Dynamic changes in growth factor levels over a 7-day period predict the functional outcomes of traumatic brain injury
2018-10-22ShuaiZhouDongPeiYinYiWangYeTianZengGuangWangJianNingZhang
Shuai Zhou , Dong-Pei Yin , Yi Wang Ye Tian Zeng-Guang Wang , Jian-Ning Zhang
1 Department of Intensive Care Unit, Tianjin Medical University General Hospital, Tianjin, China
2 Tianjin Neurological Institute, Tianjin, China
3 Key Laboratory of Post-Trauma Neuro-Repair and Regeneration in Central Nervous System, Ministry of Education, Tianjin, China
4 Tianjin Key Laboratory of Injuries, Variations and Regeneration of Nervous System, Tianjin, China
5 Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, China
Abstract Traumatic brain injury (TBI) can result in poor functional outcomes and death, and overall outcomes are varied. Growth factors, such as angiopoietin-1 (Ang-1), vascular endothelial growth factor (VEGF), and granulocyte-colony stimulating factor (G-CSF), play important roles in the neurological functions. This study investigated the relationship between serum growth factor levels and long-term outcomes after TBI. Blood samples from 55 patients were collected at 1, 3 and 7 days after TBI. Blood samples from 39 healthy controls were collected as a control group. Serum Ang-1, G-CSF, and VEGF levels were measured using ELISA. Patients were monitored for 3 months using the Glasgow Outcome Scale-Extended (GOSE). Patients having a GOSE score of > 5 at 3 months were categorized as a good outcome,and patients with a GOSE score of 1–5 were categorized as a bad outcome. Our data demonstrated that TBI patients showed significantly increased growth factor levels within 7 days compared with healthy controls. Serum levels of Ang-1 at 1 and 7 days and G-CSF levels at 7 days were significantly higher in patients with good outcomes than in patients with poor outcomes. VEGF levels at 7 days were remarkably higher in patients with poor outcomes than in patients with good outcomes. Receiver operating characteristic analysis showed that the best cut-off points of serum growth factor levels at 7 days to predict functional outcome were 1,333 pg/mL for VEGF, 447.2 pg/mL for G-CSF, and 90.6 ng/mL for Ang-1. These data suggest that patients with elevated levels of serum Ang-1, G-CSF, and decreased VEGF levels had a better prognosis in the acute phase of TBI (within 7 days). This study was registered with the Chinese Clinical Trial Registry(registration number: ChiCTR1800018251) on September 7, 2018.
Key Words: nerve regeneration; traumatic brain injury; vascular endothelial growth factor; angiopoietin-1; granulocyte-colony stimulating factor; outcomes; secondary brain injuries; blood-brain barrier; brain edema; acute phase; clinical trial; neural regeneration
Introduction
Traumatic brain injury (TBI) is a devastating neurological disease that is associated with significant mortality, morbidity and societal and economic costs worldwide. Each year,more than 1.7 million individuals are estimated to suffer TBI annually in the United States (Jin et al., 2017; Nissinen et al.,2017), and TBI is predicted to be the third leading cause of disease burden by 2020 (Feigin et al., 2013). Typically, neurological deficits following TBI do not immediately occur,but instead, evolve over the post-trauma time-course. This late-developing damage is remarkable because of TBI-associated damage due to cerebral ischemia (Bratton et al.,2007). No effective treatment exists for TBI, despite recent clinical trials (Hirschi et al., 2017; Zhang and Wang, 2017;Hart et al., 2018), and several biological factors are thought to be involved in the pathophysiology of TBI.
Growth factors such as angiopoietin-1 (Ang-1), vascular endothelial growth factor (VEGF), and granulocyte-colony stimulating factor (G-CSF) play important roles in the regulation of vascular structures and neurological functions,which can be disturbed in experimental models of TBI. In experimental studies, TBI can induce cerebral angiogenesis and up-regulation of VEGF, simultaneously increase vasopermeability, and cause the destruction of endothelial tight junctions (Gao et al., 2015; Li et al., 2016). The involvement of VEGF up-regulation has been implicated in the breakdown of the blood-brain barrier (BBB), thereby aggravating the development of brain edema. G-CSF has been studied in experimental studies of stroke and TBI and reported to alleviate brain insults, decrease cell death and neuroinflammation, and improve prognoses (Shyu et al., 2006; Solaroglu et al., 2006). Ang-1 and its receptor tyrosine kinase receptor-2 have been shown to have a close relationship with angiogenesis, vascular endothelium maturation, and an intact BBB in the rat brain after TBI (Ge et al., 2014). Based on this previous evidence, we tested the hypothesis that higher Ang-1 and G-CSF levels might predict better neurologic and functional outcomes in the acute phase after TBI, whereas high serum VEGF levels might be associated with poor outcomes.
