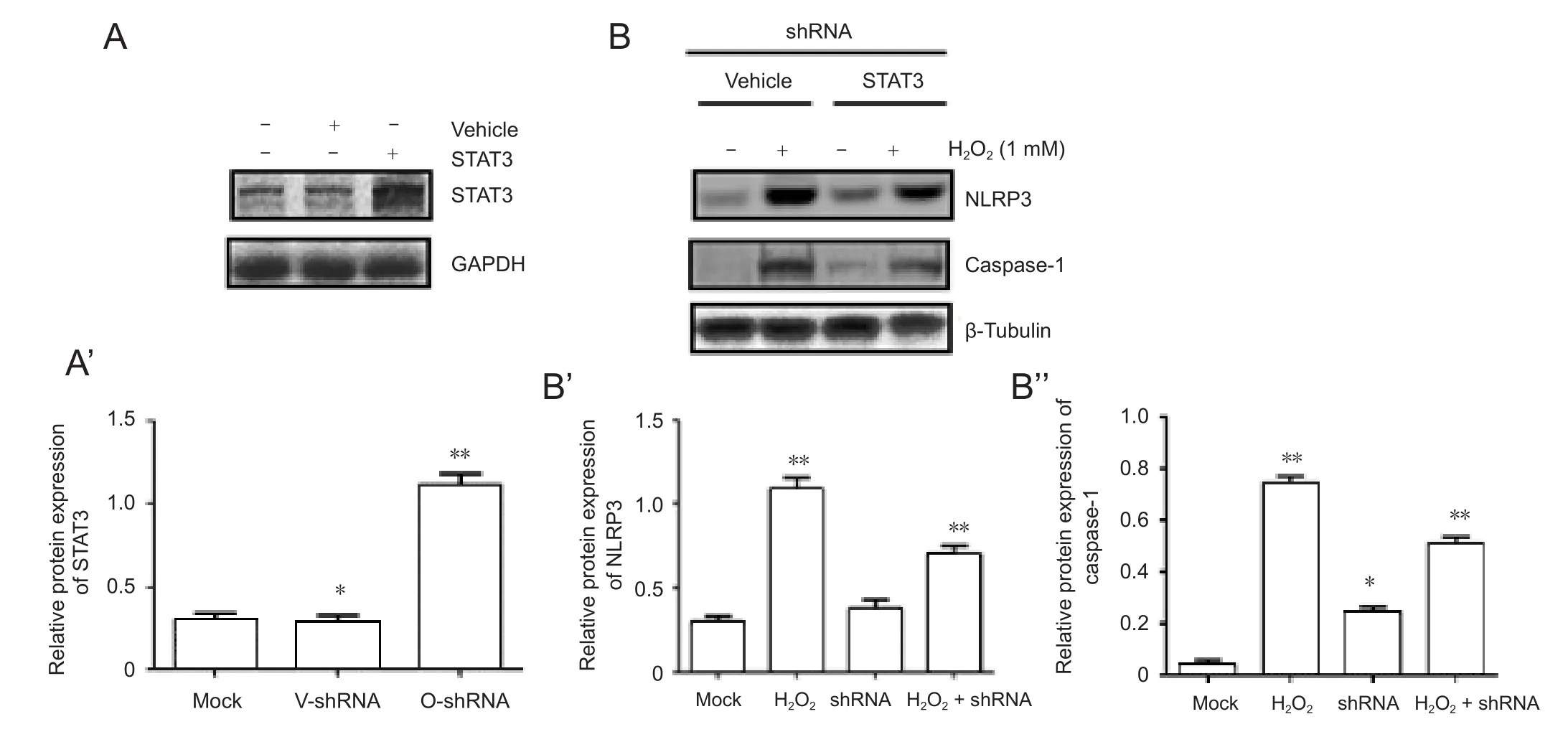
Figure 5 BAPTA-AM partly inhibits H2O2-induced NLRP3 expression in cells with STAT3 overexpression.
