Novel translational rat models of dopamine transporter deficiency
2018-10-22DamianaLeo,IlyaSukhanov,RaulR.Gainetdinov
Dopamine (DA) is one of the brain’s fundamental neurotransmitters. Despite the fact that the dopaminergic synapses constitute less than 1% of all brain synapses, DA is implicated in a number of critical physiological functions and in the pathogenesis of important psychiatric diseases such as schizophrenia, attention-deficit/hyperactivity disorder(ADHD), Parkinson’s disease (PD) and others. Genetically modified animals represent important tools in understanding the role of DA in the regulation of brain functions and pathology. The most known transgenic animal models to investigate DA abnormalities are dopamine transporter (DAT)knock-out (KO) mice, DAT knock-down mice and DAT over-expressing mice (Giros et al., 1996; Zhuang et al., 2001;Gainetdinov, 2008; Efimova et al., 2016). It is not surprising,since DAT (Slc6a3), the major target of psychostimulant drugs of abuse such as amphetamine (AMPH) and cocaine,plays a key role in maintaining both the extraneuronal and intraneuronal DA levels via selective re-uptake of released DA back in the presynaptic dopaminergic neurons (Giros et al., 1996; Efimova et al., 2016). The data gained from studies using the transgenic mice have provided numerous advances in understanding not only basic principles of DA transmission, but also the mechanisms of psychotropic drugs action,interaction between neurotransmitter systems, pathological mechanisms involved in the DA-related brain disorders and uncovered new principles of therapy of these disorders(Gainetdinov, 2008; Efimova et al., 2016). However, certain questions, particularly concerning the investigation of cognitive behavioural processes that are critical for modelling neuropsychiatric conditions can be more reliably addressed in transgenic rats. A rat has larger brain size for neurosurgery and electrophysiological recordings, richer behaviour and demonstrate more robust and reproducible performance in cognitive tasks (Abbott, 2004). Thus, rats with deficient DAT function were developed by two groups independently(Vengeliene et al., 2017; Leo et al., 2018). DAT-KO rat line from Leo and colleagues was created in outbred Wistar Han rats by using zincfinger nuclease technology. DAT-KO rats have no DAT protein and function due to the 5 bp deletion and an early stop codon insertion (Leo et al., 2018). DATN157K mutant rats from Vengeliene et al. were generated in an inbred F344 line by N-ethyl-N-nitrosourea (ENU)-induced spontaneous mutation. The rat DAT-N157K protein is transcribed and translated but is not correctly processed to the cell surface resulting in dramatically decreased (> 95%)of DAT function. Thus, by the level of DAT deficiency DATN157K mutant rats are more comparable to DAT knockdown (Zhuang et al., 2001), rather than DAT-KO mice (Giros et al., 1996). Since a well-known draw-back of the ENU technology is the induction of bystander mutations, DAT-N157K founder rats were backcrossed with intact female rats for up to 13 generations to reduce the chance of contribution of additional mutations to the observed phenotype (Vengeliene et al., 2017).