Subjects and Methods
Study population
A total of 55 patients with acute TBI admitted to the Neurosurgical Intensive Care Units of four medical centers in Northern China were enrolled in this study between October 2014 and March 2016. These medical centers included the General Hospital of Tianjin Medical University, the Second Hospital of Tianjin Medical University, the Binhai Hospital of Tianjin Medical University and the Airport International Hospital of Tianjin Medical University. The study protocol was approved by the Ethics Committee of Tianjin Medical University of China (No. IRB2014-YX-066) in September 2014. Written informed consent was obtained from all patients or their relatives at the time of enrollment. The study followed was in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with principles of the Declaration of Helsinkin 1975, as revised in 2000. This study was registered with the Chinese Clinical Trial Registry (registration number: ChiCTR1800018251) on September 7, 2018.
Inclusion and exclusion criteria
TBI patients may suffer consciousness disorders, or exhibit positive signs of the nervous system, thus the computed tomography (CT) may be helpful for diagnosis of TBI (Jin et al., 2017; Nissinen et al., 2017). For this study, the inclusion criteria were: (1) evidence of acute TBI by cerebral computed tomography; (2) closed craniocerebral injury; (3)time from trauma onset to hospital admission of fewer than 8 hours. No differences in age, sex or ethnicity were found among the enrolled patients or the healthy controls.
Exclusion criteria were: (1) a history of stroke; (2) trauma after stroke; (3) severe bodily injury; (4) subacute or chronic TBI; (5) recent infectious diseases; (6) evidence of tumor diseases; (7) severe hepatic or renal diseases; (8) acute open craniocerebral injury.
A total of 39 healthy volunteers were from the Health Examination Department of Tianjin Medical University Airport International Hospital.
Laboratory tests
Blood samples drawn from TBI patients were collected at 1,3 and 7 days post-trauma. Control blood samples were collected from healthy individuals. Samples were obtained by centrifugation at 3000 × g for 10 minutes within 2 hours after blood collection and were immediately frozen and stored at−80°C. Serum VEGF, Ang-1, and G-CSF levels were measured with available quantitative enzyme-linked immunosorbent assay kit kits (R&D systems, Minneapolis, MN, USA).Determinations of growth factor levels were performed in an independent laboratory blinded to the clinical data.
Follow-up
Glasgow Outcome Scale-Extended (GOSE) is the most widely used outcome measure after TBI. After a 3-month follow-up (Wilson et al., 1998), the severity of brain damage among TBI patients was evaluated by a standard assessment tool known as the GOSE (Jennett and Bond, 1975). Patients having a GOSE score of > 5 at 3 months were categorized into a favorable outcome (good) group and patients having a GOSE score of 1–5 were placed in an unfavorable outcome(bad) group. The good outcome and poor outcome groups were compared to discern factors that influence prognoses.
Statistical analysis
Data are expressed as the mean ± SD. All analyses were performed using SPSS 19.0 software (IBM, Armonk, NY, USA).GF levels were compared between TBI patients and healthy controls. Important covariates were also compared between TBI patients and healthy controls, and changes in GF levels were compared at different time points. An independent sample t-test was used to assess continuous variables. GF levels were dichotomized based on the best predictive values obtained by receiver operating characteristic (ROC) curve analysis. Multivariable logistic regression analysis was used to examine the association between outcome (good vs. bad)and growth factor levels. Results are express as adjusted odds ratios (ORs) with corresponding 95% confidence intervals(95% CIs). A P value of < 0.05 was used to determine statistical significance for all analyses.
Results
Baseline characteristics of the study population
A total of 55 TBI patients were recruited into the statistical analysis. The baseline data of the 55 TBI patients are shown in Table 1. Of the 55 TBI patients, 45.5% (n = 25) had a poor prognosis and 54.5% (P = 30) had a good prognosis.Important covariates were compared, such as age and sex, as well as clinical information such as vascular risk factors between the patients with good outcomes and poor outcomes.Comparisons between the TBI patients with good outcomes and those with poor outcomes regarding demographic, clinical, and laboratory characteristics revealed no significant differences (P > 0.05).
