Is ATP a key player in conditioning neurons to support axonal regeneration?
2018-10-22XuenongBo
Neurons in the central nervous system (CNS) of adult mammals have a weak intrinsic regenerative capacity,which is one contributing factor to the failure of axonal regeneration. Finding the means to elevate the regenerative capacity of axotomised neurons is one requirement for successful regeneration. Forty-five years ago, it was reported that crushing of the sciatic nerves of adult mice two weeks before cutting the nerves accelerated the regrowth of their axons (McQuarrie and Grafstein, 1973).The nerve injury two weeks before triggered the regeneration machinery in the injured neurons, leading to faster axonal regrowth after a subsequent lesion. Later it was found that a lesion to a peripheral nerve also strongly enhanced the regeneration of the central branches of the appropriate primary sensory neurons (Richardson and Issa, 1984). This phenomenon is termed preconditioning lesion (or conditioning lesion if the central branches of the sensory neurons are injured after a concomitant injury to their peripheral branches). Conditioning lesion of peripheral nerves has been used in combination with other strategies in promoting sensory axon regeneration in injured spinal cord in animal models. Although conditioning lesion cannot be translated into clinical practice as intentional injury to the peripheral nerves of the patients would be counterproductive, studying the underlying molecular events will reveal how neurons respond to axonal injuries, leading to the development of practical therapeutic strategies for axonal regeneration.
In the last four decades, considerable progress has been made in identifying the molecules stimulating regenerative events in the neurons after preconditioning lesion,in particular, neuropoietic cytokines including ciliary neurotrophic factor (CNTF), leukaemia inhibitory factor (LIF), and interleukin-6 (IL-6), and on the signalling pathways and transcription factors such as STAT3 that mediate growth-enhancing changes in gene expression(Richardson et al., 2009). However, the injury signal molecule(s) released from the site of nerve injury that triggers a regenerative propensity in neurons through induction,release, or activation of neurotrophic factors and neuropoietic cytokines has not been identified. Identification of the early injury signal molecule(s) for conditioning effect may open a new window for mimicking conditioning lesion in promoting axon regeneration of both peripheral nerves and CNS.
It has been known for many years that the effect of conditioning lesion can also be induced by several approaches without causing significant damage to the axons, although the conditioning effect was not as strong as nerve transection or crush. Chronic compression of rat sciatic or tibial nerves using silicone tubes before sciatic nerve crush was shown to enhance sensory nerve outgrowth(Dahlin and Kanje, 1992). Compression of nerves causes disruption of blood supply to the nerves and lead to oedema and mild demyelination although the axons in the compressed nerves remain intact. The higher the pressure is applied the severer the insult is to the nerves, therefore,the conditioning effect was positively correlated with the pressure applied to the nerves. Dahlin et al. (1992) also reported that the outgrowth of crush-injured rat sciatic nerves was much faster if the hindlimbs were subjected to vibration for 5 hours per day and for 2 or 5 days. Unlike nerve compression, vibration should not be too traumatic to the nerves, yet it also mimicked conditioning lesion,indicating that some molecules capable of elevating the regenerative state of the dorsal root ganglion (DRG) neurons were released without damaging the cells and axons in the nerves.
Another approach that mimics conditioning lesion without nerve damage is electrical stimulation. Several studies showed that electrical stimulation of peripheral nerves could enhance the regeneration of peripheral nerves and the central projections of primary sensory neurons while raising levels of cyclic AMP (Udina et al., 2008), growth associated protein-43 (GAP-43), and brain derived neurotrophic factor (Geremia et al., 2007)in DRG neurons. Electrical stimulation also increased the levels of brain derived neurotrophic factor, trkB, and GAP-43 in motor neurons.