From the neurochemical point of view, DAT-KO and DAT-N157K mutant rats show a lack or dramatic reduction of DAT ability to re-uptake DA from the synaptic cleft. To evaluate the consequences of DAT deficiency on the extracellular dynamics of DA, Leo et al. (2018) used the Fast Scan Cyclic Voltammetry (FSCV) technique on brain slices, while Vengeliene et al. (2017) directly measured [3H]DA uptake in the striatal synaptosomes. FSCV studies have shown that the time to clear released DA from extracellular space was 1.3,10, and 50 seconds, in wild type (WT), heterozygous (HET)and DAT-KO rats, respectively, comparable to the values found in DAT-KO mice (Giros et al., 1996). Furthermore,the application offluoxetine, a selective serotonin transporter (SERT) blocker, did not affect DA clearance in any of the genotypes in DAT-KO rats indicating that SERT cannot provide extracellular DA clearance via promiscuous uptake. At the same time, in DAT-N157K mutant rats, DAT deficiency resulted in reduced norepinephrine transporter (NET) and increased SERT expression as revealed by the binding assay with [3H]-nisoxetine and [3H]-citalopram, respectively. In both strains, DAT deficiency caused robustly augmented extracellular DA levels in the subcortical areas. Quantitative“low perfusion rate” microdialysis measurements have revealed that DAT-KO rats display about 7-fold increased basal extracellular DA levels, as well as significantly augmented levels of the DA metabolites – 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the striatum of awake rats. Similarly, DAT-N157K mutants presented strong augmentation in striatal DA extracellular levels and robust increase in HVA levels. In conventional microdialysis experiments, both mutant strains responded to AMPH administration in a genotype-dependent manner (Vengeliene et al., 2017; Leo et al., 2018). As was described earlier in DATKO mice (Efimova et al., 2016), AMPH induced a significant increase in extracellular DA in WT and to a lesser degree in HET rats (measured only in DAT-KO strain), but caused no effect in DAT deficient animals. Moreover, the treatment with mGluR2/3 antagonist LY341495 caused a minor increase in the extracellular DA levels in WT animals but did not produce the same effect in DAT-N157K rats. At the same time this treatment induced reduction in the hyperactivity of mutant animals (Vengeliene et al., 2017). This evidence suggests an involvement of the glutamate system in the compensation of the consequences of augmented DA transmission. In fact,DAT deficiency in mice is causing potent dysregulation in the glutamatergic transmission (Efimova et al., 2016). In addition, DAT-KO rats showed an increased expression of brain derived neurotrophic factor (BDNF) leading to the activation of TrkB and its downstream intracellular signalling culminating in glutamate synapse re-arrangement (Leo et al.,2018). Thus, the removal of DAT leads to increased BDNF expression in the cytosol while reducing its expression, together with its downstream signalling, in the postsynaptic density. This observation may have a relevant and functional effect, given the well-established role of BDNF in the control of glutamatergic spine density and since reduced spine density and postsynaptic density-95 (PSD-95) levels were reported in the striatal medium spiny neurons of DAT-KO mice (Efimova et al., 2016). Other monoamine neurotrans-mitter systems seem to be indirectly affected in DAT mutants as well (Vengeliene et al., 2017; Leo et al., 2018). According to the high performance liquid chromatography results, a significant decrease in serotonin tissue levels were observed in the striatum of both strains with no changes observed in its metabolite 5-hydroxyindoleacetic acid (5-HIAA) levels.Likewise, in DAT-N157K, norepinephrine tissue levels were reduced in several brain areas (Vengeliene et al., 2017). Furthermore, the DAT deficiency causes remarkable changes in DA extracellular levels that result in alterations in genes critical for DA homeostasis. DAT-KO animals responded to this imbalance with a decrease in tyrosine hydroxylase (TH)mRNA in the midbrain and with an even more pronounced decrease of TH protein levels in the striatum. Persistently elevated extracellular DA levels also cause down-regulation of the dopamine D1 receptor and the dopamine D2 receptor expression in the striatum in both rat mutant strains(Vengeliene et al., 2017; Leo et al., 2018).
