Molecular detection of Leishmania species in human and animals from cutaneous leishmaniasis endemic areas of Waziristan, Khyber Pakhtunkhwa, Pakistan
2018-09-08MubashirHussainShahzadMunirAbdullahJalalTajAliKhanNiazMuhammadBaharUllahKhattakAbdullahKhanIrfanAhmedZulqarnainBalochNawazHaiderBashirMuhammadAmeenJamalKashifRahimHumairaMazharMairaRiazNohaWatany
Mubashir Hussain, Shahzad Munir, Abdullah Jalal, Taj Ali Khan, Niaz Muhammad, Bahar Ullah Khattak, Abdullah Khan,Irfan Ahmed, Zulqarnain Baloch, Nawaz Haider Bashir,Muhammad Ameen Jamal, Kashif Rahim, Humaira Mazhar, Maira Riaz, Noha Watany
1Vector Borne Diseases Lab, Department of Microbiology, Kohat University of Science and Technology Kohat, KP, 26000, Pakistan
2Faculty of Plant Protection, Yunnan Agricultural University, Kunming 650201, Yunnan, China
3Institute of Biotechnology and Genetic Engineering, The University of Agriculture, Peshawar, Pakistan
4Yunnan Provincial Key Laboratory of Animal Nutrition and Feed, Yunnan Agricultural University, Kunming 650201, Yunnan, China
5College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642, China
6Department of Animal Breeding, Genetics and Reproduction, Yunnan Agricultural University, Kunming 650201, Yunnan, PR China
7Beijing Key Laboratory of Genetic Engineering Drug and Biotechnology, Institute of Biochemistry and Biotechnology, College of Life Sciences, Beijing Normal University, Beijing 100875, China
8Vector Biology Research Lab, US Naval Medical Research Unit 3, Cairo, Egypt
Keywords:Leishmania major Rodents Cutaneous leishmaniasis Domestic animals ITS 1 PCR Waziristan
ABSTRACT Objectives: To detect Leishmania species in human patients, animal reservoirs and Phlebotomus sandflies in Waziristan, Pakistan. Methods: Tissue smears and aspirates from 448 cutaneous leishmaniasis (CL) suspected patients were analyzed. To sort out role of the reservoir hosts, skin scrapings, spleen and liver samples from 104 rodents were collected.Furthermore, buffy coat samples were obtained from 60 domestic animals. Sandflies were also trapped. All human, animals and sandfly samples were tested by microscopy,kinetoplastic PCR and internal transcribed spacer 1 (ITS1) PCR followed by restriction fragment length polymorphism for detection of Leishmania species. Results: An overall prevalence of 3.83% and 5.21% through microscopy and ITS1 PCR respectively was found.However, the statistically non-significant correlation was found between area, gender, and number of lesions. The presence of rodents, sandflies, domestic animals and internally displaced people increased the risk of CL. Using ITS1-PCR-RFLP, Leishmania tropica (L.tropica) was confirmed in 106 samples while 25 of the isolates were diagnosed as Leishmania major (L. major). Similarly, 3/104 rodents were positive for L. major and 14 pools of DNA samples containing Phlebotomus sergenti sandflies were positive for L. tropica. None of samples from domestic animals were positive for leishmaniasis. Conclusions: In the present study, L. tropica and L. major are found to be the main causative agents of CL in study area.Movement of internally displaced people from CL endemic areas presents a risk for nearby CL free areas. To the best of our knowledge, we report for the first time L. major infection in rodents (Rattus rattus) and L. tropica in Phlebotomus sergenti sandflies trapped in Waziristan,Pakistan.
1. Introduction
Cutaneous leishmaniasis (CL) appeares as an emerging endemic disease in Afghanistan and Pakistan[1]. It was first reported from Pakistan in 1960 and was limited to northern mountainous region, but now is widely spread in most parts of the country[2]with annually approximately 20 000 CL infected cases have been reported[3,4]. In Khyber Pakhtunkhwa (KP) province of Pakistan,epidemiological and clinical patterns of CL was reported in armed forces deployed in tribal areas for military operation[5].
World Health Organization enlisted this as one of the most serious diseases due to increase number of new cases every year (more than 2 million)[6]. The disease is spread through bloodsucking sand flies(Phlebotomus), consisting of 37 species reported from Pakistan[7,8].The habitat ranges from tropical rainforest to desert and human,dogs, chickens, vertebrates, livestock as well as some mammals serve as hosts[9].
