Predictive Modeling for Growth and Enterotoxin Production of Staphylococcus aureus in Milk
2018-10-10DangFangfangJiangYujunPanRuiliZhuangKejinWangHuiSunLuhongWangRuiZhaoFengLiTiejingandManChaoxin
Dang Fang-fang, Jiang Yu-jun, Pan Rui-li, Zhuang Ke-jin, Wang Hui, Sun Lu-hong, Wang Rui, Zhao Feng,Li Tie-jing, and Man Chao-xin
Key Lab of Dairy Science, Ministry of Education, College of Food Science, Northeast Agricultural University, Harbin 150030, China
Abstract: Predictive microbiology was utilized to model Staphylococcus aureus (S. aureus) growth and staphylococcal enterotoxin A (SEA) production in milk in this study. The modified logistic model, modified Gompertz model and Baranyi model were applied to model growth data of S. aureus between 15℃ and 37℃. Model comparisons indicated that Baranyi model described the growth data more accurately than two others with a mean square error of 0.0129. Growth rates generated from Baranyi model matched the observed ones with a bias factor of 0.999 and an accuracy factor of 1.01, and fit a square root model with respect to temperature;other two modified models both overestimated the observed ones. SEA amount began to be detected when the cell number reached 106.4 cfu · mL-1, and showed the linear correlation with time. Besides, the rate of SEA production fitted an exponential relationship as a function of temperature. Predictions based on the study could be applied to indicate possible growth of S. aureus and prevent the occurrence of staphylococcal food poisoning.
Key words: Staphylococcus aureus, staphylococcal enterotoxin A, milk, predictive model
Introduction
Staphylococcus aureus (S. aureus) is a well-documented pathogen and can cause many foodborne diseases. Staphylococcal food poisoning is one of the most common foodborne diseases in the world(Ángeles Argudín et al., 2010; Torlak and Mustafa,2012) and caused by ingestion of staphylococcal enterotoxin (SE) produced in food by certain strains of S. aureus (Yuko and Masatsune, 2009). Evenson et al.(1988) reported an amount as small as 100-200 ng of staphylococcal enterotoxin A (SEA) can produce symptoms of staphylococcal food poisoning, such as vomiting, abdominal cramps and diarrhoea.
Many cases of staphylococcal food poisoning occurred among patients who ingested dairy products,including an extensive outbreak of illness in Japan in 2000 (Stewart et al., 2002). According to investigators,exposure of the raw milk to abuse temperatures, due to a period of power supply loss during milk production may be the potential contributing factor, which led to S. aureus growth and subsequent SEA production in the contaminated milk (Fujikawa and Morozumi,2006). It is known to all that heat treatments can kill S. aureus in raw milk, but cannot thoroughly destroy SEA because of its thermal stability (Ángeles Argudín et al., 2010); so dairy products which are manufactured by S. aureus contaminated raw milk may induce safety problem. The key to control staphylococcal food poisoning is an understanding of the factors that influence S. aureus growth and SEA production in raw milk and the manipulation of those factors in order to limit potential risks.
Environmental factors that affect bacterial growth in food are mainly temperature, pH and water activity(aw) (Juneja et al., 2016). Values of pH and aware commonly stable even in different batches of raw milk, so it is more practical and valuable to investigate S. aureus growth and SEA production contaminated raw milk under the environmental factor of temperature. Some researchers have reported that raw milk is easily contaminated by S. aureus (Martin et al.,2016); moreover, raw milk after milking is usually stored under incorrect temperature by milk farmers before factory collection, thus longer storage time for such contaminated raw milk will trigger higher risk(Christidis et al., 2016). Therefore, it is important to predict S. aureus growth and SEA production in contaminated raw milk from its storage temperature in order to ensure food safety.
