Polyphenols-gut microbiota interplay and brain neuromodulation
2018-10-22StefaniaFilosaFrancescoDiMeoStefaniaCrispi
Stefania Filosa , Francesco Di Meo Stefania Crispi
1 Institute of Biosciences and Bioresources, National Research Council, National Research Council, via P. Castellino, Naples, Italy
2 Istituto di Ricovero e Cura a Carattere Scientifico Neuromed, Pozzilli, Italy
Abstract Increasing evidence suggests that food ingested polyphenols can have beneficial effects in neuronal protection acting against oxidative stress and inflammatory injury. Moreover, polyphenols have been reported to promote cognitive functions. Biotransformation of polyphenols is needed to obtain metabolites active in brain and it occurs through their processing by gut microbiota. Polyphenols metabolites could directly act as neurotransmitters crossing the blood-brain barrier or indirectly by modulating the cerebrovascular system. The microbiota-gut-brain axis is considered a neuroendocrine system that acts bidirectionally and plays an important role in stress responses. The metabolites produced by microbiota metabolism can modulate gut bacterial composition and brain biochemistry acting as neurotransmitters in the central nervous system. Gut microbiota composition can be influenced by dietary ingestion of natural bioactive molecules such as probiotics, prebiotics and polyphenol. Microbiota composition can be altered by dietary changes and gastrointestinal dysfunctions are observed in neurodegenerative diseases. In addition, several pieces of evidence support the idea that alterations in gut microbiota and enteric neuroimmune system could contribute to onset and progression of these age-related disorders. The impact of polyphenols on microbiota composition strengthens the idea that maintaining a healthy microbiome by modulating diet is essential for having a healthy brain across the lifespan. Moreover, it is emerging that they could be used as novel therapeutics to prevent brain from neurodegeneration.
Key Words: polyphenols; gut-microbiota; gut-brain axis; metagenomic; neurodegeneration; neurotransmitters;prebiotics; probiotics
Introduction
Neurodegenerative disorders are increasing with the ageing of human population. In addition, environmental stressors such as oxidants determine progressive neurons degeneration affecting cognitive functions.
Diet and lifestyle play an important role in the onset and progression of age-related disorders, and there is a growing interest on the bioactivity of polyphenol-rich foods. Indeed,polyphenols are able to protect neurons against injury,suppressing neuroinflammation, thus promoting memory,learning, and cognitive functions (Pandey and Rizvi, 2009).
Recent evidences suggest that polyphenols exert beneficial effects acting through multiple pathways involved in oxidative/inflammatory stress signalling and leading to the expression of antioxidant enzymes neurotrophic factors, and cytoprotective proteins. All these processes act to maintain brain homeostasis.
The mechanisms by which polyphenols act on cognitive functions have not been fully elucidated. It has been reported that polyphenols carry out brain protection through direct and indirect actions. Direct actions depend on their ability to cross the blood-brain barrier. In fact, some polyphenol metabolites can modulate directly neuronal receptors(Youdim et al., 2004). Moreover a recent study using different neuronal systems reported that metabolites from dietary polyphenols exert neuroprotective effects after reaching the brain by crossing blood-brain barrier (Figueira et al., 2017).
Polyphenols indirect actions involve mechanisms that improve the peripheral cerebrovascular health. Several studies in humans indicated that dietary polyphenols improve vasodilatatory response and increase levels of circulating nitric oxide (NO) species that are essential in the control of vascular tone; vasodilation and bloodflow in the body and in cerebral circulation (Bondonno et al., 2012;Laranjinha et al., 2012).
Bioavailability of polyphenols is related to their chemical structure. The majority of dietary polyphenols are present as esters, glycosides or polymers that cannot be absorbed in this native form. After ingestion, to gives rise to beneficial effects at human body, polyphenols must become bioactive.This is realized through gut intestinal transformations that produce bioavailable and active phenolic metabolites.
Polyphenols are usually hydrolysed by intestinal enzymes or by gut microbiota. These modifications produce metabolites completely different from those present in food. In this form they reach blood, tissues and brain where exert biological activities.
In this review we briefly discuss the protective effects that polyphenols-gut microbiota interaction exert on the onset and progression of neurodegenerative diseases.
