Neuroprotective effect of baicalin on focal cerebral ischemia in rats
2018-10-22JiongDaiYongMingQiuZhengWenMaGuoFengYanJingZhouShanQuanLiHuiWuYiChaoJinXiaoHuaZhang
Jiong Dai, Yong-Ming Qiu, Zheng-Wen Ma, Guo-Feng Yan, Jing Zhou, Shan-Quan Li, Hui Wu, Yi-Chao Jin, Xiao-Hua Zhang,
1 Department of Neurosurgery, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
2 Department of of Laboratory Animal Science, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Abstract
Abstract Baicalin, aflavonoid compound from the root of the herb Scutellaria baicalensis Georgi, has been widely used to treat patients with inflammatory disease. The aim of this study was to assess the efficacy of baicalin in a rat model of focal cerebral ischemia. Adult male Sprague-Dawley rat models of cerebral artery occlusion were established and then randomly and equally divided into three groups: ischemia (cerebral ischemia and reperfusion), valproic acid (cerebral ischemia and reperfusion + three intraperitoneal injections of valproic acid; positive control),and baicalin (cerebral ischemia and reperfusion + intraperitoneal injection of baicalin for 21 days). Neurological deficits were assessed using the postural reflex test and forelimb placing test at 3, 7, 14, and 21 days after ischemia. Rat cerebral infarct volume was measured using 2,3,5-triphenyltetrazolium chloride (TTC) staining method. Pathological change of ischemic brain tissue was assessed using hematoxylin-eosin staining. In the baicalin group, rat neurological function was obviously improved, cerebral infarct volume was obviously reduced, and the pathological impairment of ischemic brain tissue was obviously alleviated compared to the ischemia group. Cerebral infarct volume was similar in the valproic acid and baicalin groups. Thesefindings suggest that baicalin has a neuroprotective effect on cerebral ischemia.
Key Words: cerebral ischemia; neurological function; cerebral infarct volume; middle cerebral artery occlusion; rat model; valproic acid; neural regeneration
Introduction
Cerebral ischemia is caused by metabolic dysfunction secondary to insufficient blood supply to the brain (George and Steinberg, 2015). Cerebral ischemia is classified as either global ischemia or focal ischemia (Woodruff et al., 2011). In clinical practice, the most common cause of global cerebral ischemia is cardiac arrest which may cause the reduction or abolishment of the blood supply to the brain, resulting in extensive brain injury. Focal cerebral ischemia is often caused by a thrombus in a blood vessel. Focal cerebral ischemia usually causes focal brain dysfunction and may further result in cerebral stroke(Woodruff et al., 2011). Stroke is a leading cause of mortality and disability worldwide (Kim and Johnston, 2011). In the United States, it is the leading cause of disability and ranks third among all causes of death (Ramee and White, 2014). In China, stroke is thefirst leading cause of mortality (GBD 2016 Causes of Death Collaborators, 2017) and morbidity (GBD 2016 DALYs and HALE Collaborators, 2017) in 2016.
The rapid restoration of bloodflow to the brain is essential in the treatment of acute ischemic stroke. Currently, thrombolysis and thrombectomy are the main therapeutic strategies for restoring bloodflow to the brain (Raychev and Ovbiagele,2011; Ramee and White, 2014). After cerebral stroke, the brain tissues at the central necrotic area are in a completely ischemic and hypoxic environment due to bloodflow interruption secondary to acute cerebral infarction and these tissues quickly become softened and necrotic, neuronal dysfunction occurs,and it is hard to restore viability in this area (Cao et al., 2016;Yuan et al., 2017). However, there is an ischemic penumbra at the transition zone between the necrotic area and normal area(Ramee and White, 2014). Although there is insufficient blood supply for electrophysiological activities, cells in this area may maintain ion channels. But, as time passes this area is adversely affected by metabolic processes emanating from the ischemic area which results in the ischemic area expanding with deleterious effects on clinical status. Therefore, quick treatment is necessary to counter these damaging events. In the effort to improve treatment of cerebral ischemia, restoration of cellular function in the penumbra, reduction of infarct area, and protection and repair of neurological function have been the focus of recent studies (Ramos-Cabrer et al., 2011).