Serum growth factor levels after TBI are higher in TBI patients than healthy controls
Of the 55 TBI patients and 39 healthy controls included in this study (Figure 1), blood samples were drawn from healthy controls (mean 50.3 years of age) and TBI patients(mean 43.3 years of age) aged over 18. The proportions of males and females were similar among healthy controls and TBI patients. The proportions of males and females were similar among the healthy controls and TBI patients; 46.2%(n = 18) of the healthy controls were male, and 52.7% (n =29) of the TBI patients were male. Therefore, no significant differences in age and sex were found between TBI patients and healthy controls. Serum GF levels were higher in TBI patients than in healthy controls at 1 day (P < 0.01; Table 2).Serum GF levels demonstrated gradual increases within thefirst 7 days (P < 0.001; Figure 2).
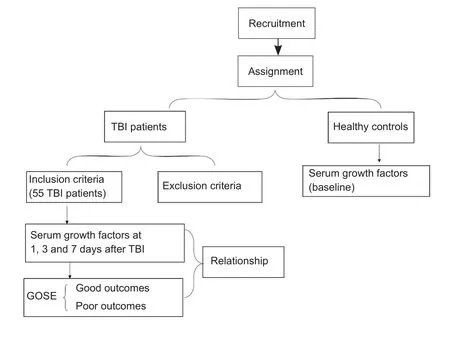
Figure 1 Trialflow chart.
Higher Ang-1 and G-CSF levels have improved outcomes after TBI
Table 3 contrasts the growth factor levels at different time periods (1, 3 and 7 days post-trauma) between patients with good or poor outcomes. Generally, patients classified as having a good outcome exhibited lower VEGF levels at all time points (1, 3 and 7 days post-trauma; P < 0.01),higher Ang-1 levels (1 and 7 days post-trauma; P = 0.044; P< 0.01), and higher G-CSF levels (7 days post-trauma; P <0.01) compared with patients classified as having poor outcomes. However, no significant differences in G-CSF levels(1 and 3 days post-trauma; P > 0.05) or Ang-1 levels (3 days post-trauma; P > 0.05) were found between patients with good and poor outcomes.
Binary logistic regression analysis at 7 days post-trauma
Binary logistic regression analysis (Table 4) shows the association between growth factor levels at 7 days and functional outcomes among TBI patients. For example, patients with high serum Ang-1 levels at 7 days were more likely to have good outcomes than patients with low serum Ang-1 levels at 7 days (OR = 1.108; 95% CI: 1.051–1.169; P < 0.001; Table 4).An odds ratio of 1.152 with a P value of 0.002 was obtained after adjustment for GCS score, sex, and age at admission.
ROC curve analysis at 7 days post-trauma
Figure 3 shows the results of ROC curve analysis of growth factor levels at 7 days post-trauma. ROC analysis showed that the best cut-off points of serum levels of growth factors at 7 days to predict functional outcomes were 1,333 pg/mL(area under curve: 0.887, sensitivity: 80%, specificity: 80%;P < 0.001) for VEGF; 447.2 pg/mL (area under curve: 0.845,sensitivity: 87%, specificity: 72%; P < 0.001) for G-CSF; and 90.6 ng/mL (area under curve: 0.910, sensitivity: 90%, specifi city: 88%; P < 0.001) for Ang-1.
Discussion
This clinical study investigated the relationship between serum growth factors and functional outcomes in TBI patients. In this trial, we did not administer any drugs that would affect serum growth factors during the follow-up. We found that serum growth factor levels gradually increased within 7 days post-trauma in TBI patients. Increases in serum Ang-1 and G-CSF levels within 7 days were associated with good outcomes after TBI, whereas higher serum VEGF levels were associated with poor outcomes. Our results offer novel insight into the biological mechanisms linking growth factor levels to functional outcomes of TBI patients.
