The rat model of placental ischemia as a model of postpartum posterior cortical atrophy?
2018-10-22AshtinB.Giambrone,JunieP.Warrington
Potential link between preeclampsia (PE) and posterior cortical atrophy (PCA): PCA is a neurodegenerative disorder affecting the parietal, occipital, and occipital-temporal brain regions, often manifesting as a decline in visual processing and perception skills in affected individuals. The most common underlying pathology of PCA is Alzheimer’s disease (Crutch et al., 2012), which disproportionally affects women. Indeed, approximately two-thirds of Alzheimer’s disease patients are women and some studies suggest that PCA is more common among women than men, although other studies report no sex differences in PCA incidence (Crutch et al., 2012).Taken together, these findings point to the potential involvement of sex-specific factors such as pregnancy and pregnancy disorders like PE, in driving the increased risk of neurodegenerative diseases in women. While there are no studies showing a direct link between PE and PCA, a recent study reported decreased gray matter volume in the posterior brain region of women with a history of PE and subsequent late-life hypertension (Raman et al., 2017). Importantly,during pregnancy, not only do PE patients often present with visual symptoms but abnormal brain imagingfindings are consistent most commonly with posterior cerebral or occipital lobe abnormalities,suggesting that PE-induced abnormalities occur most frequently in the posterior cerebrum. These findings support the hypothesis that women with a history of PE may be more likely to present with PCA later in life; however, no studies have specifically addressed this question. Furthermore, because of low awareness of the disorder,not much is known regarding the underlying pathophysiological mechanisms, and prevention and treatment strategies are lacking. A recent publication provided evidence that reduced utero-placental bloodflow and resulting placental ischemia during pregnancy, led to neuroinflammation and increased water content in the posterior cortex at two months postpartum while other brain regions such as anterior cerebrum, striatum, and hippocampus were spared (Clayton et al., 2018). Herein, we present that the rat model of placental ischemia may be a suitable tool to assess the underlying mechanisms of postpartum posterior cortical abnormalities.
PE is a hypertensive disorder of pregnancy affecting 3–8% of pregnancies in the United States and results in significant morbidity and mortality risks for both the mother and offspring. Placental abnormalities are thought to initiate and mediate the characteristic symptoms of the disorder and babies born to PE patients tend to be intrauterine growth restricted or small for gestational age. Because PE symptoms remit after delivery of the fetus and placenta for most patients, delivery has been considered the only “cure” for PE; however, emerging evidence suggests that this is not the case since women with a history of PE have increased risk of long-term postpartum complications. For example, prior PE patients were shown to have increased risk of mortality from Alzheimer’s disease and stroke years after index pregnancy (Theilen et al., 2016). Additionally, a recent meta-analysis showed that women with a history of (pre)eclampsia had an 80% increased risk of stroke compared to those who had normal, healthy pregnancies (Wu et al., 2017). Moreover, women with a history of PE performed worse on cognitive tasks and reported higher cognitive difficulties in their daily lives (Postma et al., 2014; Mielke et al., 2016) and were more likely to have white matter lesions(Siepmann et al., 2017) when compared to women with history of normal pregnancies. White matter lesions, hyper-intense areas observed on brain imaging, are also prevalent in brains of Alzheimer’s diseases patients, supporting a link between PE and Alzheimer’s disease. An important question is whether hypertension (not associated with pregnancy) in women, contributes to increased neurodegenerative disorders. As reviewed by Nagai et al. (2010), hypertensive patients have an increased risk of developing dementia and cognitive impairments later in life, without an increased risk for Alzheimer’s disease, suggesting that hypertension alone does not contribute to Alzheimer’s disease risk. Whether hypertensive women have a higher risk of developing Alzheimer’s disease compared to normotensive women is mostly unexplored. Thus, an important question to be addressed is whether hypertensive women with or without a history of hypertensive disorders of pregnancy, have increased risk of dementia and/or Alzheimer’s disease compared to normotensive women and hypertensive men. Furthermore, the underlying pathophysiological mechanisms contributing to the increased risk of prior PE patients developing white matter lesions, Alzheimer’s disease,and other cerebrovascular complications, are not known.