Previously, in some parts of Pakistan, new outbreaks of CL resulted due to frequent movement of internally displaced people (IDPs) to settled urban areas of KP. Positive cases of CL were also reported recently in region of Bannu, Karak, and Kohat because most of IDPs migrated from Waziristan agency settled in these areas (Unpublished data). The recurrent epidemics of leishmaniasis have emerged due to military operations in the tribal areas of KP, Pakistan. As a neglected consequence, a large number of local people migrated from tribal to urban areas. The presence of domestic animals (cows, buffaloes,sheep, goats and pet dogs) in the houses of CL-infected patients or close to these houses have been associated with the prevalence of CL. A positive correlation between prevalence of disease and the presence of domestic animals have been reported in some previous studies[10,11]. Sandflies, animals, and rodents act as carriers for spread of CL from person to person within population.
The purpose of this study was to determine the prevalence of CL in IDPs in Waziristan, molecular detection ofLeishmaniaspecies from human patients, sand flies and reservoir animals by using molecular tools. Moreover, we also performed the identification of the vector species responsible for transmission of CL in this area for the first time.
2. Materials and methods
2.1. Study area and sample collection
The present study was conducted in selected CL endemic villages of North and South Waziristan, Pakistan during 2015-2016.Waziristan covers 15 000 square kilometers with latitude of 33°02’ 59” N and longitude: 70° 01’ 10” in Federally Administered Tribal Areas (FATA). Samples from patients were collected from ulcerating skin lesion scar on clean glass slides with completion of a questionnaire after obtaining informed consent (ethical considerations approved by KUST ethical committee) of the patients including patient’s information’s regarding name, age, sex, lesions number, site and duration of lesion, present and permanent address,occupation of the patient, type of house, species of domestic animals, physical examination and history of any previous treatment with anti-leishmanial drugs. Furthermore, 104 rodents were captured in steel traps (35 cm × 12 cm × 12 cm) according to methodology standardized for capture of small mammals. A total of 35 traps were set every night in different locations such as corn field and in wild plantations surrounding the houses of CL patients. Rodent species were identified on the basis of anatomical criteria by Department of Zoology, Kohat University. Rodents were euthenised humanely and under aseptic conditions, the spleen and liver were removed and stored at -20 ℃ until further processing. Skin scrapings from suspected skin lesions on ears and body of rodents were obtained. In addition to trap sandflies, reference points were set from houses of CL infected patients. The suspected resting sites and trapping sites of sandflies were within a 1.5 km radius from houses of CL patients.The collection of sandflies was done with sticky papers (castor oil)and CDC light traps (Model 512, John Hock and Co., USA) installed outside and inside of houses in endemic areas. Throughout the study period, 2 traps per site per week were installed in suspected houses of CL patients. The captured sandflies were analyzed soon after their collection. Sandflies to be identified were killed by chilling them in freezer for short time and stored in 70%-80% ethanol until further processing by PCR[12]. Buffy coat was extracted from blood samples obtained from domestic animals sheep (n=8), goat (n=8), cows(n=10), Dogs (n=8), horses (n=8), donkey (n=8), chicken (n=10).
2.2. Microscopy and molecular detection
2.3. Restriction fragment length polymorphism (RFLP)
The ITS1-PCR product was digested to carry out the RFLP. Ten μL of amplified product was digested in a 20 μL total mixture containing 10 U ofHaeⅢ (Fermentas) and 2 μL of the appropriate restriction buffer at 37 ℃ water bath overnight. All 20 μL of the digest was used for electrophoresis on 2.5% agarose gel. DNA length standard(100 bp ladder DNA size marker, CinnaGen Co) was used to check the estimated fragment size.
2.4. Identification of sand flies
The sandflies were morphologically identified on the basis of anatomical keys. Detergent solution (1%) was used to wash the sand flies for 5 min and under a stereoscope, the dissection was carried out in 1× phosphate buffer saline. The morphological identification was done by studying anatomical features of heads and genitalia[13].
2.5. Prevalence rate
Prevalence rate of CL was determined by the formula as described[14].
Prevalence rate = No. of patient infected with CL/ total No. of patient examined × 100.