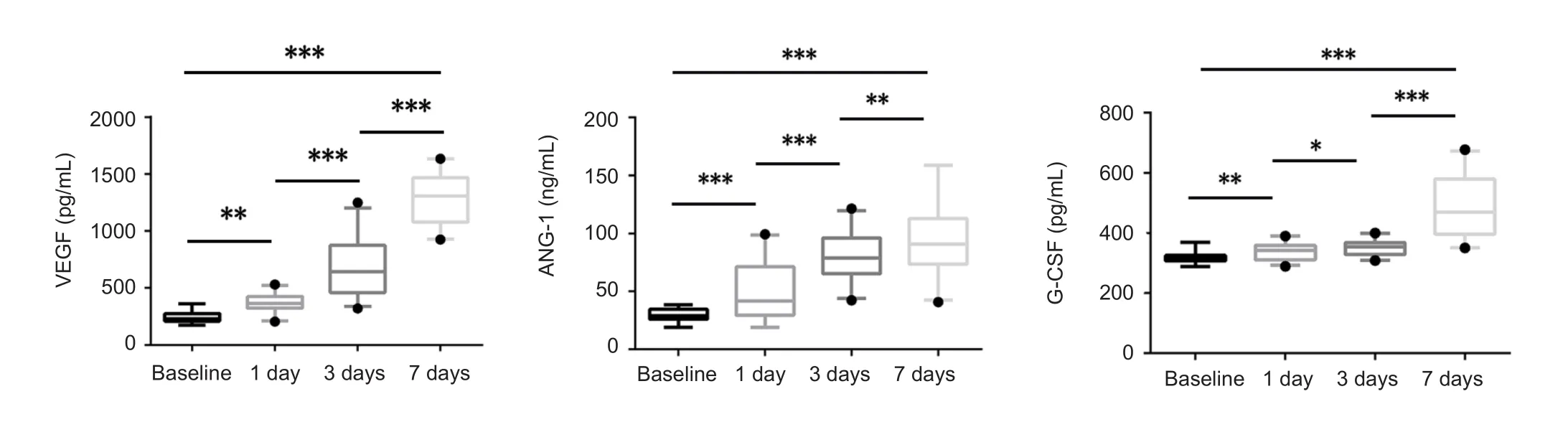
Figure 2 Growth factors parameters at 1, 3, and 7 days post-trauma in traumatic brain injury patients.
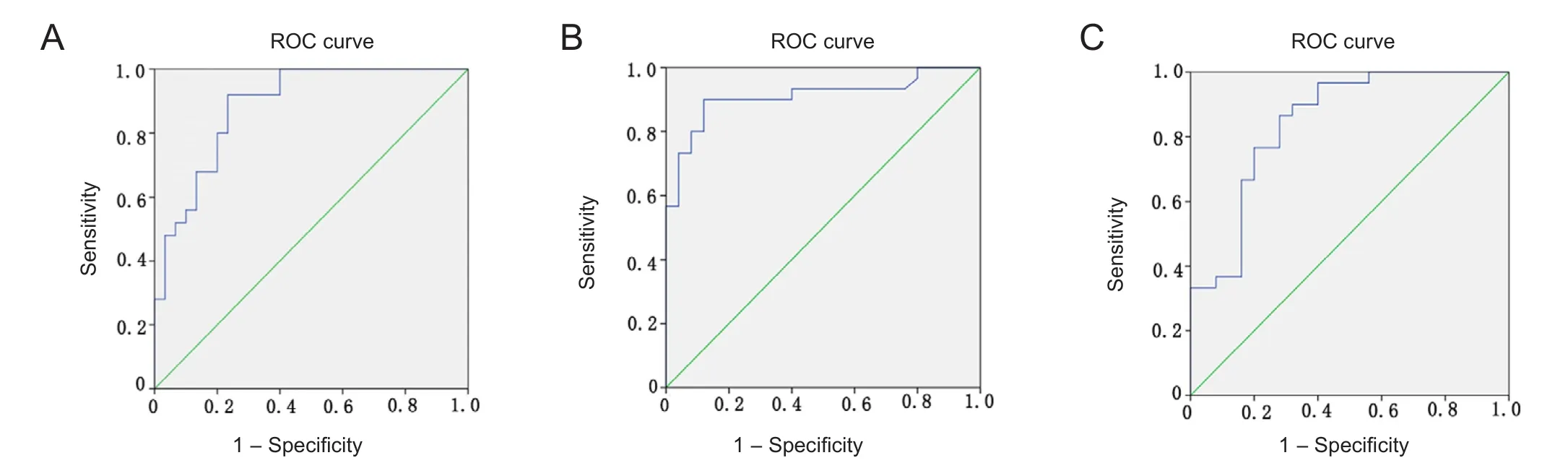
Figure 3 ROC curve analysis of the optimal cut-off points of serum levels of Ang-1 at 7 days post-trauma for prediction of functional outcomes.