Polyphenols and Gut Microbiota
Polyphenols are a class of organic chemicals characterized by the presence of multiples phenol structural units. They are produced by plants as secondary metabolites as defence against exogenous stresses. Specifically, polyphenols protect plants against reactive oxygen species (ROS), ultraviolet radiation (UV), pathogens, parasites and plant predators.In humans, the antioxidant properties of polyphenols are effective in different diseases and in cancer (Zhang, 2015).These molecules act as natural antioxidants thanks to the metal-chelating and free radical scavenger properties. The scavenging activity is linked to their structure, since compounds with similar structures exhibit similar antioxidant activity. In general, the antioxidant activity increase is directly linked to the number of hydroxyl groups and indirectly to glycosylation (Di Meo et al., 2018).
Polyphenols bioactivity is related to their absorption rate,metabolism and bioavailability. These activities depend on the direct interaction with other dietary nutrients, such as proteins, carbohydrates, fat andfibres. In addition, the biological activity of the derivative metabolites may differ from the native compound. Interestingly, the properties of polyphenols depend from bioactive metabolites produced when they are metabolised by microbiota (Carmody and Turnbaugh, 2014).
In particular, polyphenols are characterized by low bioavailability, and after ingestion they are recognized as xenobiotics. The majority of them, due to the structural complexity, reach the large intestine without modifications. On the contrary, small polyphenols can be directly adsorbed in the small intestine. Therefore, complex polyphenols are converted into low-molecular-weight metabolites by the gut microbial community and become readily absorbable(Aura, 2008).
Dietary polyphenols biotransformations such as deglycosylations can be carried out by different gut microbial species. On the contrary, other reactions such as the generation of urolithins or (S)-equol require the presence of specific bacteria strains (Marin et al., 2015).
Gut microbiota and dietary polyphenols affect each other: microbiota enzymatically transforms polyphenols improving bioavailability and health effects, while polyphenols modulate microbiota community composition avoiding the growth of pathogens (Figure 1). In fact, polyphenols can modify microbiota composition and functions by acting on their growth or metabolism (Cardona et al.,2013). Polyphenols metabolism can be different among individuals since everyone has own microbiota compositions.Thus, similar daily intake of polyphenols can have different effect on health in people with a different bacterial content.
Specific polyphenols are able to inhibit/increase the growth of specific bacteria resulting in changes of gut microbial composition. For example the ingestion of different polyphenols result in different ratio between the healthy gut bacteria, Bacteroides and Firmicutes favouring the growth of Bacteroides that possess a higher number of glycan-degrading enzymes (Rastmanesh, 2011).
Polyphenols metabolites produced by gut microbiota are well absorbed in the intestine and are able to persist in the plasma for a longer time. Polyphenols absorption through the gut barrier can be increased after specific conjugation such as methylation, sulfation, and glucuronidation. These processes facilitate biliary and urinary elimination by increasing their hydrophilic properties.
Due to their “prebiotic-like” effect, polyphenols can also modify gut microbiota composition. In vitro and in vivo studies showed that different polyphenols can modulate the growth of specific bacterial strains. Indeed, polyphenols can increase beneficial strains as Bifidobacteria Lactobacilli,reducing the number of pathogens, such as C. perfringens and C. histolyticum (Duenas et al., 2015).
The Gut-Brain Axis
In humans, the gut microbiota is one of the most densely populated microbial ecosystems. Gut microbiota contributes to healthy state and several diseases can be linked to dysbiosis. Microbial interactions are important in infections, being able to block pathogens activities. Furthermore, microbiota composition is tightly linked to diet.
The connection between microbiota present in the gastrointestinal tract and the central nervous system, the gutbrain axis, plays an important role in stress response and it is widely recognized as neuroendocrine system. Communication between the gut and the brain involves multiple overlapping pathways: the enteric nervous system, the neuroimmune and the neuroendocrine systems. By acting on the nervous, endocrine and immune system, gut microbiota can influence brain functions. In addition, microbiota,being able to produce both neurotransmitters and neuropeptide, can directly influence the brain functions by acting on neuroactive metabolites production (Cryan and Dinan,2012).
The gut microbiota can synthesise neurotransmitters or regulate their levels acting on their precursors. This is the case of Bifidobacterium infantis that influence central serotonin transmission by increasing plasma tryptophan levels(O’Mahony et al., 2015).
On the contrary, different Lactobacillus and Bifidobacterium species are able to produce γ-aminobutyric acid(GABA) (Yunes et al., 2016) while Streptococcus, Escherichia and Enterococcus spp. produce serotonin (Lyte, 2011;Nzakizwanayo et al., 2015).
