Cell-based therapy in Alzheimer’s disease: can human fetal cholinergic neurons “untangle the skein”?
2018-10-22GiuliaGuarnieri,EricaSarchielli,GabriellaB.Vannelli等
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder and the most common form of old-age dementia. The disease is characterized by a progressive decline in cognitive functions, gradual loss of memory and ability to perform everyday activities, and leads to inevitable death within 3 to 9 years after diagnosis. The pathological hallmarks of the disease are the accumulation of intracellular neurofibrillary tangles, composed of the microtubule associated protein tau, and extracellular deposits of amyloid-β (Aβ) plaques, which are overall responsible for an extensive loss of neurons and synaptic connectivity.
The pathophysiology of late-onset, non-familial, AD is complex and include a cascade of pathogenic events not yet fully understood. Among the several competing theories on the underlying neurodegenerative mechanisms,the cholinergic hypothesis has revolutionized thefield of AD research allowing advancements into interventions to arrest or delay the disease progression [see Hampel et al. (2018) for review]. According to this hypothesis, the AD-related pathological changes occur in those brain regions predominantly involved in the cholinergic neurotransmission, critically important for memory, learning,and attention. In particular, the basal forebrain (BF) cholinergic neurons and their cortical projections have been identified as the most selectively vulnerable to degeneration in AD (Liu et al., 2015). Accordingly, while there is no strict correlation between AD-related cognitive impairment and number of cortical Aβ plaques, neuronal and synaptic loss within the nucleus basalis of Meynert (NBM),the main source of cholinergic neurons within the BF,represents the principal clinical correlate in AD patients(Kilimann et al., 2014). As recently reviewed (Hampel et al., 2018), several studies have shown that the cholinergic lesion is an early and prodromal event for AD pathogenesis, mainly based on the degeneration of NBM cholinergic neurons. Neuroimaging studies with the use of Aβ biomarkers demonstrated that NBM degeneration precedes and predicts the cortical spread and memory impairment of AD pathology. In particular, a subsequent anterograde cholinergic deafferentation of the cerebral cortex, hippocampus and amygdala, as well as alterations of both nicotinic and muscarinic cholinergic receptors represent key neurochemical pathogenic events for AD onset (Hampel et al., 2018). The relation between cholinergic activity and the etiology of AD is also corroborated by the negative pharmacological effects of anticholinergic drugs on human memory and learning, while early treatments that promotes cholinergic function in individuals with asymptomatic AD, or at risk for developing the disease, were associated with substantially reduced cortical injury and basal forebrain atrophy over time (Hampel et al., 2018).Moreover, multidisciplinary investigations have demonstrated that dysfunctions of the basal forebrain cholinergic network may interact with other well known pathogenic factors of AD, including formation of Aβ plaques and neurofibrillary tangles, inflammation, oxidative stress, neuroplasticity, and cerebral hemodynamic processes.
Given its crucial implication in the disease development,the major therapeutic efforts have been directed toward the cholinergic system. Current strategies, based on targeting at cholinergic neurotransmission, include cholinesterase inhibitors- to increase acetylcholine (Ach) levels - and Ach receptor agonists. Only four drugs have been approved for treating AD-associated dementia so far, and three of them- donepezil, galantamine, and rivastigmine - are cholinesterase inhibitors. Galantamine is also active as a modulator at nicotinic Ach receptors. In addition to these drugs,memantine is the most recently approved pharmacological treatment, which is an N-methyl-D-aspartate (NMDA)receptor antagonist able to modulate glutamate and glutaminergic pathways. These drugs are generally considered symptomatic treatments for AD, being able to significantly improve cognition, and some behavioral manifestations of the disease (Massoud and Gauthier, 2010). However, none of them are disease-modifying therapies since they do not exert any impact on the underlying primary pathological processes of the disorder. Therefore, they are administered as palliative therapy with the aim of slowing the decline in quality of life, but with no long-term beneficial effects.