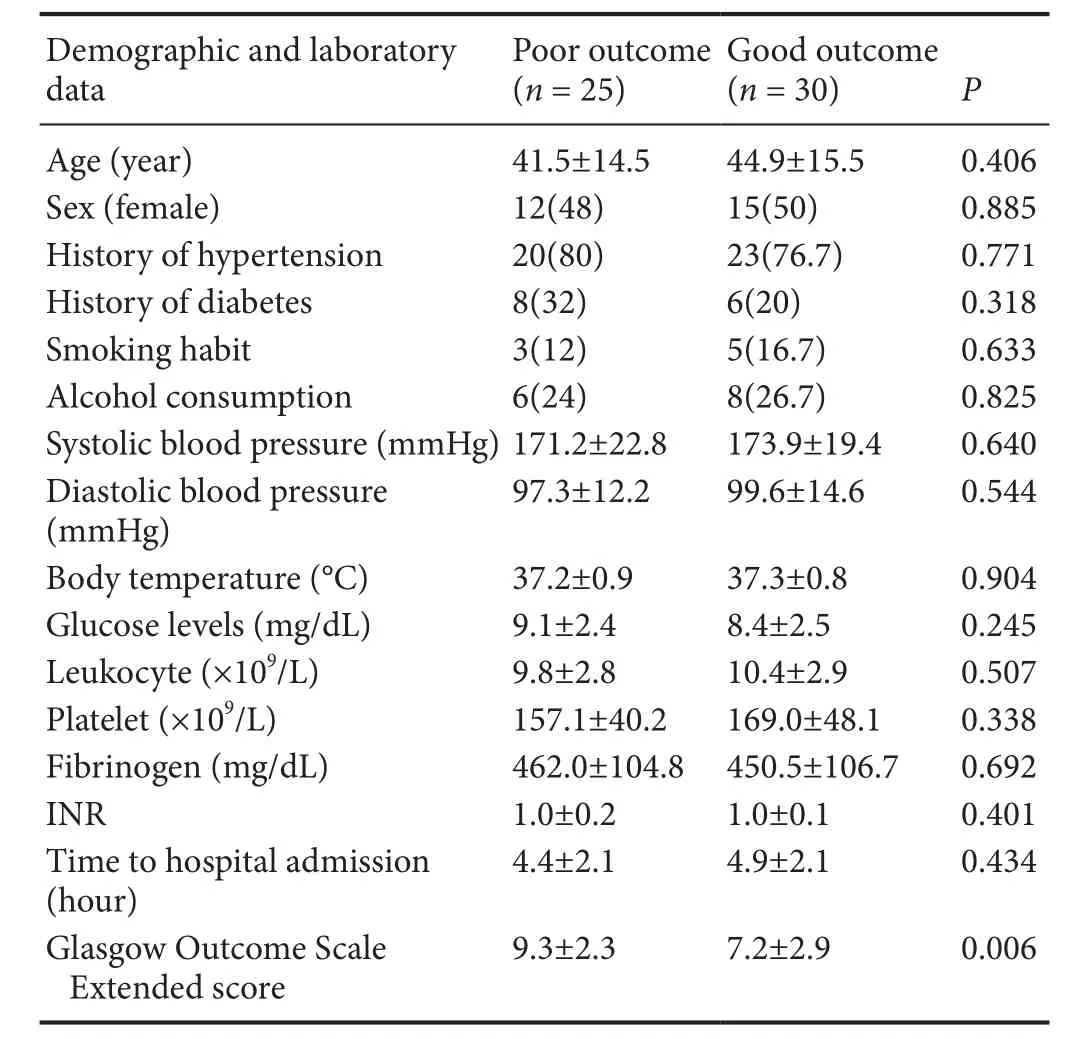
Table 1 Baseline characteristics, including demographic and laboratory data, of the study population
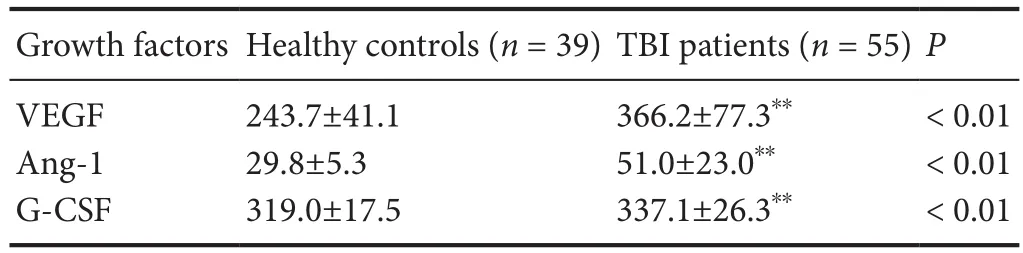
Table 2 Serum growth factor levels in TBI patients and healthy controls at 1 day post-trauma
TBI comprises primary and secondary brain injuries. Primary brain injury, which occurs immediately after physical trauma, can induce biochemical changes and ultimately lead to secondary BBB disruption and dysfunction in the microvasculature over the course of hours to days (or weeks)(Raghupathi and Huh, 2017; Sun et al., 2017). A BBB disruption is likely the key pathological development after a TBI(Wurzelmann et al., 2017; Xiong et al., 2017). Circulatory biomarkers can play an important role in characterizing both TBI severity and its time course (Agoston et al., 2017).Several studies have found an increase in the expression of several blood biomarkers such as GFAP, neurofilament heavy chain protein, ubiquitin C-terminal hydrolase-L1,and neuron-specific enolase, which have important roles in outcomes following TBI (Yokobori et al., 2013; Zetterberg et al., 2013; Strathmann et al., 2014). Dynamic changes in blood neurofilament heavy chain protein predict brain injury severity and the possibility of good versus poor outcomes(Gyorgy et al., 2011). Increased serum GFAP and its degradation products have been associated with injury severity following TBI. Higher serum levels of ubiquitin C-terminal hydrolase-L1 following TBI have been associated with injury severity (Li et al., 2015). In the acute post-traumatic period,serum neuron-specific enolase levels are biomarkers of the severity of brain injury (Agoston et al., 2017).