All these neurotransmitters crossing the mucosal layer of the intestines, reach the brain where they could mediate different physiological events.
Large-scale metagenomic sequencing analyses have allowed understanding the functional relationship existing between functional components of the microbiome, microbial metabolism and metabolites production. These studies have indicated the existence of a high inter-individual diversity in microbiota composition and a strong association between unbalance in composition or stability and disease states (hmpdacc.org; human-microbiome.org).
Alteration in the homeostasis of gut-brain axis has been associated also to neurological disorders and neurodegen-erative diseases (Zhu et al., 2017). Moreover, it is emerging that the regulation of microbiota composition can be realized using natural bioactive molecules such as polyphenols derived by plants, suggesting that polyphenols could be used to restore the altered brain functions that characterize neurodegenerative diseases.
The homeostasis of the gut microbiota can be modified by changing dietary ingestion of probiotics and prebiotics.Probiotics are live organisms that can exert health benefits to the host, while prebiotics are food ingredients able to induce in the host the growth beneficial microorganisms.
The use of probiotics and prebiotics can be viewed as novel strategy to change the gut microbiota composition thus modulating the gut-brain axis (Petschow et al., 2013).
Several studies both in humans and animals showed that these molecules could increase the levels of neurotransmitters allowing modulating mood and cognition (Sudo et al.,2004; Savignac et al., 2013; Alherz et al., 2017).
Thesefindings suggest that communication between gut microbiota and brain isflexible and they also contribute to the identification of specific targets to be used in patients with altered stress responses associated with gut dysbiosis.
Microbiota and Neurodegeneration
Neural aging is characterized by progressive loss of function that involves central and peripheral neurons and neural stem cells. This degenerative process leads to neurodegeneration and can be considered the principal cause of cognitive impairment and sensory and motor deficits during aging.
It has been described that the onset of neurodegeneration starts earlier than the symptoms appears. At this time neurons have already been destroyed and this can explain why therapeutic approaches have little or no effect.
Gut microbiota can interact with central nervous system through different mechanisms and pathways such as the neurosystems implicated in stress and stress-related disorders (sympathetic and parasympathetic branches of the autonomic nervous system, neuroendocrine and neuroimmune systems). The communication between these pathways occurs through vagus nerve, cell wall, metabolites,and through neurotransmitters and brain neurotrophic factors. Gut microbiota are able to synthetize neurotransmitters thus microbiota homeostasis can impact on complex neurodegenerative disorders. Example are short-chain fatty acids (SCFAs), tryptophan, GABA and brain-derived neurotrophic factor (BDNF) (Rieder et al., 2017).
SCFAs represent the most abundant product of bacterial fermentation and have neuroactive properties. They modulate the release of serotonin as well as peptide YY, an important neuropeptide acting at multiple levels of the gut brain axis. Microbial-derived SCFAs acting on the production of secondary peptides represent a mechanism through which the gut microbiota influences human behaviour. In fact, it has been reported that systemic administration of the SCFA butyrate determines an antidepressant-like behavioural response (Jiang et al., 2018). In addition a recent study reported that SCFAs are active in Alzheimer’s disease by inhibiting the generation of beta-amyloid peptides (Ho et al., 2018).
Tryptophan is an essential amino acid a precursor of many biologically active agents, including the synthesis of serotonin in the central nervous system. Serotonin, a neurotransmitter active both in the central nervous system and in the gut, plays an important role maintaining mood and cognition (Szapacs et al., 2004). Alterations in the levels of serotonin can be associated to the onset of gastrointestinal and mood disorders, and tryptophan dysregulation is linked to many disorders both in the brain and in the gastrointestinal tract. The direct regulation of tryptophan on serotonin by microbiota has been demonstrated in germ free animals in which that increased levels of circulating tryptophan and decreased levels of serotonin were restored after bacteria colonization of these animals (Clarke et al.,2013).
GABA, is the main inhibitory neurotransmitter that regulates neuronal excitability. It is produced from glutamate metabolism by different bacteria species, as demonstrated by in vitro studies. Dysfunction of the GABA system has been implicated in the pathophysiology of several chronic neurological diseases. GABA treatments were shown to decrease cytotoxicity induced by beta-amyloid fibers (Sun et al., 2012). GABA system represents an important mechanism through which bacteria can modulate brain chemistry.