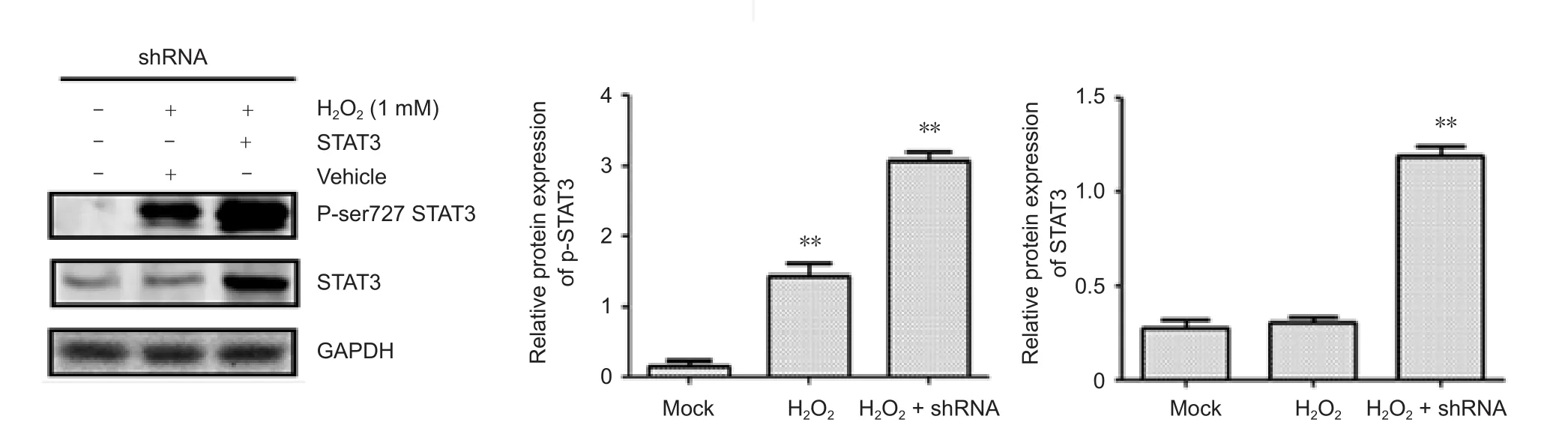
Figure 6 H2O2 activates the phosphorylation of STAT3 serine 727 in an oxidative stress model of SH-SY5Y cells.
Oxidative stress plays an essential role in neuroinflammation (Kim et al., 2017). NLRP3 inflammasome activation-induced IL-1β is a major proinflammatory cytokine in neuroinflammation related to oxidative stress (Liu et al., 2013).However, it is unclear if the molecular mechanism underlying oxidative stress activates NLRP3 inflammasomes. Here,we used SH-SY5Y cells, which are derived from human neuroblastoma and widely used to explore AD and other neurodegenerative diseases. Our results demonstrated that STAT3 expression regulates NLRP3 expression and activation in response to oxidative stress.
A previous study has reported that K ion efflux, lysosomal break, production of ROS, and calcium mobilization are the main mechanisms of NLRP3 inflammasome activation(Tan et al., 2013). Our experiments found that downregulation of STAT3 may be a new mechanism for activating NLRP3 inflammasomes. The NLRP3 inflammasome has recently been thought of as an important signaling receptor involved in the classic innate immune response and various cell responses including endogenous and exogenous signaling pathways. The various components of inflammasomes involving the innate immune response are obvious to AD in the acute-phase adaptation and chronic pathological changes of the nervous system (Heneka and O’Banion, 2007).Until now, it was unclear if inflammation is a cause, contributor, or secondary factor in the pathogenesis of AD, despite evidence that inflammasomes take part in the occurrence of AD (Zhang et al., 2013a). Ourfindings will provide some new clues for studying the role of NLRP3 inflammasomes in AD.
It remains unknown why downregulated expression of STAT3 induces activation of NLRP3 inflammasomes. A possible mechanism for the effect could be disruption of mitochondria and promotion of ROS production so as to promote NLRP3 inflammasome activation. STAT3 is activated by phosphorylation in response to various cytokines and growth factors, including interferons, epidermal growth factor, IL-5, IL-6, hepatocyte growth factor, leukemia inhibitory factor, and bone morphogenetic protein-2, all of which may play key roles in many cellular processes, such as cell growth and apoptosis (Duyckaerts et al., 2008).