Many molecules, including growth factors, also act simultaneously at different periods during BBB destruction(Sobrino et al., 2009; Chittiboina et al., 2013). Ang-1 is an important member of the angiopoietin family and is typically constitutively expressed in endothelial cells and interacts with the endothelial-specific tyrosine kinase receptor-2 receptor (Augustin et al., 2009). Ang-1 reduces vascular solute permeability and contributes to vascular maturation and BBB stabilization by increasing the expression of BBB-related tight junction proteins (Sobrino et al., 2009; Herbert and Stainier, 2011). Pericyte-derived Ang-1 will activate the tyrosine kinase receptor-2 tyrosine kinase pathway and exert strong neuroprotective effects on the BBB (Hori et al., 2004).Lower Ang-1 levels are associated with poorer outcomes,including severe sepsis, subarachnoid hemorrhage and cerebral malaria (Sobrino et al., 2009; David et al., 2010; Wang et al., 2015). Our study suggests the possibility that serum Ang-1 levels increase within 7 days after TBI and higher Ang-1 levels might predict better neurologic and functionaloutcomes in the acute phase after TBI. Therefore, we speculate that Ang-1 may be an important factor involved in vascular repair and BBB stabilization after TBI.
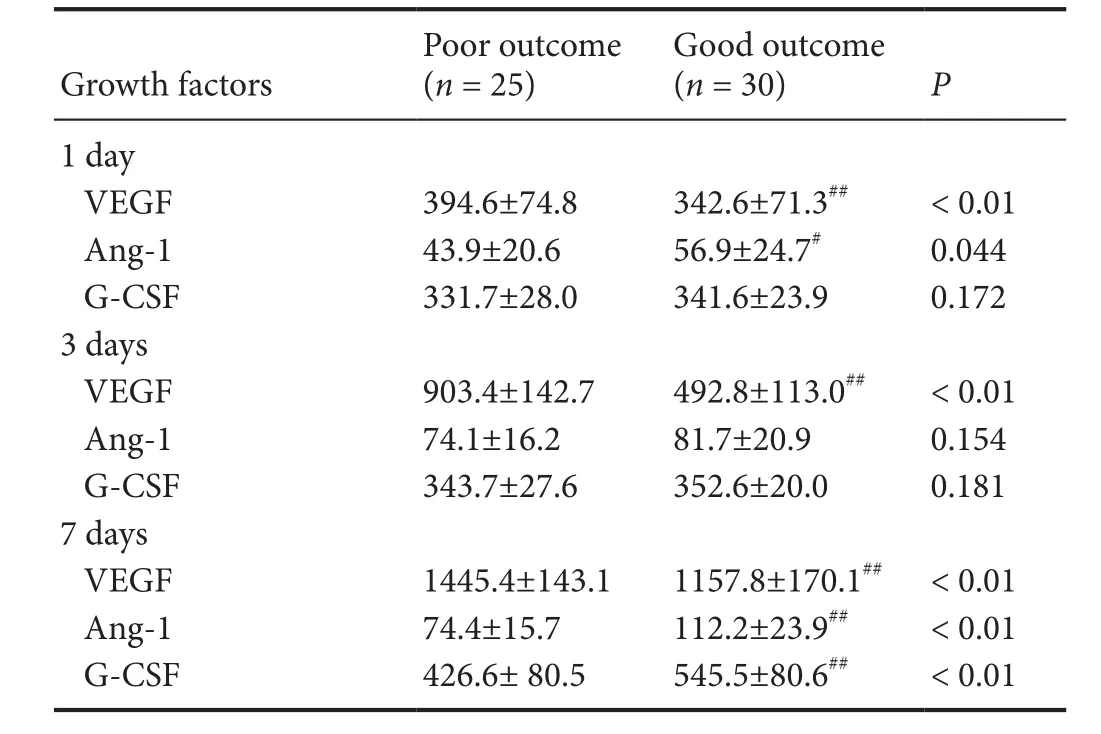
Table 3 Growth factor levels at different time points after TBI by functional outcome
G-CSF is a member of the cytokine family of growth factors that readily permeate the BBB (Song et al., 2016).Although G-CSF has been used for the treatment of leukopenia, it has been studied in experimental studies of stroke and TBI and has been reported to alleviate brain injury and improve prognoses (Solaroglu et al., 2006; Yang et al., 2010).G-CSF promotes the proliferation of hematopoietic cells and can mobilize endothelial progenitor cells in the bone marrow into the peripheral blood in cases of trauma, ischemia and inflammatory response, thereby redistributing endothelial progenitor cells in the body. Endothelial progenitor cell proliferation and differentiation are involved in angiogenesis and regeneration (Wang et al., 2012). Endothelial progenitor cells have been shown to migrate to areas of brain lesions after TBI (Zhang et al., 2013). The results of our study indicated that serum G-CSF levels increased gradually within 3 days after TBI and reached a peak at 7 days post-injury,which is consistent with previous studies of the dynamic changes