Baicalin is a flavonoid compound extracted from the root of the herb Scutellaria baicalensis Georgi which has been widely used in traditional Chinese medicine for the treatment of inflammatory disease and is also used for ischemic stroke(Zhuang et al., 2013; Zhu et al., 2016). It has been shown that baicalin can delay neuronal death, exerting protective effects on focal ischemia/reperfusion injury and the time window of baicalin’s neuroprotective effect in a rat model has been found to be within 4 hours after stroke (Cheng et al., 2013). DNA microarray expression analysis indicates that baicalin may induce the expression of genes related to the differentiation of neuronal stem cells (such as NGF, a signaling molecule of neuritogenesis and neurogenesis, and Adam23, which plays a role in regulating neuronal differentiation) (Li et al., 2012).In the rat cerebral ischemia/reperfusion model, baicalin can inhibit COX-2 expression and reduce the number of apoptotic neurons in the hippocampus, leading to the improvement of spatial memory following brain injury (Cheng et al., 2012).In global ischemia/reperfusion injury, baicalin also exerts neuroprotective effects that have been found to be related to the activation of GABAergic molecules (GABAARα1, GABAARγ2, and KCC2), heat shock protein 70, and mitogen-activated protein kinase pathway (ERK, JNK and p38) (Dai et al., 2013b). Another study using an ischemia/reperfusion rat model found evidence that the neuroprotective effect of baicalin against ischemic injury involves the PI3K/Akt and PTEN pathway (Liu et al., 2010). Thesefindings indicate that baicalin may significantly improve neurological function and attenuate the ischemia-induced damage to neurons. In addition, in focal cerebral ischemia/reperfusion injury, baicalin has also been found to exert neuroprotective effects which are related to the inhibition of protease-activated receptor 1 and the suppression of programmed cell apoptosis (Zhou et al., 2014).
There is considerable evidence that baicalin can provide neuroprotection in cerebral ischemia/reperfusion injury in rats. The aim of this study was to assess the neuroprotective efficacy of baicalin in a focal cerebral ischemia rat model. A growing number of studies demonstrate that valproic acid(VPA), an anti-convulsant and mood-stabilizing drug, is neuroprotective against various insults including transient global ischemia (Xuan et al., 2012). In this study, we set valproic acid as a positive control to compare the effects of baicalin in a rat model of focal cerebral ischemia.
Materials and Methods
Animals
Fifteen healthy adult male Sprague-Dawley (SD) rats, weighing 250–280 g, aged 10–14 weeks, were purchased from the Experimental Animal Center of Shanghai University of Traditional Chinese Medicine (laboratory animal usage license number: SYXK (Hu) 2013-0050). The rats were randomly assigned to three groups withfive rats in each group: ischemia (cerebral ischemia and reperfusion), VPA (cerebral ischemia and reperfusion + three intraperitoneal injections of valproic acid; positive control), and baicalin (cerebral ischemia and reperfusion + intraperitoneal injection of baicalin for 21 days). Prior to the experiment, the rats were deprived of food for 12 hours. The surgical procedures and postoperative animal care were conducted according to the Guide for the Care and Use of Laboratory Animals (National Research Council 1996) and the Guidelines and Policies for Rodent Survival Surgery provided by the Animal Care and Use Committees for Shanghai Jiaotong University.
Establishment of rat models of cerebral ischemia
The protocol used for establishing rat models of cerebral ischemia by occlusion of the middle cerebral artery (MCA)was the same as that used in a previous study (Dai et al.,2013a). The animals were anesthetized by intraperitoneal injection of 10% chloral hydrate (350 mg/kg, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China).
A thick 3-0 surgical nylon suture was used for occlusion.The modified suture was inserted from the right external carotid artery (ECA) into the lumen of the right internal carotid artery (ICA) where it blocked the origin of the right MCA. When the suture entered the right pterygopalatine artery (PPA) (about 10 mm in length), evident resistance was felt. Then the suture was retracted and the PPA was clamped. The direction of the head of the nylon suture was adjusted, and then the suture was inserted further. When the head of the suture was 17–20 cm away from the stump, there was no feeling of resistance. The clamp on the common carotid artery (CCA) was released and the skin was closed. The suture was left in place for 2 hours.The CCA and PPA were occluded temporarily.