BDNF is a neurotrophin widely expressed in the central nervous system with neuroprotective functions. BDNF play an important role in the growth and plasticity of synapses as well as in the survival and differentiation of neurons. Levels of BDNF are decreased in the cerebral cortex in Alzheimer’s disease and several clinical trials aims to use BDNF as therapeutics for different neurodegenerative diseases (Nagahara and Tuszynski, 2011). Neurological mood diseases have been reported to be linked to BDNF levels and they have been associated with the gut-brain axis. It has been reported that microbiota fecal transplants in pathogen free mice increased the levels of hippocampal BDNF (O’Sullivan et al., 2011).
Several pre-clinical and clinical studies have indicated that microbiota, maintaining physiological homeostasis,can significantly interfere with human for brain functions and cognitive systems studies (Savignac et al., 2013).
Aged healthy people do not show changes in the microbiota diversity. Studies in centenarians have stressed the importance of microbial diversity in maintaining health with age (Biagi et al., 2016). On the contrary, dysbiosis have been observed in neurodevelopmental diseases as autism and in neurodegenerative including Alzheimer’s disease,Huntington’s disease and Parkinson’s disease (Di Meo et al., 2018).
The role of the gut microbiome in the pathogenesis of chronic neurodevelopmental and neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases is beginning to emerge.
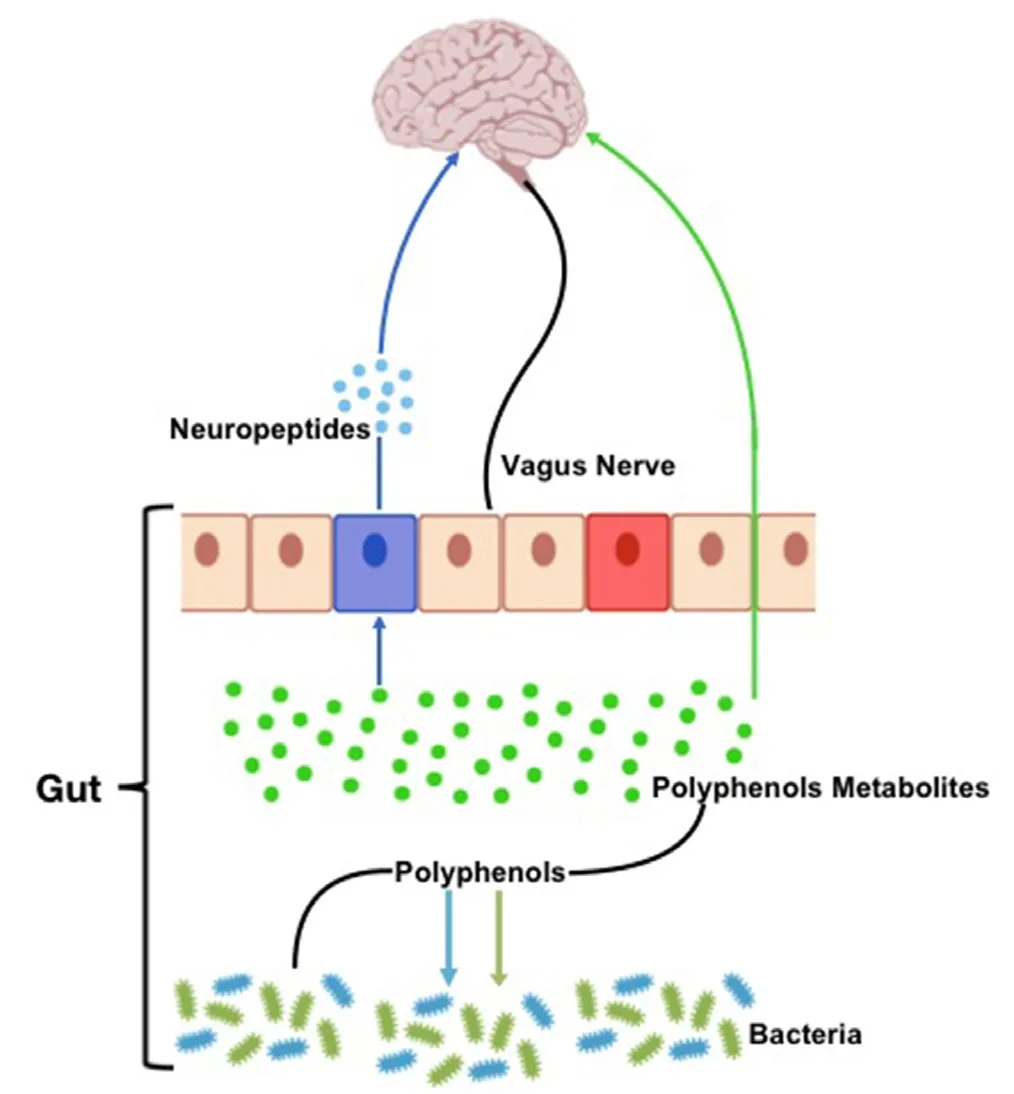
Figure 1 Schematic representation of the gut microbiota-brain interaction.