A number of mathematical models and equations have been developed in predictive microbiology to predict the microbial growth in food (Fang et al.,2015; Baka et al., 2016). Many of these models are based on some basic mathematical models, such as logistic model and Gompertz model; however, they cannot generate a sigmoid curve on a semi-logarithmic plot, which is not consistent with bacterium growth curves. To overcome this disadvantage, Gibson et al.(1987; 1988) modified logistic model and Gompertz model to fit the bacterial growth data and the two modified models have been used to model the growth kinetics of several foodborne pathogens by some researchers (Saucedo-Reyes et al., 2012; Maghsoudi,2014; Guan et al., 2017). Although based on theoretical considerations, the two modified models were not originally developed for modeling bacterial growth and certainly not for modeling the logarithm of the bacterial cell concentration (Baranyi et al., 1993).Then, Baranyi and Roberts (1994; 1995) developed a mechanistic model– Baranyi model to predict bacterial growth and the model has been used successfully to fit a variety of bacterial growth curves (Baka et al., 2014;Glaser and Venus, 2017; Li et al., 2017), including applied in a dairy and other food microbiology predictive model (Lee, 2014; Ačai et al., 2016; Lobete et al., 2016). Recently, Baranyi model is being increasingly adopted over modified Gompertz model and modified logistic model. For example, Lee (2014)demonstrated that Baranyi model was more suitable than modified Gompertz and logistic models, when performed to develop a predictive growth model of Listeria monocytogenes.
The objective of this work was to predict S. aureus growth and SEA production in milk at different storage temperatures by predictive microbiology. In this study, sterilized liquid milk which was artificially contaminated with pure culture of S. aureus was used to simulate S. aureus contaminated raw milk. Three growth models, modified logistic model, modified Gompertz model and Baranyi model were applied to analyze S. aureus growth data, and then performance among models was compared. A relationship between SEA production and time as well as S. aureus growth was also studied. Additionally, models of rate constant of growth and SEA production in milk were established.
Materials and Methods
Strain and milk
S. aureus (ATCC 13565), which only produced SEA,was used in this study. This strain was purchased from the National Center for Medical Culture Collections(CMCC). S. aureus (ATCC 13565) was stored at–80℃ in Nutrient Broth (NB) supplemented with 25%glycerol.
Sterilized whole homogenized milk (Inner Mongolia Yili Industrial Group Co., Ltd., China) for study was confirmed to be bacteriologically negative, when samples were evaluated by a standard spread plate count assay (Messer et al., 1985) and was determined to be SEA-free based on the toxin assay described below.
Artificial contamination and incubation
S. aureus (ATCC 13565) from –80℃ was grown in NB with shaking (200 revolutions per minute) at 37℃ for 10 h. Cultured cells were streaked on brain heart infusion (BHI) agar plates, and grew at 37℃for 15 h. One well-grown colony from the plate was transferred to NB with shaking at 37℃ for 8 h, then enumerated the culture on nutrient agar (NA) with spread plate method, and stored at 4℃ for 10 h until the results of the enumeration were available. Five μL of this cultured cells were washed with 5 mL of sterile phosphate buffer (pH 7.0), harvested by centrifugation at 6 200 g, and then resuspended in 5 mL of buffer.Based on the previous enumeration, the cell density of suspension was approximately 106cfu · mL-1.
Then, the cell suspension was added to sterilized milk at volume ratio of 1 : 1 000 to achieve an initial inoculum of approximately 103cfu · mL-1. The inoculated milk was dispensed in 5 mL portions to sterile glass screw-cap test tubes (10 mL) using a pipette (Fujikawa et al., 2004). Tightly capped tubes were then placed in a test tube rack, and the rack was placed in a controlled temperature incubator (HPS-160,Donglian Electron Technology Corporation, Harbin,China), which was set at 15, 18, 20, 23, 25, 30 and 37℃, respectively. After each incubation period at a constant temperature, duplicate sample tubes were removed from the incubator and cooled in ice water for measurement of growth and SEA production.Each experiment at one temperature was performed in triplicate.
Enumeration of viable cells
The number of viable cells in duplicate samples removed from the incubator was determined with the spread plate method. Each sample was serially diluted with 0.85% sterile NaCl and 100 μL of properly diluted (or undiluted) cell suspension was spreadplated on triplicate plates of NA. The plates were incubated at 37℃ for 10 h, and then colonies were counted as viable cells of S. aureus. The measured cell counts were transformed to a base 10 logarithm.Average and standard deviations of the transformed values were then calculated. In the whole process of spread-plating, the two sample tubes were kept in ice water. The remanent samples in tubes were used for SEA measurement.