2.6. Statistical analysis
The data was analyzed by using univariate ANOVA using IBM SPSS Statistics 21 and thePvalue <0.05 means significant difference.
3. Results
The CL prevalence rate was assessed based on gender, area and number of lesion using two different methods. Firstly, microscopy was used followed by amplification of kDNA and ribosomal internal transcribed spacer 1 region (Table 1). The overall prevalence was significantly higher in male compared to female through microscopy(2.34% male and 1.48% female) and PCR (3.18% male and 2.02%female) (Table 2). The results demonstrated a high prevalence of CL in children of 0-15 years compared to other age groups (Data not shown). The microscopic as well as molecular analysis revealed that patients with more than two lesion had a high frequency of cases(1.35%) of CL (P<0.05) as compared to other (Figure 1). One of the patient belonging to Razmak of North Waziristan had 8 scar lesions on different parts of the body. Mostly affected persons with active lesions had sought medical intervention within 3-6 months after appearance of the lesions. As CL in Waziristan and KP is thought to be anthroponotic caused byL. tropicain which the duration of lesion varies from 3-12 months, while in the case ofL. major, infectious lesions are thought to be self-healing within 3-6 months, although in the field conditions the story is not always true.

Figure 1. Lesion wise comparison of infected people in South and North Waziristan.
ITS1-PCR-RFLP analysis was performed for identification of different species ofleishmania. A total of 150 (75 from each region)ITS1 PCR positive random samples were carried out only with the products. ITS-PCR product was incubated and digested byHaeⅢenzyme. Three bands approximately 185 bp, 57 bp and 24 bp ofLeishmania tropica(L. tropican) were produced (confirmed throughgene bank). Similarly, forLeishmania major(L. major) two bands of about 203 bp and 132 bp and three bands forLeishmania infantumof about 184 bp, 72 bp, 55 bp size were produced. The ITS1-PCR-RFLP confirmed 76.0% ofL. tropicaand 14.0% ofL. majorspecific bands in North Waziristan. However, 65%L. tropicaand 18%L. majorspecific bands were obtained in samples from South Waziristan (Table 3). Sand flies of the genusPhlebotomusandSergentomyawere identified both in North and South Waziristan(Table 4). The presence of domestic animals normally influences the populations of the sand fly vectors by providing them with an additional blood source or acting as attractant to make residing people of that house more vulnerable to sand fly bite. Many stray dogs were found in CL endemic villages but none of the dogs was found positive for leishmaniasis. In fact, their increased number in streets can attract sand fly for blood meal thus expanding the risk of CL to the inhabitants. In the present study none of the domestic animal was found PCR positive for leishmaniasis. Previous studies have also shown that domestic animals in CL endemic areas are usually found seropositive but PCR negative. Sand flies and rodents were also collected from different CL endemic villages of the study areas. All the rodents (n=104) were of the same species(Rattus rattus) that could be trapped in the present study near houses of CL patients. This is the predominant rodent specie found in study area. PCR analysis showed only 3 of the trapped rodents(Rattus ratus) with skin lesions on ears were positive forL. major.We trapped different species of sandlfies in present study but the predominant one wasPhlebotomus sergenti(P. sergenti) (n=88)followed byPhlebotomus papatasi(71)sergentispecies (141). So the PCR results showedL. tropicain 14P. sergentisandflies (15%of allP. sergenti) and overall infectivity percentage of infected sandflies was 4.6%. Samples collected from domestic animals were negative for leishmaniasis (Table 5).

Table 1 Overall prevalence of CL in Waziristan.

Table 2 Gender CL prevalence (%) in South and North Waziristan.
We reported for the very first-timeL. tropicainfection in 14 femaleP. sergentiandL. majorinfection in this study area.
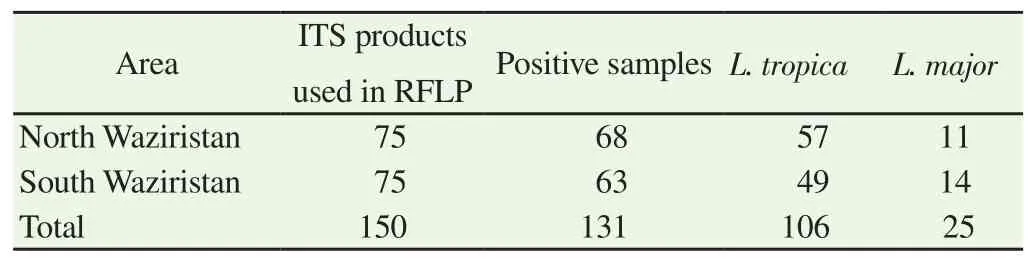
Table 3 Species identification using RFLP analysis of ITS1-PCR products.