The rodent model of placental ischemia:Placental ischemia is thought to occur as a result of incomplete remodeling of the maternal uterine arteries that supply the developing utero-placental unit.The ischemic placenta then secretes numerous factors including pro-inflammatory and anti-angiogenic factors into the maternal circulation, inducing hypertension and other characteristic symptoms of PE. To mimic the clinical condition, the placental ischemia model was developed in the rat. To do this, silver clips are surgically placed around the abdominal aorta and both branches of the uterine arteries between the ovary andfirst pup (Granger et al., 2006), resulting in reduced bloodflow to the utero-placental unit by approximately 40%. Placental ischemic rats have increased placental and circulating levels of pro-inflammatory cytokines and anti-angiogenic factors,develop hypertension, and have smaller pups (Granger et al., 2006).Importantly, placental ischemia induces impaired cerebral bloodflow autoregulation, increased blood-brain barrier permeability, and cerebral edema during pregnancy (Warrington et al., 2014), indicating impaired cerebrovascular function and suggesting potential neuronal damage. Using this rodent model, a recent manuscript reported that following placental ischemia, rats present with neuroinflammation (increased posterior cortical tissue levels of pro-infl ammatory cytokines (interleukin (IL)-17, IL-1α and β, leptin, and macrophage inflammatory protein 2, increased density of astrocytes,and increased activated microglia) at two months postpartum (Clayton et al., 2018). These rats also had reduced blood-brain barrier-associated protein, occludin in the posterior cortex, indicating that potentially blood-brain barrier integrity is lost. Thus, it is possible that the postpartum observations could be reflective of persistent damage that was unresolved after delivery.
While the authors found features of neuroinflammation and edema in the posterior cortex, they did not determine whether neurodegeneration is increased within the posterior cortex of postpartum rats, or if these abnormalities result in functional changes such as learning and memory deficits or visuo-spatial differences, characteristic of PCA patients. Because the analysis of neuroglia activation was performed in the retrosplenial, visual, and parietal cortex, the findings of increased astrocyte and microglia activation in those brain regions support the hypothesis that functional visual deficits would be present in these rats at two months postpartum. It should be noted that the regions assessed in the postpartum placental ischemia rat model correspond to similar regions affected by PCA. To further characterize the model, cortical thickness, neuronal density,and behavioral tasks will be performed in rats subjected to placental ischemia at different time-points during the postpartum period.
Link between neuroinflammation and neuronal damage:Neuroinflammation is generally characterized as either acute or chronic depending on the time of assessment after initial injury. Acute neuroinflammation occurs in response to tissue injury and usually serves a neuroprotective or neuro-reparative role. Chronic inflammation on the other hand exists when persistent or recurring damage creates a vicious cycle that ultimately leads to further neuronal and glial cell damage. In the postpartum placental ischemic rat model, reduced density of microglia was observed in the posterior cortex where pro-inflammatory cytokines were elevated (Clayton et al., 2018). Reduced density of microglia can be detrimental in the presence of ongoing and unresolved tissue damage since microglia act to restore homeostasis, functioning to phagocytose cell debris. As reviewed in Jellinger (2010), neuroinflammation and the contribu-tion of microglia to Alzheimer’s disease progression are important considerations as studies report increased activation of microglia,while others show reduced number or increased degeneration of microglia in the setting of Alzheimer’s disease. Interestingly, of the remaining microglia found in the postpartum placental ischemic rat posterior cortex, a higher percentage were in the activated, type 4 category, suggesting chronic neuroinflammation and potential neuronal damage. Thus, neuronal density, neurodegeneration markers,and cortical thickness can be assessed to determine whether neuronal degeneration and damage occurs in this rat model.