SEA measurement
SEA in milk was measured using VIDAS Staph enterotoxinⅡ(SET2, bioMerieux, Marcy-l' Etoile,France), which was based on an enzyme-linked fluorescent assay, with mini-VIDAS automated instrument. Purified SEA (Sigma-Aldrich. Inc., USA) added to sterilized liquid milk at concentrations of 0.125,0.25, 0.5, 1.0, 2.0 and 4.0 ng · mL-1together with the negative control was used to establish a standard curve with respect to their corresponding TV (test value, a reading on the result sheet which was automatically given by mini-VIDAS automated instrument once the assay was completed). The remanent samples after spread-plating were used for SEA measurement.The standard curve was used to determine SEA concentration in the experimental samples. The average of each measurement was calculated for each data point.Each sample was performed in triplicate.
Analyzing growth of S. aureus with growth models
Experimental growth data were analyzed with three different growth models, modified logistic model,modified Gompertz model and Baranyi model. DMFit software and Baranyi model used in this study were kindly provided by Dr. J. Baranyi (Institute of Food Research, Norwich Research Park, Norwich NR4 7UA, United Kingdom). The mean square error (MSE)was used to assess the performance of predictive growth models. In order to further assess the performance of these three models, the rate constants of growth derived from the three models were compared with the observed values by calculating values of the bias factor (Bf) and the accuracy factor (Af) which were both modified by Baranyi et al (1999). Ideally, a predictive model should ensure that Af=Bf=1; however,Afusually increased by 0.10 to 0.15 for each variable in the model.
Modeling temperature dependency parameters
The rate constants of growth and SEA production were both temperature dependency parameters.According to the best fit model, the data of both rates were obtained at each constant temperature and then established models of the rate constant of growth and SEA production with respecting to temperature by MATLAB 6.5.
Statistical analysis
For statistical evaluation of data, one-way analysis of variance (ANOVA) was applied using the program SPSS 17.0 for windows. This was followed by post hoc comparisons using the Tukey's test. Significant differences were considered significant at p<0.05.Microsoft Excel 2007 was also used for data processing.
Results
Growth models comparison
Three models, modified logistic model, modified Gompertz model and Baranyi model, were successfully used to predict the observed growth data of S. aureus at constant temperatures of 15, 18, 20,23, 25, 30 and 37℃, respectively. Performances of one certain model for growth data at different temperatures were similar, it could infer that these growth models were temperature independency.Thus, profile of these three models at 23℃ was shown as an example (Fig. 1). From the curves generated by the three models, the curve of Baranyi model gave an excellent fitness, when compared with other two models and there were no remarkable differences between modified logistic model and modified Gompertz model. To verify the conclusion,the values of the growth rate, MSE, Bfand Afwere determined.