Creation of local inflammation in DRG or sciatic nerve can also mimic conditioning lesion. Lu and Richardson(1991) injected Corynebacterium parvum into rat DRG to induce inflammation, which accelerated axonal regeneration of the associated dorsal roots. Dahlin (1992) induced local inflammation by placing a catgut suture close to sciatic or tibial nerve for four weeks, significantly promoting axonal outgrowth after nerve crush without significant axonal injury. Transposition of macrophage-rich tissues to the sciatic nerves of the same rats also accelerated axonal outgrowth in the crushed sciatic nerves, indicating that certain factors released by macrophages can activate the regeneration mechanisms of the injured neurons.Chemokine (C-C motif) ligand 2 (CCL2) (also known as monocyte chemoattractant protein-1, MCP-1) has been identified as a crucial chemokine in the recruitment of macrophages to injured nerves and the associated DRG.Viral-vector mediated over-expression of CCL2 in DRG induced a conditioning-like increase in neurite outgrowth (Kwon et al., 2015). However, the authors pointed out that CCL2 was dispensable for the initial growth response, therefore, some other molecules are responsible for the initiation of conditioning effect.
In summary, conditioning lesion can be mimicked to a certain degree by nerve compression, vibration, electrical stimulation, and induced inflammation or administration of macrophage-derived factors without significantly damaging the peripheral nerves or DRG neurons. Although each approach may involve a different mechanism and a different set of molecules, it is also possible that there exists a common trigger in all these approaches. If there is one, a likely candidate molecule is ATP. Extracellular ATP has a broad range of biological activities including glia-glia and glia-neuron communication. ATP released from damaged cells acts as an injury signal in several types of tissues including brain. ATP can be released from cells upon mechanical stimulation and electrical stimulation such as that from DRG neurons (Zhang et al., 2007). ATP and its hydrolysis products ADP, AMP,and adenosine can act via seven subtypes of P2X receptors (ATP-gated cation channels) or eight subtypes of P2Y receptors (G-protein coupled), or four subtypes of adenosine receptors (G-protein coupled) and exert a large array of biological functions including acting as a chemoattractant to microglia and macrophages. Sciatic nerve crush or transection, mechanical stimulations such as vibration and compression, and electrical stimulation can all cause ATP release, which would trigger a cascade of molecular events in the adjacent cells or axons, such as the up-regulation and release of neurotrophic factors and cytokines, and activation of transcription factors, finally leading to activated regeneration state of the injured neurons. If that were the case, injection of ATP into a peripheral nerve should also mimic the conditioning lesion.We found that L4 and L5 DRG neurons removed from rats 3 days after a single injection of 6 μL of 150 μM ATP into rat sciatic nerve grew more and much longer neurites than neurons associated with nerves injected with saline (Wu et al., 2018). In a dorsal column transection model, a single injection of ATP quadrupled the number of axons growing into a lesion epicentre in spinal cord.Interestingly a second boost ATP injection one week after the first one markedly reinforced the stimulatory effect of a single injection, with many axons growing across the lesion centre and some even entering the rostral spinal cord. Injection of 6 μL of ATP at 150 μM did not cause significant histological or functional damage to the injected nerves. The results in this study support our hypothesis that ATP may be the initiator for conditioning lesion.I believe that any treatment that causes local release of ATP in the peripheral nerves can condition neurons. We tested intraneural injection of hypotonic or acidic saline and both treatments increased the expression of GAP-43 and activated STAT3 in DRG neurons, although to a lesser degree compared with ATP injection (unpublished observations).