Drug administration
The rats in the baicalin group were administered baicalin (Cat#572667, Sigma-Aldrich, St. Louis, MO, USA) using a method adapted from a previously established method (Dai et al.,2013b; Zhuang et al., 2013). Baicalin was diluted in sodium hydrogen phosphate buffer (Na2HPO4, 0.2 M) at a concentration of 100 mg/mL (stock solution) and incubated under agitation for 1 hour at 37°C. The solution wasfiltered with a 0.22 mm filter. Baicalin was administered intraperitoneally immediately after ischemia and at a dose of 100 mg/kg body weight (Zhuang et al., 2013; Zhou et al., 2014) once daily for 3 weeks. The rats in the VPA group were administered VPA (Cat# P4543-10G), which served as a positive control,according to a procedure as previously described (Ren et al.,2004). Valproic acid (300 mg/kg) was dissolved in normal saline and injected intraperitoneally at 48, 60, and 72 hours after ischemia. The rats in the ischemia group were injected daily with sodium hydrogen phosphate buffer (Na2HPO4, 0.2 M), the solvent for baicalin, and used as a negative control.
Evaluation of neurological deficits
Neurological deficits were assessed at 3, 7, 14, and 21 days after ischemia based on a previously described procedure(Belayev et al., 1996).
Neurological deficits were assessed by two tests: (1) The postural reflex test, which was developed to assess upper body posture while the rat is suspended by its tail, and (2) the forelimb placing test, which was developed to assess sensorimotor integration in forelimb placing responses to visual, tactile,and proprioceptive stimuli. Total neurological score was calculated as the sum of scores on both tests, and ranged from 0 to 12, with the maximally abnormal score of 12. Convulsions and sustained disturbance of consciousness were exclusion criteria but none of the rats exhibited these abnormalities.
Measurement of infarct volume
Infarct volume was measured according to the protocol from Zhang et al. (2012) as follows:
The whole brain was removed from the skull on day 21 after ischemia. The brain was placed into the matrix with the ventral side up. The brain was lined up longitudinally with the matrix and sliced with wet blades (68709, Rwdmall,China) at intervals of 2 mm. Then the brain slices and blades together were lifted off the matrix. Six or seven slices were expected from the procedure.
2,3,5-Triphenyltetrazolium chloride (TTC) (Sigma, Cat#T8877) was dissolved in normal saline at a concentration of 2%. Then 25 mL of 2% TTC solution was poured into a Petri dish or a small beaker (Shanghai Yuanye Biological Technology Co., Ltd., Shanghai, China). The TTC solution then was prewarmed to 37°C in a water bath. The brain slices were gently brushed off the blade one at a time, and the slices were carefully dropped into TTC solution. The slices were spreadflat on the bottom, making sure that they were totally immersed in the solution. The brain slices were maintained at 37°C for 30 minutes in the dark. Then the slices were carefully transferred into formalin and fixed for 24 hours.Finally the slices were transferred into saline.
The MCID computer imaging analysis system (Image Pro-Plus software, Media Cybernetics, MD, USA) was launched and images were loaded. To calibrate measurements, the ruler was used as a reference. The regions of interest (ROIs)were selected manually or automatically with the threshold setting. Typical ROIs are the whole area of contralateral hemisphere and the healthy tissue of ipsilateral hemisphere.Both are shown in red. Another ROI is the infarct which is shown in white. The infarct area and volume on each slice were calculated using the following equations:
Infarct area = Contralateral hemisphere area – healthy area of ipsilateral hemisphere.
Infarct volume = Infarct area × thickness of slice.
Also, the ratio of mean infarct volume/brain section volume was calculated.
Hematoxylin and eosin (H&E) staining
Three brain sections for each case taken from the injury area were stained with H&E (Sigma-Aldrich). Briefly, the sections were warmed at 37°C overnight. After OCT was eluted with PBS for 10 minutes, the sections were stained with hematoxylin solution for 8 minutes. After differentiation in 0.5% acid alcohol for 10–15 seconds, the sections were washed in running tap water for 10 minutes, followed by dehydration in 75% and 85% alcohol for 5 minutes each.The sections were then counterstained in eosin solution for 25 seconds, quickly dipped in 95% alcohol four times, dehydrated in 95% alcohol, with two changes of absolute alcohol for 5 minutes each, followed by clearing with two changes of xylene for 5 minutes each. The sections were cover-slipped using PermountTMMounting Medium (0909129, Shanghai Zhanyun Chemical Co., Ltd., Shanghai, China).