Hence, it is undoubted that new approaches to treat and understand the disease need to be urgently explored. Indeed, AD progression could be potentially modified only by interventions able to replenish lost neurons and/or avoid neuronal death. In this regard, novel therapeutic approaches are based on delivering to the brain neurotrophic factors, such as the nerve growth factor (NGF), able to promote neuronal survival (Tuszynski et al., 2005). More importantly, the clinical application of the so-called “regenerative medicine” aimed at replacing diseased tissues started to be investigated also in the case of nervous tissue loss. Nevertheless, the proper strategy to functionally replace the damaged brain areas in AD patients is still under investigation.
When we consider cell-replacement therapy for neurodegenerative diseases, the major questions are: 1) is the cell loss limited to a precise region of the brain? 2) what phenotype/differentiation grade of transplanted cells should be the most appropriate? 3) may the pathological environment within the damaged brain (neurofibrillary tangles,Aβ plaques, inflammatory insult, loss of glial and vascular trophic support) obstacle the therapeutic intervention? 4)how long may the desirable positive effects of cell transplantation persist although the pathogenic primary causes are not removed? Answering these questions is the first step before thinking of the clinical application of any kind of cell-replacement strategy for AD treatment. Thefirst occurrence (i.e., involvement of a precise brain region) certainly facilitated the advances obtained in the case of neurodegenerative diseases such as Parkinson’s disease (PD),caused by focal degeneration of dopaminergic neurons of substantia nigra, or Huntington’s disease, caused by focal loss of medium spiny neurons in the corpus striatum of basal ganglia. Protocols of intervention by cell-based therapies for both diseases are under development (Paganini et al., 2014; Barker et al., 2017; Parmar, 2018). In particular,PD may be taken as the prototypical disease where regenerative medicine is close to clinical translation (Barker et al., 2017). Differently, AD pathogenesis encompasses several brain regions, such as the BF, the neocortex and hippocampus, thus impairing an intricate system of neuronal connections, and making difficult the establishment of the most appropriate cell source for the replacement approach. A significant advancement toward this aim may be reachable through the identifications of those mechanisms underlying the generation of neurons mainly damaged by the disease, especially during the early phases of its development, such as the NBM cholinergic neurons. Hence, it appears mandatory to assess physiologically relevant in vitro models enabling to identify the differentiation steps through which NBM cholinergic neuronal precursors acquire their key morpho-functional properties (excitability,release of the appropriate neurotransmitter, the peculiar gene and protein expression signature, the ability to communicate through neurite and synapsis formation and the receptorial machinery for responding to either healthy or pathological environmental cues). As an initial step to achieve this objective, we recently isolated and fully characterized human cholinergic neurons from the fetal NBM(hfNBM; Morelli et al., 2017). Here we propose the potential of these “young” cholinergic neurons in providing new models that enable researchers to study the disease and develop novel therapeutic approaches using a human neuronal setting. Indeed, fetal tissue is a rich source of cells already committed toward a specific cell fate, but they also retain immature features. The characterization of this cellular plasticity may represent a resource to identify the factors involved in the developmental steps responsible for morphological and functional differentiation of neurons specifically affected during AD progression. Accordingly, pioneering studies on the applicability of regenerative medicine in PD patients demonstrated that human fetal neurons may serve as an important reference model for identifying those phenotypic properties related to a therapeutic potential for cell replacement purposes (Grealish et al., 2014).