Our working hypothesis for ATP as an initiator for conditioning lesion is illustrated in Figure 1. ATP released from Schwann cells and axons upon mechanical stimulation or electrical stimulation or ATP injected intraneurally may initiate the injury responses via three routes: (1) activating purinoceptors on axons to signal the neuronal soma on potential injury to their axons, for example by inducing IL-6 synthesis in neurons; (2) stimulating Schwann cells to secrete neuropoietic cytokines such as LIF, nerve growth factor and other neurotrophic factors, and chemokines, in particular CCL2; (3) attracting macrophages to the nerves directly. The last action may be weak due to rapid degradation of ATP. Macrophages and Schwann cells would interact with each other and release more cytokines and neurotrophic factors.In our study we found that nerve crush was more potent than intraneural injection of ATP. One reason may be that the concentration of injected ATP (150 μM) is lower than that released by nerve crush. Further studies need to be carried out to test higher concentrations of ATP. However, ATP concentrations higher than 1 mM may induce pore formation on Schwann cells and lead to cell death, due to the activation of P2X7 receptors on Schwann cells. It should also be pointed out that other injury signals may exist in addition to ATP. For example,nothing is known about how the neuropoietic cytokine CNTF, which shares the same signalling pathway as IL-6 and LIF, is presented to its receptors on axons. CNTF is synthesised in Schwann cells but lacks the signal peptide for targeting to secretory pathway and is not secreted under normal circumstances but thought to be released by some unknown mechanisms in the distal segments of injured nerves.
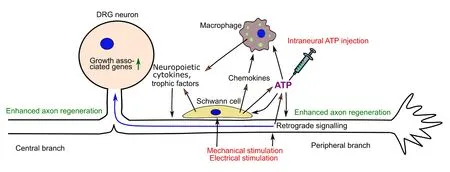
Figure 1 Schematic model illustrating the role of ATP as the initiator in mimicking conditioning lesion in promoting regeneration of sensory nerves.
The rapid degradation of ATP would limit its efficacy in therapeutic usage. Identification of the purinoceptors that mediate ATP-induced injury responses could lead to administration of a more stable and/or more potent purinoceptor agonist than ATP. However, the action of ATP may be mediated by multiple subtypes of purinoceptors on axons, Schwann cells, and macrophages. We found that P2Y2 receptor partially mediates the conditioning effects of ATP (Wu et al., 2018), indicating the involvement of other subtypes of purinoceptors in the process. In any case, more than one administration of ATP is required for sustained conditioning effect as the conditioning effect begins to subside after few weeks. The efficacy of ATP may be enhanced by local administration of ectonucleotidase inhibitors before the administration of ATP.
If intraneural ATP administration can be translated clinically to treat nerve injury, the most likely application in the foreseeable future is to enhance axonal regeneration of injured peripheral nerves. Specifically, regenerative processes need to be rebooted when the need for them is prolonged by delay in repair or requirement for regeneration over a long distance. First, of course, the efficacy of intraneurally administrated ATP in promoting peripheral nerve regeneration needs to be confirmed.Also, since most of the studies on conditioning lesion involved sensory nerves, the responses of spinal motor neurons to intraneurally administrated ATP must be investigated.
In summary, conditioning lesion can be mimicked by several different approaches including mechanical and electrical stimulation of a peripheral nerve or induction of local inflammation. Since conditioning lesion and those approaches that mimic conditioning lesion can all cause ATP release from axons and Schwann cells, I postulate that ATP may be the injury signal that initiates the subsequent molecular events in Schwann cells, macrophages, and axons, leading to elevated regeneration state in injured neurons. This hypothesis is supported by our recent publication on promoting sensory axon regeneration in injured spinal cord by intraneural injection of ATP. After further optimization, such knowledge may be exploited in the treatment of nerve injuries, especially,peripheral nerve injury in the not-too-distant future.
I would like to thank Professor Peter M. Richardson (Queen Mary University of London) for his valuable comments to this perspective.
I apologize to the authors for citing their studies without providing the associated references due to the limit on the number of references.
Xuenong Bo*
Centre for Neuroscience and Trauma, Blizard Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK
*Correspondence to:Xuenong Bo, MB, MSc, PhD,x.bo@qmul.ac.uk.
orcid:0000-0002-9202-3562 (Xuenong Bo)
Received:2018-06-20
Accepted:2018-08-16
doi:10.4103/1673-5374.241447
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- MicroRNAs of microglia: wrestling with central nervous system disease
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Apomorphine effects on the hippocampus
- Roles and functions of Atp6ap2 in the brain
- Magnesium sulfate and fetal neuroprotection:overview of clinical evidence
- Polyphenols-gut microbiota interplay and brain neuromodulation