The slices from anterior to posterior, with left hemisphere on the left, were lined up on a piece of blue plastic. Excess water was removed on and around the slices with a paper towel. A ruler was placed alongside the slices. Photos were taken with a digital camera (Olympus BX 53 microscope, Tokyo, Japan).
Statistical analysis
The neurological scores were presented as the mean ± standard deviation (SD) with a line plot during postoperative days 3, 7, 14, and 21 for each of the treatments. The infarct volumes were presented as the mean ± SD with a bar graph for each of the treatments. The dispersion of neurological score over time (from day 3 to day 21) among the three treatments was compared using repeated measures analysis of variance (ANOVA) along with a Bonferroni pair-wise comparison. Differences among the three treatments were compared using one-way ANOVA with a post-hoc Bonferroni pair-wise comparison. All statistical assessments were twotailed and considered significant at P < 0.05. All statistical analyses were carried out with IBM SPSS statistical software version 22 for Windows (IBM Corp., Armonk, NY, USA).
Results
Effects of baicalin on neurological function in cerebral ischemia rats
The dispersion of neurological score at 3, 7, 14, and 21 days postoperatively for the ischemia, VPA, and baicalin groups were shown in Figure 1. The baicalin group had a lower mean neurological score from day 3 to day 21 postoperatively compared with the ischemia group (P < 0.05). However,the statistical significance was borderline (P = 0.046). The baicalin group had a lower mean neurological score at 14 and 21 days postoperatively compared with the VPA group(day 14: P = 0.044; day 21: P = 0.025). The baicalin group also had a lower mean neurological score than the ischemia group at 14 days postoperatively (P = 0.044).
Effects of baicalin on infarct volume of cerebral ischemia rats
Figure 2 displays the comparison of the ratio of mean infarct volume to brain section volume among the ischemia,VPA, and baicalin groups. The results showed that this ratio was significantly decreased in the VPA and baicalin groups compared with the ischemia group (P = 0.007 and P < 0.001,respectively). There was no significant difference in the ratio of mean infarct volume to brain section volume between the VPA and baicalin groups (P = 0.217).
Effects of baicalin on pathomorphology in cerebral ischemic tissue of rats
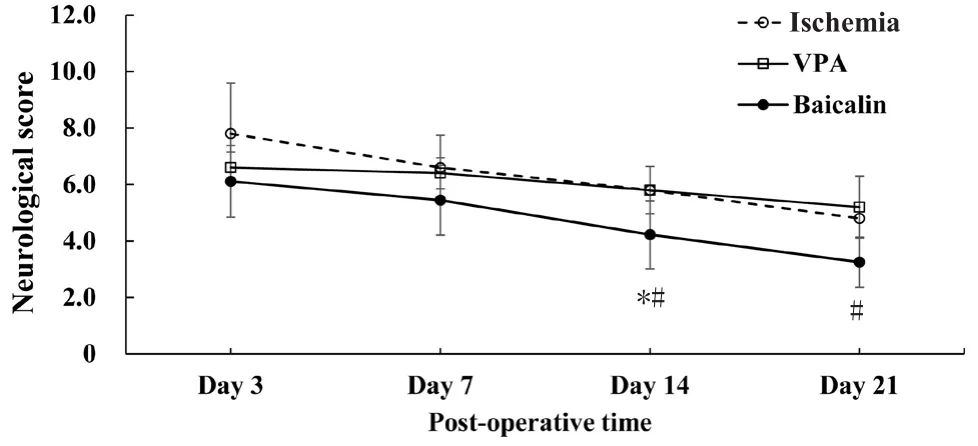
Figure 1 The dispersion of neurological function of cerebral ischemic rats on days 3, 7, 14, and 21 post-operatively.
The results of H&E staining are shown in Figure 3. The contralateral (left) side of the brain was used as normal control(Figure 3A–C). Cavities formed and were observed in the ipsilateral (right) striatum in the ischemia group (Figure 3G and H). Baicalin treatment significantly attenuated the injury, and cavities were not observed (Figure 3D–F). In the contralateral (left) side of the brain in rats, there were clear layers in the striatum and cortex (Figure 3B and C)and cells were arranged closely. In the ipsilateral (right) side of the brain in rats, clear cavities were observed in the striatum, the tissues were loose, cells were ruptured, normal cell structure was almost absent, and the wholefield was blurred(Figure 3G–I). In the baicalin group, tissues were regularly arranged, there were no cavities, and small space was observed between cells with a clear borderline, and infiltration of inflammatory cells was also noted (Figure 3D–F).