The application of cell-based therapies in the treatment of AD has been explored in several experimental studies,both in vitro and in vivo, using animal models and different cell sources, but studies using human cholinergic neuronal precursors of fetal origin are lacking. Indeed,the research in the field is currently based on the use of pluripotent embryonic stem cells (ESCs) and multipotent stem cells, such as mesenchymal stem cells (MSCs) and neural stem cells (NSCs), as recently reviewed (Duncan and Valenzuela, 2017). In addition, a relatively new technology consists into the generation in vitro of induced pluripotent stem cells (iPSCs) from mature somatic cells,commonly adult dermal fibroblasts genetically modified to become pluripotent and ESC-like. However, both ESCs and iPSCs possess the ability to generate cell types from all the embryonic germ layers and therefore require in vitro manipulations in order to obtain the desired cell type for transplantation purposes. At variance, the brain-derived NSCs possess the potential to generate all neural cell types(both glial cells and neurons) during development and therefore may be regarded as the best cell source for the replacement of nervous tissue. However, one of the main problems encountered by the exogenous introduction of NSCs in the diseased brain is that the engrafted cellsfind a pathological microenvironment, which strongly counteracts the differentiation and integration of new neurons. In particular, it has been demonstrated that following NSCs transplantation the surrounding microenvironmental alterations under AD pathology may determine a preferential differentiation toward the glial lineage rather than the neuronal one (Kwak et al., 2006). In this context, it appears conceivable that an optimal cell source should possess the features of neuronal precursors that have lost the multipotent capacity, are already committed toward a specific neuronal type and may complete the maturation program within the host brain. Hence, using cells phenotypically resembling the hfNBM model, i.e., with a clear-programmed cholinergic neuronal fate, could help to overcome obstacles such as glial differentiation. Indeed, we demonstrated that immediately after their isolation (early passages in culture) hfNBMs showed a cholinergic identity (expression of specific markers, such as choline acetyltransferase(ChAT), vesicular Ach transporter (VACHT) and TrkA;responsiveness to NGF; electrophysiological properties of functional neurons; Ach release ability) and that they retained this phenotype for several passages in culture.Moreover, when injected in NBM-lesioned rats, hfNBMs promoted functional effects that could be ascribed to an improvement of the cholinergic signaling in the basal forebrain. Overall, thesefindings suggest that hfNBMs could be a useful model for identifying the proper differentiation grade needed for a successful regenerative medicine approach. The precise mechanisms underlying the overall experimental observations remain to be defined and represent the main challenge for future research. It would be interesting to assess in further studies how the hfNBM cell phenotype could be affected by the insults characterizing the AD brain.
In conclusion, as schematized in Figure 1, the availability of physiologically relevant in vitro preclinical tools is crucial to cover the gap between “income” and “outcome”considerations concerning the feasibility of cell-based therapy for AD. Prior to clinical application, preclinical research needs to identify the most appropriate cell source(differentiation grade and survival ability in AD microenvironment) satisfying the basic knowledge (income)required for a successful outcome (neuronal circuitry integration and long term functional recovery). We firmly believe that the data obtained using fetal cell sources, such as the hfNBM model, are a valuable asset to “untangle the skein” in order to decipher the most effective regenerative potential required by the transplanted cells.
Giulia Guarnieri, Erica Sarchielli, Gabriella B. Vannelli,Annamaria Morelli*
Anatomy and Histology Unit, Department of Clinical and Experimental Medicine, University of Florence, Florence, Italy
*Correspondence to:Annamaria Morelli, PhD,
a.morelli@uni fi.it.