Just like DAT-KO mice, DAT-KO rats have lower weight in comparison to HET and WT rats (Cinque et al., 2018;Leo et al., 2018). By comparison, DAT-N157K rats, much like DAT knock-down mice (Zhuang et al., 2001), have normal weight through the development and in adulthood(Vengeliene et al., 2017). Remarkably, both DAT-deficient rats strains develop normally and do not show a decrease in survival rate accompanied with neurological dysfunctions that is observed in DAT-KO mice. In concordance with the previous studies in DAT-KO mice (Gainetdinov et al.,1999), both mutant rat strains demonstrate pronounced spontaneous locomotor hyperactivity in home cages and in open field monitors (Vengeliene et al., 2017; Leo et al.,2018). The hyperlocomotion persists during the lifespan of the rat (Leo et al., 2018). Psychostimulants such as AMPH and methylphenidate, clinically used to treat ADHD symptoms, showed the ability to reduce hyperactivity in DAT-KO rats while AMPH was highly effective in reducing hyperactivity of DAT-N157K mutants (Vengeliene et al., 2017; Leo et al., 2018). These observations confirm the “paradoxical”calming effect of AMPH and methylphenidate previously observed in DAT-KO mice (Gainetdinov et al., 1999). Similarly, mGluR2/3 antagonist, LY341495 (Vengeliene et al.,2017) and partial TAAR1 agonist, RO5203648 (Leo et al.,2018) were able to revert the hyperactivity of DAT mutants,suggesting that these novel pharmacological approaches may hold potential for treatment of hyperdopaminergic disorders such as ADHD. At the same time, atomoxetine(NET inhibitor used to treat ADHD) and haloperidol (typical antipsychotic) non-selectively decreased the locomotor activity in both control and mutant animals (Vengeliene et al., 2017; Leo et al., 2018). The selective depletion of DA in dopaminergic terminals of DAT-KO rats by TH inhibitor alpha-methyl-para-tyrosine resulted in almost immediate loss of locomotor activity and development of severe akinetic phenotype, which was previously described for DAT-KO mice (DDD mice) (Sotnikova et al., 2006). In prospective,DDD rats might be used to identify compounds with potential therapeutic use for the treatment of PD.
Despite a weight difference, DAT disruption did not change food and water consumption (Cinque et al., 2018).Moreover, Cinque and colleagues noted that food restriction induced more rapid weight loss in DAT-KO than in the control groups. Both DAT-KO and DAT-N157K rats did not develop preference (Vengeliene et al., 2017) or demonstrate markedly lower preference (Cinque et al., 2018) for sweet solution in the free choice test. DAT-KO rats also displayed lower preference for highly appetitive food in comparison to both WT and HET littermates (Cinque et al., 2018).
DAT disruption can affect the wide spectrum of emotional and cognitive processes. Remarkably, freezing time was reduced in fear conditioning test in DAT-N157K rats, however,no difference between the mutant rats and WT was reported in time spent in open arm (Vengeliene et al., 2017). DATKO rats showed an impaired sensorimotor gating measured as reduced pre-pulse inhibition of the acoustic startle reflex and decreased Y-maze spontaneous alternation that indicated an impaired working memory function (Leo et al., 2018).Moreover, DAT-N157K rats spent less time both in contact with other rats in the social interaction test and exploring a novel object in the novel object recognition test (Vengeliene et al., 2017). Furthermore, DAT-N157K rats were not able to learn the stimulus-food reward association to develop lever press operant behaviour (Vengeliene et al., 2017). Also DATKO rats were reported to perform poorly operant nose-poke responding reinforced by food pellets (Cinque et al., 2018).When DAT-KO rats were tested at different ages in a novelty-seeking test using a black/white box, they showed some signs of attention impairment while more novelty-seeking traits were identified in HET rats (Adinolfiet al., 2018).
Dysfunction of DAT is believed to be involved in the pathogenesis of ADHD, however, Cinque and colleagues failed to show the increased impulsivity typical for ADHD in the DAT-KO rats in intolerance-to-delay task, in which animals should choose between large but delayed and small but immediate reward. Furthermore, the mutant rats seem to develop the side preference in a reward amount independent manner and make more inadequate nose-poke responses during intertrial intervals (Cinque et al., 2018).These findings were interpreted as a compulsive trait in DAT-KO rats behaviours. However, a highly perseverative patterns of locomotor activity reported previously in DATKO mice (Gainetdinov, 2008; Efimova et al., 2016) and observed in DAT-KO rats can in part contribute to these behavioural manifestations. Moreover, DAT-KO rats showed lack of schedule-induced polydipsia (SIP) development,which serves as a model of the compulsive behaviour in rats(Leo et al., 2018).