Studies regarding the associations between gut microbiome and neurodevelopmental or neurological disorders have been performed mainly in animal models (Bercik et al., 2011; Sampson et al., 2016; Brandscheid et al., 2017).And very few studies have been reported in humans.
Studies performed in early childhood showed that in autism there are gut microbiota perturbations. In particular Proteobacteria and Bacteroidetes are strongly increased while Firmicutes and Bifidobacteria are less abundance respect to healthy controls (Finegold et al., 2012). A recent small study in Alzheimer’s disease patients described a reduction of gut microbiota diversity respect to the healthy controls. In particular in the Alzheimer group it was described a decrease of Firmicutes and Actinobacteria and increase of Bacteroidetes (Vogt et al., 2017). In Parkinson’s disease it has been reported that the disease might begin in the gut and than spread to brain via the gut-brain axis.Indeed microbiota analysis in Parkinson’s disease patients showed a decrease of Prevotella strains and increase of Enterobacteria (Scheperjans et al., 2015).
Considering the protective role of neurotransmitters produced by gut microbiota metabolism in neuroplasticity and neurological disorders, it will be fundamental to examine in depth the associations and the conditions by which the levels of these factors are influenced or regulated by microbiota.
Additional studies are needed to better understand if gut microbiota changes are central in the pathophysiology of neurodegenerative diseases or if they represent epiphenomena.
However, the association between gut microbiota and brain disorders suggests that a healthy microbiota may be one of the keys to longevity.
Conclusion
Emerging evidence indicates that changes in the composition of the microbiota may contribute to the onset of neurodegenerative disorders that increase with age.
Adults undergo to dramatic changes in microbiota composition following diet modifications. Furthermore, ageing is related to specific changes in the microbiota diversity that result in health outcomes in the elderly. Since microbiota can significantly interfere with human cognitive system, the understanding of the microbiota composition in healthy people is fundamental for maintaining brain and mental health during all over the life.
Gut microbial composition can be modulated by polyphenols. It is becoming clear that dietary polyphenols through their metabolites contribute to the maintenance of gut health by the modulation of the gut microbial balance.Polyphenols act by enhancing the growth of beneficial bacteria and inhibiting the growth of pathogens, thus exert prebiotic-like effects.
Regulation of microbiota composition using polyphenols, or other probiotics and prebiotics, may help to restore gut equilibrium and to set up new therapeutic intervention in neuropathologies. Since brain dysfunctions are associated with dysbiosis of the gut microbiota, it is possible that a rebalance in the microbiota composition may result in a partial or complete reversion of these diseases.
Finally, a better understanding of the interplay between polyphenols and gut microbiota will provide more insight into the health effects of polyphenols and open new possibilities to develop microbiota-based therapies for treating neuronal disorders.
Acknowledgments:We thank Dr. Maria Rosaria Aletta (National Research Council, Napoli, Italy) for bibliographic support.
Author contributions:Manuscript conception: SC; manuscript writing and revising: SF, FDM and SC; literature collection andfigure design: FDM.
Financial support:This work was supported by Italian Ministry of Health ‘Ricerca Corrente” (to SF).
Conflicts of interest:None declared.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Inhibiting the kynurenine pathway in spinal cord injury: multiple therapeutic potentials?
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- MicroRNAs of microglia: wrestling with central nervous system disease
- Roles and functions of Atp6ap2 in the brain
- Magnesium sulfate and fetal neuroprotection:overview of clinical evidence
- Ketogenic diet versus ketoacidosis: what determines the influence of ketone bodies on neurons?