of endothelial progenitor cells in the peripheral blood after TBI (Gong et al., 2012). The G-CSF levels in the group with good outcomes were significantly higher than those in the group with poor outcomes, and the number of available endothelial progenitor cells was hypothesized to be insufficient after TBI, leading to up-regulated expression of G-CSF and the mobilization of endothelial progenitor cells that then migrated into the injured brain tissue and its surroundings.
VEGF is a heparin-binding growth factor that promotes angiogenesis and vascular permeability following TBI.Members of the VEGF family, VEGF-A, and VEGF-B are involved in vascular inflammation and remodeling through increased proinflammatory and angiogenic mechanisms(Holmes and Zachary, 2005). It has been reported that VEGF-B might potentiate angiogenesis by increasing the bioavailability of VEGF-A (Robciuc et al., 2016), but the majority agree that VEGF-B cannot initiate angiogenesis by itself (Zafar et al., 2017). The VEGF signaling pathway represents a critical role in physiological angiogenesis (Li et al., 2016). Previousfindings suggest an extensive role for endogenous expression of VEGF and VEGFR2 as good factorsfollowing TBI (Sköld et al., 2006). Nevertheless, some studies have found an increase in VEGF expression after TBI(Thau-Zuchman et al., 2012). Up-regulation of VEGF could activate matrix metalloproteinases, which disrupt the basilar membrane and damage the BBB (Bauer et al., 2010). In early stages of TBI, the disrupted BBB allows influxes of harmful blood compounds and induces brain edema formation. In addition, our previous research has found that TBI patients with good outcomes had lower levels of VEGF on day 7 and higher VEGF levels on day 21, which indicates that higher VEGF levels exhibit a disadvantage during the edema period(4–14 days after TBI) and a beneficial effect during the recovery period (14–21 days after TBI) (Li et al., 2016). The results of our study indicate that serum VEGF levels increased within 7 days after TBI and reached a peak at 7 days, which is consistent with the time-course of peak edema after TBI.Patients with higher serum VEGF levels at 7 days were more likely to have poor outcomes than patients with lower serum VEGF levels. Therefore, we suggest that TBI induces VEGF up-regulation, with a persistent detrimental impact on the BBB, thereby aggravating the development of brain edema during the acute phase after TBI.