Discussion
The findings of this study showed that in a rat model of MCAO, baicalin was effective for reducing neurological deficits and reducing infarct volume. These results provide further evidence of the effectiveness of baicalin for providing neuroprotection in cerebral ischemia injury. Our results suggest that baicalin potentially plays a wider therapautic role in clinical practice.
Baicalin has been shown to significantly improve learning and memory in a gerbil model of global cerebral ischemia/reperfusion. Wang et al. (2016) found that baicalin attenutated the phosphorylation of a key protein involved in calcium ion signaling and that it also prevented hippocampal neuronal apoptosis. Cheng et al. (2012) found that baicalin improved spatial learning ability after global cerebral ischemia by reducing hippocampal apoptosis via the inhibition of COX-2 expression. Dai et al. (2013b) investigated the mechanism underlying the neuroprotective effect of baicalin in a gerbil model of cerebral global ischemia/reperfusion and they found that the underlying mechanism is related to baicalin activating GABAergic signaling, heat shock protein 70, and mitogen-activated protein kinases (MAPSs) cascades. Zhou et al. (2014) found that baicalin had neuroprotective effects on focal cerebral ischemia/reperfusin injury through inhibiting the expression of PAR-1 and apoptosis in a rat model of MCAO. In a study by Zheng et al. (2015), administration of baicalin reduced neurological deficit and also reduced infarct volume. Neurological function was assessed 2 hours after ischemia and 24 hours after reperfusion and infarct volume was measured at the same time points (Zheng et al., 2015).Our results were similar but we did not begin to assess neurological function until day 3 postoperatively and we measured infarct volume at day 21 postoperatively.
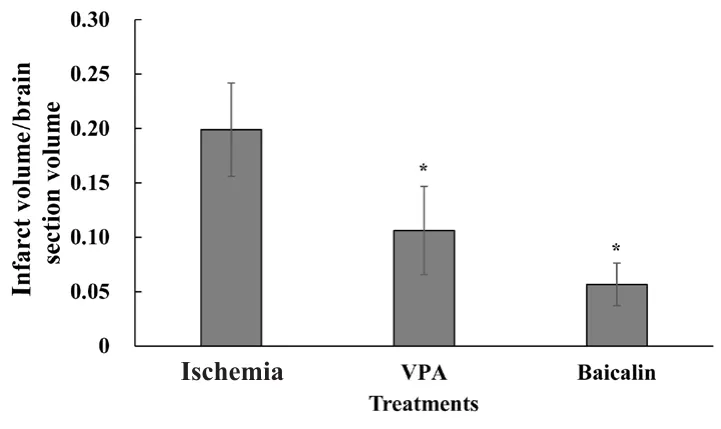
Figure 2 Quantification of infarct volume in rats at 21 days postoperatively.
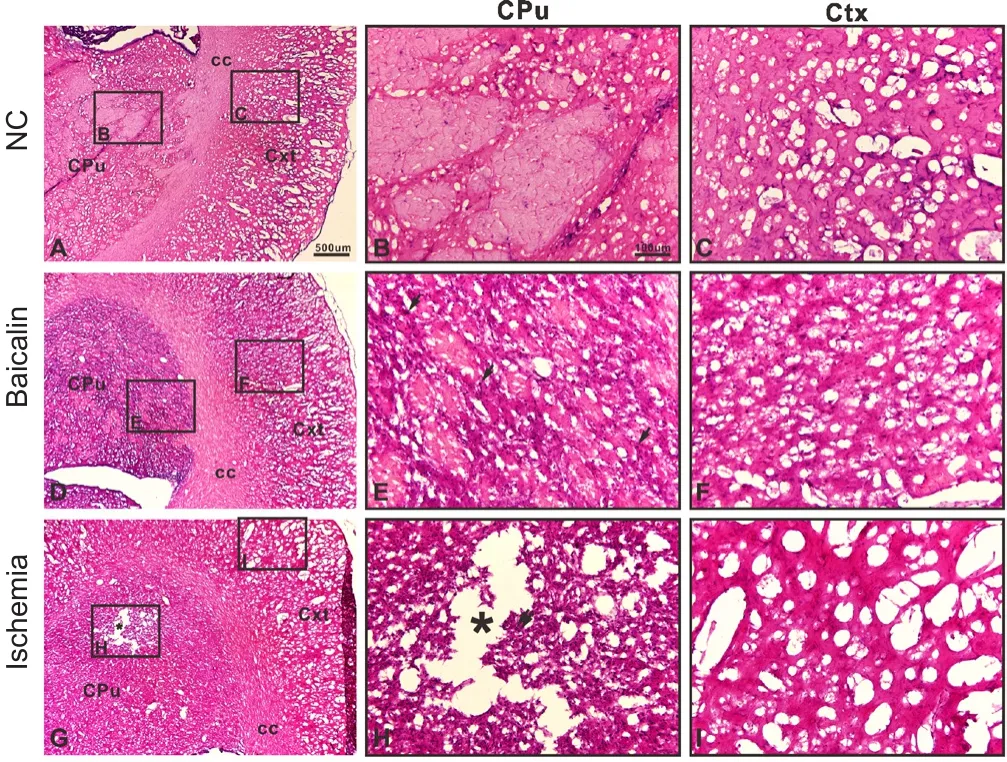
Figure 3 Baicalin reduced damaged area in rats at 21 days postoperatively (hematoxylin and eosin staining).
Baicalin is one of the constituents in one of the most widely used Traditional Chinese Medicine natural products, Huang-Lian-Jie-Du-Decoction (HUJDD) (Zou et al., 2016). Berberine, baicalin, and jasminoidin were major active ingredients of Huang-Lian-Jie-Du-Decoction (HUJDD) and they were reportedly to be responsible for the neuroprotective effects of HLJDD on the treatment of ischemic stroke in a rat model of MCAO (Zou et al., 2016). There is evidnece that the neuroprotective effect of baicalin can be enhanced by preparation of solid lipid nanoparticles (Liu et al., 2015). They found that the OX26 antibody modified PEGylated cationic solid lipid nanoparticles improved uptake of baicalin across the bloodbrain barrier, and elevated bioavailability of baicalin in cerebral spinalfluid in rats with cerebral ischemia/reperfusion injury. This suggests that administration of nanoparticles loaded with baicalin provides better therapeutic effects on stroke. This can potentially help overcome the problem of needing to begin treatment only in short time following the onset of stroke to reduce damage to the brain.
A limitation of this study is small sample size, as there was only five rats in each group. Our findings therefore should be confi rmed in a study using a larger number of rats in each group.
In conclusion, our results demonstrated that baicalin was effective for neuroprotection in focal cerebral ischemia. It improved neurological function for up to 21 days after ischemia, reduced infarct volume, and improved the pathomorphology of the injured cerebral tissue. Baicalin appears to be a potentially effective therapeutic agent for the treatment of ischemic stroke.
Author contributions:Guarantor of integrity of the entire study: JD and XHZ; study design: YMQ; experimental studies: ZWM and GFY; data acquisition and analysis: JZ; manuscript editing: SQL; statistical analysis: HW and YCJ; manuscript preparation: JD; manuscript review: XHZ. All authors approved thefinal version of this paper.
Conflicts of interest:None declared.
Financial support:This study was supported by the Cross Foundation Major Project of Engineering and Medicine of Shanghai Jiao Tong University, No.YG2016MS50 (to JD) and Foundation for Fostering Project of Clinical Study on Multi-disciplinary Team of Renji Hospital, No. PYMDT-012 (to JD). None of the funding bodies play any role in the study other than to provide funding.
Institutional review board statement:The study was approved by the Animal Care and Use Committee of Shanghai Jiao Tong University and performed in accordance with the Guide for the Care and Use of Laboratory Animals(National Research Council 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Olga Chechneva, University of California Davis, USA;Yixin Chen, Sano fi-Aventis US LLC, USA.
Additionalfile:Open peer review reports 1 and 2.
杂志排行
中国神经再生研究(英文版)的其它文章
- MicroRNAs of microglia: wrestling with central nervous system disease
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Apomorphine effects on the hippocampus
- Roles and functions of Atp6ap2 in the brain
- Magnesium sulfate and fetal neuroprotection:overview of clinical evidence
- Polyphenols-gut microbiota interplay and brain neuromodulation