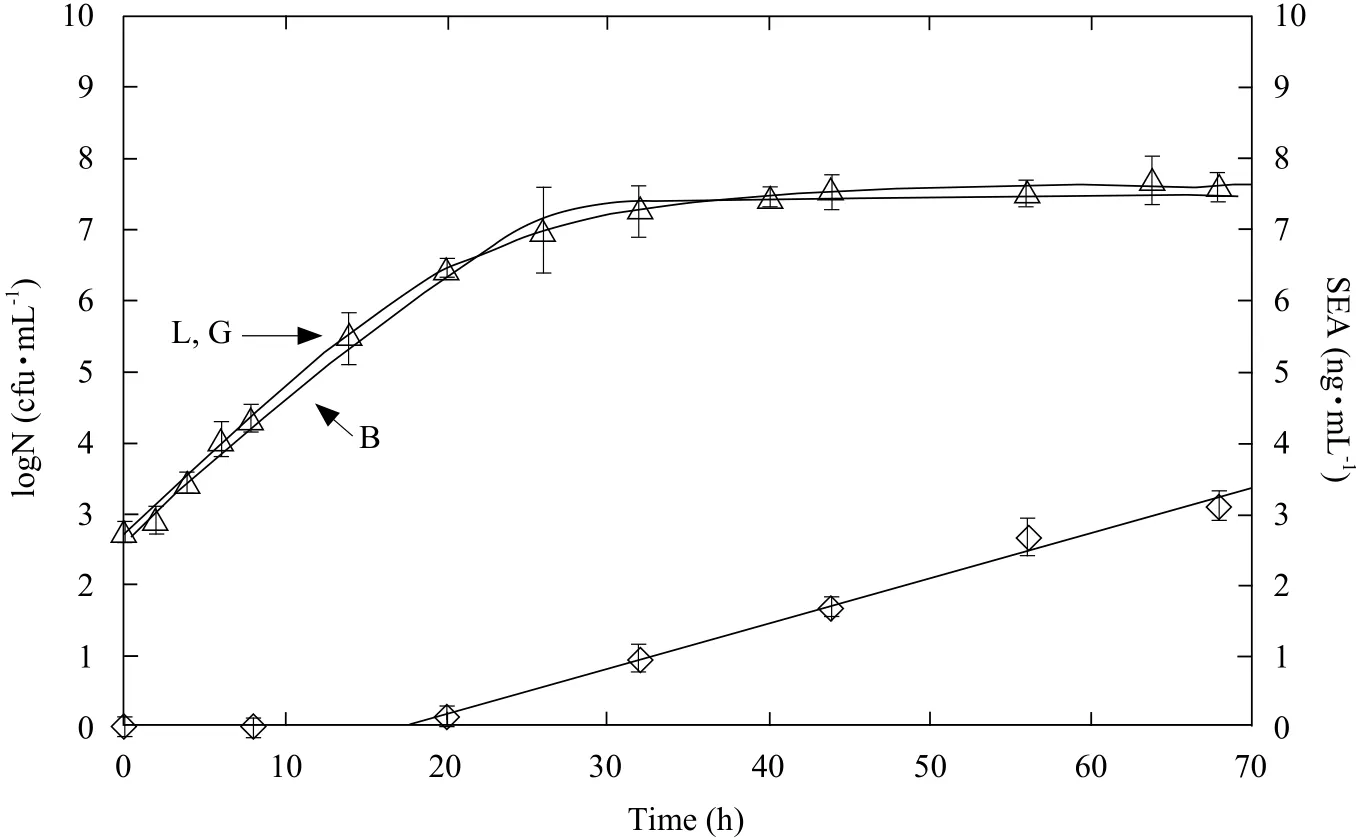
Fig. 1 Staphylococcus aureus growth and staphylococcal enterotoxin A (SEA) production in milk at constant temperature of 23℃
When compared the observed growth rate values with the values estimated by the three models (Fig. 2),it showed that most parts of observed rate constant values were lower than predicted values generated from modified logistic model and modified Gompertz model, while observed values and predicted values from Baranyi model were fit well. The values of MSE, Bfand Affor growth rate listed in Table 1 also indicated that Baranyi model gave the best estimate for the rate constant of growth. Statistical analysis showed that MSE from Baranyi (0.0129±0.0015) was lower than that from modified Gompertz model (0.0294±0.0089) and modified logistic model (0.0298±0.0084)(p<0.05). In addition, there was no statistical difference between MSE values of modified Gompertz model and modified logistic model (p>0.05). These comparisons showed that Baranyi model provided predictions were the most closely matched observed growth rate data than other two models. What was more, Bfof Baranyi model was close to one, which indicated good agreement between observed and predicted values;whereas, the values of Bfproduced by modified logistic model and modified Gompertz model was more than 1, suggesting that the two models tended to give the 'fail-safe' predictions (Ross et al., 2000).Values of Affor three models were also indicated that Baranyi model estimated more accurately than other two models. These results showed that Baranyi model described the growth curves of S. aureus in milk more accurately than those of modified logistic model and modified Gompertz model. Therefore, Baranyi model was employed in follow-up experiment.

Fig. 2 Comparison of rate constant of Staphylococcus aureus growth predicted using modified logistic and Gompertz models and Baranyi model

Table 1 Values of mean square error, bias factor and accuracy factor for rate constant of growth at temperatures ranging from 15 to 37℃ with modified logistic model, modified Gompertz model and Baranyi model
SEA production at constant temperatures
Purified SEA in sterilized milk at six concentrations of 0.125, 0.25, 0.5, 1.0, 2.0 and 4.0 ng · mL-1and negative control were used to establish a standard curve.According to TVs given by mini-VIDAS automated instrument, linear regression was established to model the relationship between SEA concentrations and their corresponding TVs, which was similar to the research reported by Asao et al. (2003) and Fujikawa and Morozumi (2006). Except 4.0 ng · mL-1, the former five SEA concentrations together with the negative control showed excellent linearity with their corresponding TVs (Fig. 3). The regression line was expressed as SEA (ng · mL-1)=1.2089TV+0.0081 with the correlation coefficient of 0.9994.