Furthermore, neurodegenerative disorders, such as AD and Parkinson’s disease, have been linked to oxidative stress and ROS-mediated cell apoptosis. H2O2is not only a ROS, but it also induces the production of ROS in the brain, which can lead to DNA damage and lipid peroxidation (Gao et al., 2005). The oxidative stress damage model induced by H2O2in SH-SY5Y cells is a simple model, which has been used to study AD and other neurodegenerative diseases. In some experiments, the injury and apoptosis to SH-SY5Y cells were induced by H2O2in a dose-dependent manner, and damage could be blocked by the antioxidant dehydroepiandrosterone after H2O2pre-treatment (Konat et al., 2001). Our results showed that H2O2can induce NLRP3 mRNA expression, and increase caspase-1 and IL-1β protein expression, which could activate the NLRP3 inflammasome.However, NLRP3 mRNA, caspase-1, and IL-1β levels were significantly reduced after adding BAPTA-AM. Murakami et al. (2012) have shown that buffering of intracellular calcium with BAPTA-AM abolishes chromatin fragmentation,and calcium is required for H2O2-induced activation of the MAR-associated endonuclease. In NLRP3 inflammasome activation, Ca2+signaling may have a critical role, as it is likely to attenuate calcium release from the IP3R, enhancing IP3R function to further block inflammasome activation(Gilabert et al., 2001; Martinon and Tschopp, 2007). Those studies have made somewhat inconsistent conclusions regarding the role of calcium in NLRP3 inflammasome activation through treating with BAPTA-AM and incubation in calcium-free media (Xi et al., 2010).
It is worth noting that NLRP3 mRNA, caspase-1 and IL-1β all had a higher expression in the H2O2group than in the mock group. Nevertheless, NLRP3 mRNA, caspase-1, and IL-1β expression was significantly decreased after adding BAPTA-AM. Those changes suggest that downregulation of STAT3 expression can enhance oxidative stress mediated by NLRP3 inflammasomes, which may not depend on the calcium signaling pathway. Ca2+concentration for activation of NLRP3 inflammasomes is indeed important, but increasing of extracellular Ca2+levels or some calcium-sensing receptor agonists normally induces intracellular Ca2+signals by the interaction between phospholipase C and calcium-sensing receptors (Tschopp and Schroder, 2010). Eliciting injury to mitochondria by controlling mitochondrial autophagy inhibits ROS-induced inflammasome activation. In the deficiency of autophagy, sensitization of the NLRP3 inflammasome is remarkably augmented (Halle et al., 2008). Heneka et al. (2013) have suggested that cathepsin B discharged from lysosomes triggers formation of the NLRP3 inflammasome and activation of caspase-1, leading to the secretion of mature IL-1β.
STAT3 is usually activated by phosphorylation of a conservative tyrosine residue in response to cell signals and some oncogenes (Wu et al., 2017). STAT3 phosphorylation and its transcriptional process are coordinated and regulated by temporal and spatial regulation during the progression of AD (Gartel and Kandel, 2006). To explore NLRP3 inflammasome activation in STAT3 downregulated expression,NLRP3 mRNA, caspase-1, and IL-1β expression levels in SH-SY5Y cells transfected with pFLU-EGFPshSTAT3 plasmid, treated with or without H2O2, were detected by RTPCR, western blot assay, and ELISA, respectively. The results showed that NLRP3 mRNA, caspase-1, and IL-1β were not significantly expressed in transfected cells, but NLRP3 mRNA, caspase-1, and IL-1β expression levels were all increased after the transfected cells were treated with H2O2.Furthermore, this increase was not blocked by BAPTA-AM.RNAi is commonly used to silence the expression of some target genes because of its high specificity and non-toxicity(Kim et al., 2005). In this study, a lentivirus vector was used for delivery of the shRNA because it can infect the requisite SH-SY5Y cells with high efficiency and sustain long-term gene expression through integrating into the target genome.Long-lasting silencing and maximal inhibition of gene expression can be made by shRNA at low concentrations(Zheng et al., 2014). As the inhibitory effect of shRNA is associated with the specificity to its target sequence, RT-PCR and western blot assay were used to detect NLRP3 mRNA and caspase-1 protein, respectively, indirectly analyzing the efficacy of STAT3 siRNA in SH-SY5Y cells. In this study, the rescue test to return STAT3 gene expression after silencing was performed; our results demonstrated that STAT3 expression was significantly increased in the O-shRNA group compared with the vehicle group or mock group. Above studies confirmed that the experiment is reliable or feasible.