orcid:0000-0001-8027-9870 (Annamaria Morelli)Received:2018-06-05Accepted:2018-08-13
doi:10.4103/1673-5374.241459
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
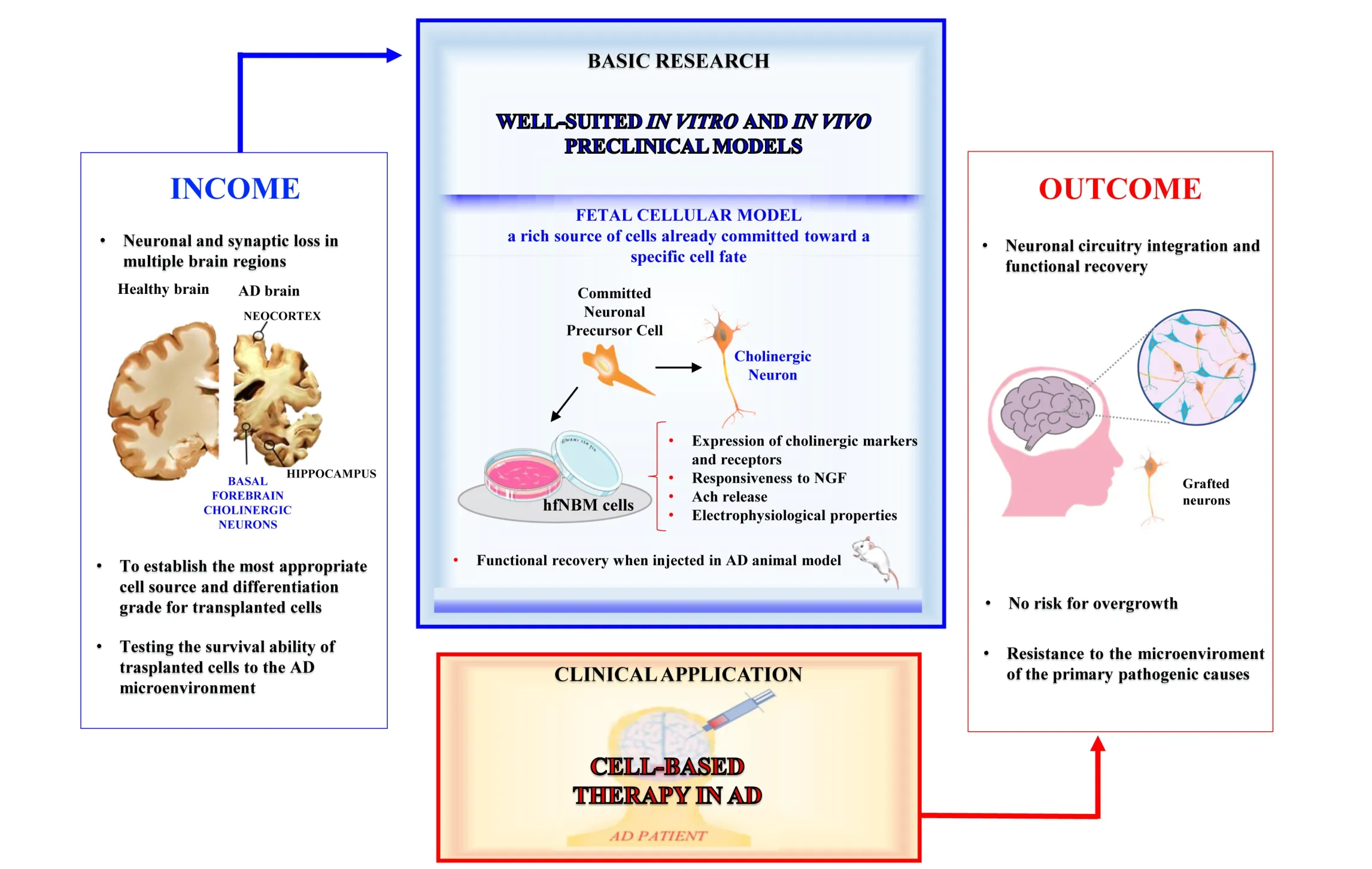
Figure 1 Applicability of cell-based therapy in Alzheimer’s disease (AD) patients.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Alessandra Bitto, University of Messina, Italy.
杂志排行
中国神经再生研究(英文版)的其它文章
- MicroRNAs of microglia: wrestling with central nervous system disease
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Apomorphine effects on the hippocampus
- Roles and functions of Atp6ap2 in the brain
- Magnesium sulfate and fetal neuroprotection:overview of clinical evidence
- Polyphenols-gut microbiota interplay and brain neuromodulation