Fig. 3 Standard curve for staphylococcal enterotoxin A(SEA) determination in milk
This standard curve indicated that SEA concentration which would be determined by this regression line should be no more than 2.0 ng · mL-1. Therefore,in order to satisfy this request, SEA extract from milk samples should be diluted with a dilution buffer in SET2 (bioMerieux, Marcy-l'Etoile, France) if necessary.
SEA amount increased linearly with time even after S. aureus growing to the stationary phase and the linear,besides, correlation coefficients were all about 0.99 for all the experimental temperatures according to Table 2.By the linear regression analysis of SEA production curve, the time when the toxin began to be detected was at the cell concentration of 106cfu · mL-1at each temperature. S. aureus growth at constant temperature of 23℃ was as an example shown in Fig. 1. This result was consistent with previous studies (Fujikawa and Morozumi, 2006).

Table 2 Functions of SEA production models and values of correlation coefficients at experimental temperatures
Models of temperature dependency parameters
The rate constant of growth generated from Baranyi model at each temperature was simulated by MATLAB 6.5 with a square root model (Ratkowsky et al., 1982).As evident from Fig. 4, the growth rate was temperature dependency, which was increased by increasing of storage temperature. The linear equation with the correlation coefficient of 0.9432 was expressed as the following:

Where, r was the rate constant of growth (1/h), and T was temperature (℃). Using this equation, r at a given temperature could be estimated. The values of maximum cell concentration called Nmaxat different experimental temperatures were almost constant; the average was estimated at about 107.52cfu · mL-1. As expected, the time taken to reach the point of Nmaxdecreased as the storage temperature increased. Eq. 1 indicated that the apparent temperature where r was zero was about 6.7℃, which was higher than 5.4℃calculated with equation reported by Fujikawa and Morozumi (2006). The two different results might be derived from different strains of S. aureus and predictive model parameters in the two studies.Calculated with Eq.1, about 0.0087 growth rate was obtained at 10℃, which overestimated the growth of S. aureus in milk, because in this study no growth of S. aureus was observed at 10℃ for 115 h (data not shown); therefore, this equation provided growth predictions with a bit of safety margin.

Fig. 4 Temperature dependency of rate constant of Staphylococcus aureus growth
The rate constant of SEA production that was another temperature dependency parameter, was studied afterwards. Two models, an exponential function and a quadratic equation, had been established to describe the relationship between the rate constant of SEA production and their corresponding temperatures. From MSE comparison, the exponential function gave a smaller MSE (0.0209) and was chosen for the model of the rate constant of SEA production (Fig. 5). The exponential function was expressed as the following:

Where, R was the rate constant of SEA production,T was temperature (℃) and exp was an exponential function. Calculated with Eq.2 about 0.0002 rate constant of SEA production was obtained at 10℃,while no SEA was detected at this temperature in the experiment (data not shown). Therefore, this equation slightly overestimated SEA production rate of S. aureus, when it grew in milk under the conditions described herein.