Taken together, DAT-KO and DAT-N157K rats represent novel models of persistently increased dopaminergic transmission that is presumably involved, at least in part, in the endophenotypes of disorders such as schizophrenia, bipolar disorder, ADHD, and Huntington’s disease. These rats have certain advantages over mouse models – they showed no mortality, they have larger brain size for surgeries and neuronal recordings, and also, they have better translational value being in many aspects physiologically closer to humans (Abbott et al., 2004). In particular, since the behavioural repertoire of rats is significantly more complex compared to mice with more sophisticated experimental paradigms established, mutant rats represent a more advanced model for the evaluation of the effects of novel therapeutics on cognitive functions. Their use as models in preclinical research is an important step toward the development of new innovative therapies for the treatment of DA-related neuropsychiatric conditions (Figure 1).
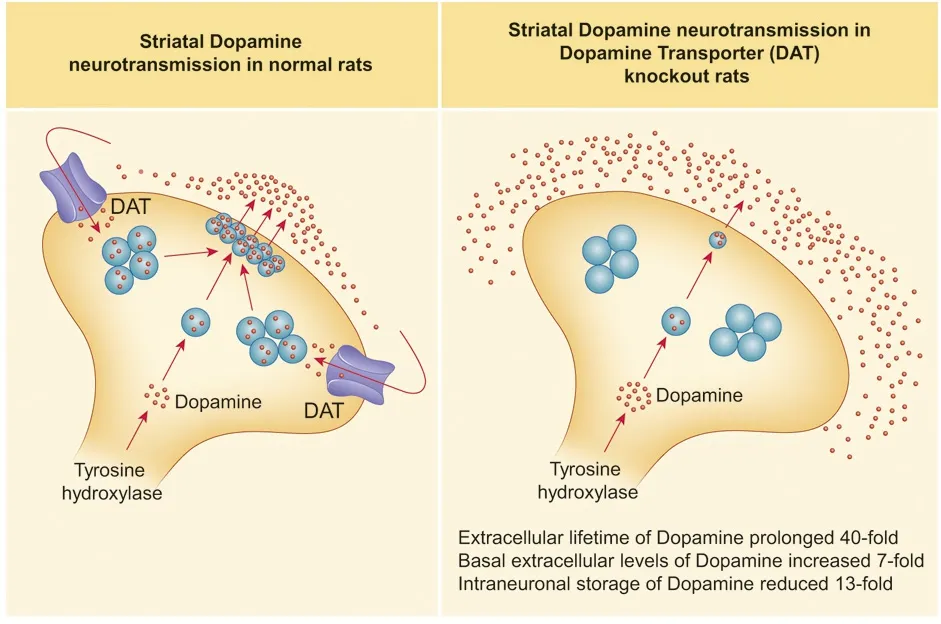
Figure 1 Major parameters of striatal dopamine neurotransmission in DAT-KO rats.
The authors would like to thank Dr. Alexander Zhivich (Department of Chemistry, Framingham State University, Framingham, USA) and Victor Zhivich (Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA) for editing the language of the manuscript.
This work was supported by the Russian Science Foundation,No. 17-75-20177 (to IS) and No. 14-50-00069 (to RRG).
Damiana Leo, Ilya Sukhanov, Raul R. Gainetdinov*
Department of Neurosciences, University of Mons, Mons,Belgium (Leo D)
Institute of Pharmacology, Pavlov Medical University, St.
Petersburg, Russia (Sukhanov I)
Institute of Translational Biomedicine, St. Petersburg State University, St. Petersburg, Russia (Gainetdinov RR)
*Correspondence to:Raul R. Gainetdinov, PhD,gainetdinov.raul@gmail.com.
orcid:0000-0003-2951-6038 (Raul R. Gainetdinov)
Received:2018-06-14
Accepted:2018-08-07
doi:10.4103/1673-5374.241453
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- MicroRNAs of microglia: wrestling with central nervous system disease
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Apomorphine effects on the hippocampus
- Roles and functions of Atp6ap2 in the brain
- Magnesium sulfate and fetal neuroprotection:overview of clinical evidence
- Polyphenols-gut microbiota interplay and brain neuromodulation