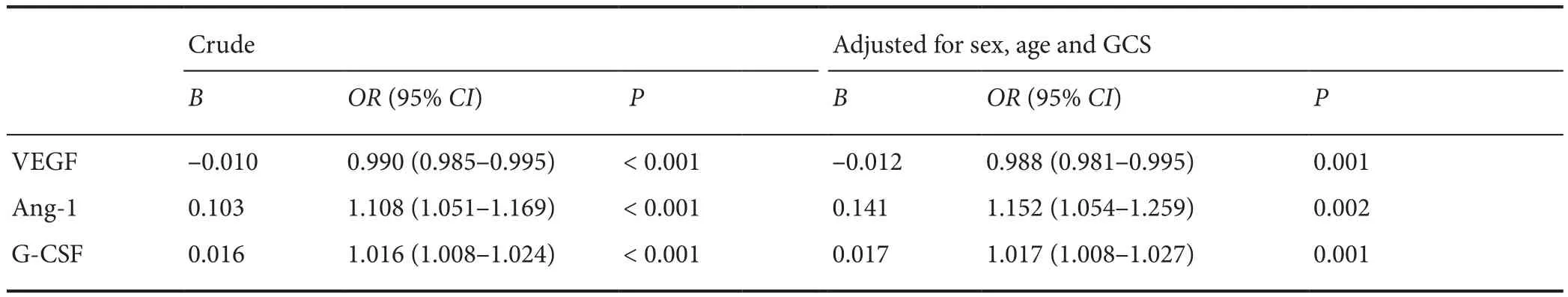
Table 4 Crude and adjusted odds ratios for the association between Ang-1 and G-CSF levels at 7 days and a good outcome, and between VEGF levels at 7 days and a poor outcome among traumatic brain injury patients
Ourfindings support the hypothesized roles of Ang-1 and G-CSF on good functional outcomes among TBI patients.However, higher serum VEGF levels were more likely to have poorer outcomes. Most of these factors must occur in the brain tissue to influence functional prognosis. Injury provoked by TBI induces BBB disruption, which would allow cerebral substances to reach the blood stream, which could explain how circulating levels of growth factors could affect brain recovery after TBI.
A limitation of this study is its small sample size, which reduces the power of the study. However, the results of this study support the clinical importance of growth factors after TBI. Our study will lead to further investigation of the relationship between growth factor levels and the functional outcomes of TBI patients.
Our study shows that higher Ang-1 and G-CSF levels at acute post-traumatic stages could be an important prognostic tool for predicting good outcomes among TBI patients.However, higher VEGF levels during the acute post-traumatic stages might be associated with unfavorable outcomes.Our study calls for additional studies to confirm the roles of growth factor levels in TBI patients.
Acknowledgments:We are very grateful to Wei-Yun Cui, Li Liu and Ping Lei from the Department of Neurosurgery, Tianjin Medical University General Hospital in China for their excellent technical support.
Author contributions:Study design: SZ, JNZ and ZGW; experiment implement: SZ, DPY and YW; data analysis YT; paper writing: SZ; paper revision: YT. All authors approved thefinal version of the paper.
Conflicts of interest:The authors have no conflicts of interest to declare.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81330029 (to JNZ), 81501057 (to YT); the Science & Technology Development Fund of Tianjin Education Commission for Higher Education in China, No. 2016YD02 (to YW); the Technology Program Fund of Tianjin Health and Family Planning Commission for the Key Field of Traditional Chinese Medicine, No. 2018001(to ZGW). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the pa-per, and in the decision to submit the paper for publication.
Institutional review board statement:The study protocol was approved by the Ethics Committee of Tianjin Medical University of China(approval No. IRB2014-YX-066). Time of ethical approval: 2014-09.The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee. This study had been registered in the Chinese Clinical Trial registry(registration number: ChiCTR 1800018251).
Declaration of patient consent:The authors certified that they have obtained all appropriate patient or their legal guardian consent forms.In the form, the patients or their legal guardians have given their consent for patients’ images and other clinical information to be reported in the journal. The patients or their legal guardians understood that the patients’ names and initials would not be published and due efforts would be made to conceal their identity, but anonymity could not be guaranteed.
Reporting statement:This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of Tianjin Medical University General Hospital in China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Individual participant data that underlie the results reported in this article, after deidentification (text, tables,figures,and appendices) will be in particular shared. Study protocol form will be available. The data will be available immediately following publication without end date. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Angélica Zepeda, UNAM, Mexico; Shan Ping Yu, Emory University School of Medicine, USA.
Additionalfile:Open peer review reports 1 and 2.
杂志排行
中国神经再生研究(英文版)的其它文章
- MicroRNAs of microglia: wrestling with central nervous system disease
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Apomorphine effects on the hippocampus
- Roles and functions of Atp6ap2 in the brain
- Magnesium sulfate and fetal neuroprotection:overview of clinical evidence
- Polyphenols-gut microbiota interplay and brain neuromodulation