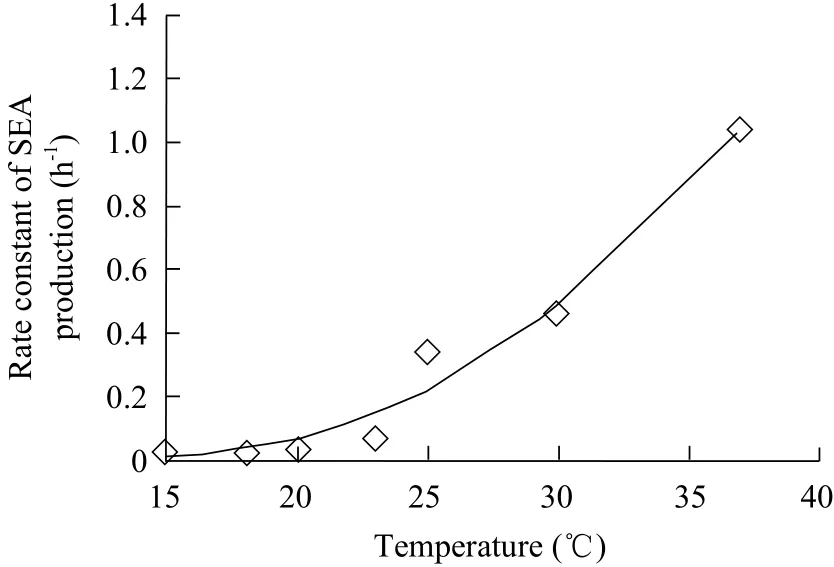
Fig. 5 Temperature dependency of rate constant of staphylococcal enterotoxin A (SEA) production
Discussion
This study aimed to predict growth and SEA production of S. aureus in milk. In order to reflect an influence of temperature on S. aureus growth and SEA production, artificially contaminated sterilized milk was utilized to simulate raw milk to exclude the interference from other factors. In fact, many factors in raw milk could affect S. aureus behaviour,such as coexistent microorganisms, the addition of preservatives and so on (Bellio et al., 2016), which might vary greatly among different batches of raw milk. Thus, it should be established a more effective imitation system to simulate raw milk in the future research to model of S. aureus growth and enterotoxin production.
When considering performance of predictive models, most were interested in whether the model was 'fail-dangerous', i.e. whether it provided estimates that underpredict the risk of toxin production or extent of pathogen growth; but the 'fail-safe'prediction was also not expected for wastage of product, consequently ideal models should predict as closely as possible the observed microorganism behaviour (Reybrouck and Mertens, 1996). Bfvalue of modified logistic model and modified Gompertz model in this study indicated 'fail-safe' prediction,which would be beneficial for consumers as a safety measure, but for milk producers it meant a higher investment or wastage of product. Therefore, it was an importance for model users (such as dairy product manufacturers) to choose a goodness-of-fit predictive model. Because Afand MSE were used for evaluating accuracy and suitability of such a model, it was essential to evaluate model performance by a combination of Bf, Afand MSE rather than only one of them. In this study, Baranyi model was proved to be the most suitable growth model for predicting growth of S. aureus in milk under a constant temperature factor, when evaluated by values of Bf, Afand MSE as reported by Lee (2014).Besides, SEA production model was constructed with significant correlation coefficients to actually describe enterotoxin production with time. Models of rate constant of growth and SEA production established in this study, square root model and exponential model, respectively, were both 'fail-safe' models with a bit of safe margin at 10℃. This result clarified that predicting growth and enterotoxin production of S. aureus in milk with these two models were safe.
As anticipated, the rate constant of S. aureus growth and SEA production were all the temperature dependency, which clearly showed a linearity with the correlation coefficients. The data firmly validated that temperature was a pivotal environmental factor affecting bacterial growth and enterotoxin production.What was more, during production and consumption,the temperature was continuously changing with time.Consequently, predictive modeling for the growth and enterotoxin production of S. aureus at constant temperatures played a key role in management staphylococcal food contamination.
Conclusions
In this report, S. aureus growth and SEA production in milk were investigated at constant temperatures by predictive microbiology. The result of this study indicated that the optimal predictive system of S. aureus growth and SEA production consisted of Baranyi model and enterotoxin production model.Predictions based on the study should be seen as an indication of possible growth of S. aureus and enterotoxin production to prevent the occurrence of staphylococcal food poisoning in a range of liquid foods.
Acknowledgments
The 1st and the 2nd authors contributed equally to this work.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Mapping and Candidate Gene Screening of Tomato Yellow Leaf Curl Virus Resistant Gene ty-5
- Mowing Height and Mowing Frequency Interactions on Turf Performance of Kentucky Bluegrass
- Molecular Cloning and Expression Analysis of AcFT3 in Allium cepa
- Screening of Actinomycetes from Medicinal Plant Rhizosphere Soils for Industrial Enzymes and Antimicrobial Activity
- Comparative Transcriptome Profiling of Glycine soja Roots Under Salinity and Alkalinity Stresses Using RNA-seq
- A Novel Bacillus thuringiensis Cry57 Protein Domain Swap In fluence on Insecticidal Activity