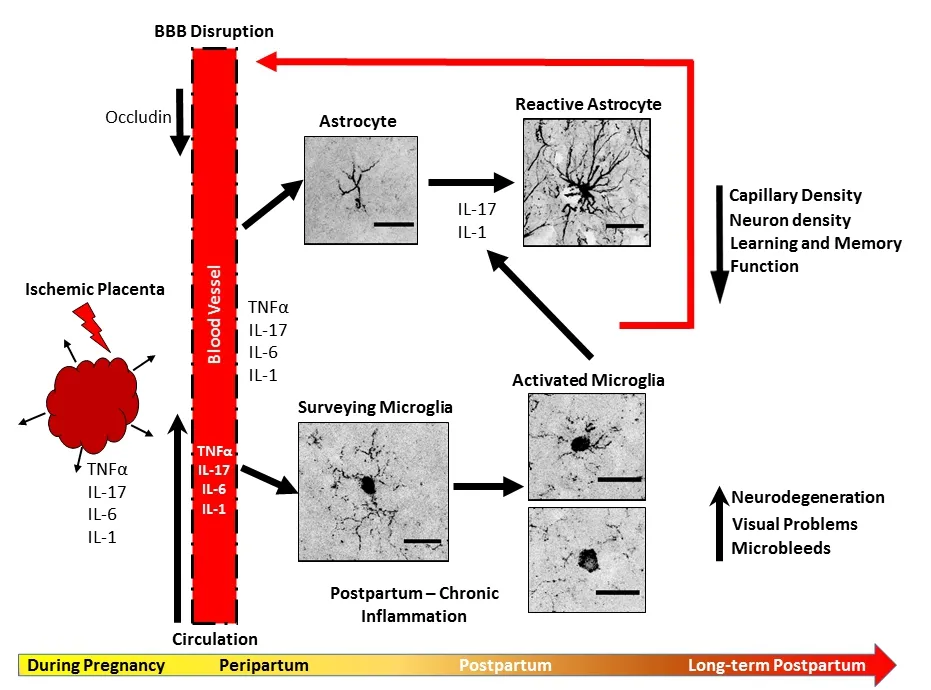
Figure 1 Summary and schematic of working hypothesis.
Implications and perspectives:PE, often referred to as an inflammatory disorder of pregnancy, is often complicated by placental under-perfusion that results in placental ischemia. Several cytokines such as tumor necrosis factor α, IL-17, IL-6, and IL-1 (α and β) are elevated in the maternal circulation of PE patients and the placental ischemic rat (Figure 1). Pro-inflammatory cytokines such as IL-17 causes decreased expression of blood-brain barrier-associated proteins like occludin, allowing for infiltration of inflammatory factors into the brain parenchyma, activating microglia and astrocytes.Activated microglia then secrete more pro-inflammatory cytokines(IL-17 and IL-1), activating astrocytes, and contributing to further vascular damage (Figure 1). Chronic inflammation could then induce rarefaction of the capillaries and weakening of the vessel wall,inducing micro-hemorrhages and cognitive impairment long-term.The rodent model of placental ischemia presents a useful model for assessing postpartum neurovascular complications after a pregnancy complicated by PE and placental under-perfusion and can be used to assess whether interventions during pregnancy can prevent these postpartum cerebrovascular complications and neuroinflammation.Whether the postpartum rodent placental ischemia model also has features of neurodegeneration in the posterior cortex and has visuo-spatial abnormalities is to be determined.
This work was supported in part by the National Institutes of Health funding, No. 4R00HL129192 (to JPW).
Ashtin B. Giambrone, Junie P. Warrington*
Department of Neurology, University of Mississippi Medical Center, Jackson, MS, USA
*Correspondence to:Junie P. Warrington, PhD,jpwarrington@umc.edu.
orcid:0000-0001-5626-8872 (Junie P. Warrington)
Received:2018-06-19
Accepted:2018-08-07
doi:10.4103/1673-5374.241454
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- MicroRNAs of microglia: wrestling with central nervous system disease
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Apomorphine effects on the hippocampus
- Roles and functions of Atp6ap2 in the brain
- Magnesium sulfate and fetal neuroprotection:overview of clinical evidence
- Polyphenols-gut microbiota interplay and brain neuromodulation