Guarda et al. (2011) observed that cucurbitacin B inhibits growth in SH-SY5Y human neuroblastoma cells and induces cell apoptosis by the JAK2/STAT3 pathway. In addition,the type I IFN can also suppress IL-1 production and activation of the inflammasome, which may induce IL-1β-dependent adaptive immunity (Ghiringhelli et al., 2009; Kiu and Nicholson, 2012). JAK/STAT3 signaling is the main anti-apoptotic pathway for the transduction of signals that are important for developmental and homeostatic processes(Krauthausen et al., 2015). Tyk2/STAT3-mediated signaling is involved in Aβ-induced neuronal apoptosis, suggesting that STAT3 works as a vital player in the progression of AD(Martinon and Tschopp, 2007; Saitoh et al., 2008; Wan et al., 2010). To explore if SH-SY5Y cell apoptosis can happen after downregulated STAT3 expression and treatment with H2O2, we performed Annexin V/PI staining in treated cells.We found that there were some apoptotic cells in the H2O2treatment and shRNA groups, but many apoptotic cells appeared in shSTAT3 cells treated with H2O2. Furthermore,after adding BAPTA-AM, no apoptosis was observed for all groups. Park et al. (2011) have shown that reduction of STAT3 gene expression obviously weakens Aβ-induced neuronal apoptosis, and STAT3 activation can help to promote neuronal apoptosis from Aβ treatment, as activation of a tyrosine kinase, Tyk2, is required for the Aβ-induced tyrosine phosphorylation of STAT3. This result has a slight contradiction with our experiment. The possible reason could be the difference between Aβ and H2O2. In some experiments, the downregulation of STAT3 function, such as by dimerization, can inhibit the upregulation of inducible nitric oxide synthase transcript induced by Aβ (Bos, 2003;Martinon and Tschopp, 2007; Mizushina et al., 2015). As a result, when STAT3 is activated by cortical neurons cultured in vitro, excitation of the target receptor on neurons by Aβ may directly trigger the signaling mechanisms during AD,which is critical for unraveling the pathophysiology of relevant diseases (Li and Rossi, 2005; Lee et al., 2012).
There are some limitations in this study. The oxidant H2O2used is relatively simple, and the cell lines used are relatively single. Further, whether the results of the experiment can be generalized in the AD model still requires verification from animal experiments.
In conclusion, activation of the NLRP3 inflammasome required Ca2+signaling in SH-SY5Y cells treated with H2O2,and STAT3 downregulation by RNAi abolished the inhibition of the Ca2+chelator on H2O2-induced NLRP3 activation,caspase-1 expression, and IL-1β release. Overexpression of STAT3 partly rescued NLRP3 and caspase-1 expressions.H2O2treatment induced serine 727 phosphorylation of STAT3, known for its low level needed to accumulate mitochondrial ROS. We conclude that inhibiting STAT3 expression may block the activation of NLRP3 inflammasomes.We have identified that inhibition of STAT3 expression can enhance the oxidative stress mediated by NLRP3 and cell apoptosis. Moreover, these effects may not depend on the Ca2+signaling pathway. Further studies are warranted to elucidate the involvement of STAT3 downregulated expression on NLRP3 inflammasome activation and in AD pathophysiology and progression.
Author contributions:Study design and concept: HB and QFZ; paper writing: HB; experiment implement, data analysis, and result interpretation: QFZ and JJD; implement of some experiments and data analysis:HB, DJY and LJL; paper revision: HB and QFZ. All authors read and approved thefinal version of the paper.
Conflicts of interest:None declared.
Financial support:This study was supported by Department of Science and Technology in Guizhou Province of China, No. Basic [2016]1131(to Qian-Ke-He; to HB); Department of Health and Family Planning Commission in Guizhou Province of China, No. 2015-326 (to HB); Less Developed Regions of the National Natural Science Foundation of China,No. 81560482; the Research Foundation for Creative Research Groups of Education Bureau of Guizhou Province of China, No. KY[2016]033 (to QFZ). All authors declare thatfinancial support does not affect the opinion of the article and the objective statistical analysis and report of the research results in this study.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- MicroRNAs of microglia: wrestling with central nervous system disease
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Apomorphine effects on the hippocampus
- Roles and functions of Atp6ap2 in the brain
- Magnesium sulfate and fetal neuroprotection:overview of clinical evidence
- Polyphenols-gut microbiota interplay and brain neuromodulation