Table 5 Screening of rodents, sandfly and domestic animals for leishmania parasites.
4. Discussion
There is a global change in the epidemiology of leishmaniasis,with the emergence of disease in different parts of the world: a change variously ascribed to population movement and manmade environmental changes. In the present study, we indicated high prevalence in male patients. Similar findings were also reported previously that male has comparatively high prevalence of CL[2,15,16]. Study in Landi Kotal, KP indicated high prevalence in the age group of 4 to 17 years. However, the researchers concluded that use of bed nets during night in the study area might reduce the incidence rate of leishmaniasis[17]. More social activity and mobility of male as they often sleep without shirt expose them to bite of sandfly. Since most of the people follow Islamic laws in the study region,females always remain covered making them less prone to sand-fly bite. Moreover, Waziristan agency is a big hub for IDPs traveling across the endemic areas of Afghanistan where CL is endemic.
So they took a sad farewell of each other, and the Princess stuck the rag in front of her dress, mounted her horse, and set forth5 on the journey to her bridegroom s kingdom. After they had ridden for about an hour the Princess began to feel very thirsty,15 and said to her waiting- maid: Pray get down and fetch me some water in my golden cup out of yonder stream: I would like a drink. 16
CL as endemic in North, South, and West of Pakistan while East and South-Eastern regions are nonendemic[18]. Moreover, in Northern Pakistan the disease was prevalent in human populations throughout the year[2]. A total of 688 cases of Old World CL from Bajor Agency, Kurram Agency, Khar, Bara, Miran shah, and southern Waziristan and endemic belt along the borders of Afghanistan were reported[19]. Travelling history to Balochistan province also resulted in CL in 256 patients with non-healing chronic ulcers. CL in young children belonging to different areas was also studied in another part of KP[20]. There were 75 (40.5%) cases from Sindh, 20 (10.0%)were from Punjab from known endemic areas, 38 (20.5%) from KP and 52 (28.0%) from Balochistan[21].
It was also reported previously that infection of theLeishmaniaparasite in children [11- 16 years) showed a high prevalence of 45.12% (2 643 out of 5 857 children)][22]. Moreover, another study reported same outcomes that children are more prone to disease as high prevalence was among 0-9 years age group. (10.96%), followed by10-19 years(6.66%), 30-39 years (5.35%), 40-49 years (5.12%),20-29 years(3.96%), and lowest prevalence rate 2.94% was found in age group 50-59 years. Furthermore, older people 51-60 years have less prevalence (4.83%)[23]. The high prevalence in children may be due to maximum exposure to sand fly bite as children mostly play outside and their innate immune system is also weak.

Table 4 Identification of Sandfly in Waziristan.
The microscopic as well as molecular analysis revealed that patients with more than two lesion had a high frequency of cases(1.35%) of CL (P<0.05) as compared to other. In a previous study a total of 50.9% of patients had single lesions while double lesions were observed in 24.6% of patients and 29.4% of patients showed multiple (3-15) lesions[24]. This difference could be a result from abundance ofP. sergentisandflies in the study area and more exposure level due to poverty and social reasons. Limited access to specific antileishmanial drugs, persistence of secondary bacterial infections, development of antimonial resistance and hygienic factors could influence the duration of CL active lesions[22].
In present study we found only 3 rodents out of 104 captured belonging to genusRattus rattuswere found positive withL.majorand none of domestic animals sampled were positive for anyLeishmaniaspecie. In a recent study from Morocco molecular analysis revealed the presence ofLeishmaniaspecies in 18 specimens: 6Rattus rattus(out of 80 captured; 7.50%),11Mus musculus(out of 50 captured; 22.00%), and oneRattus norvegicus(out of 9 captured; 11.11%)[25].L. tropicainduced CL is commonly considered an anthroponotic disease that usually does not involve an animal reservoir[26], but zoonotic transmission through animal reservoirs has been demonstrated in Palestine, Israel and Jordan[27,28]. Some of the animal reservoirs could be possible source of transmission. Similar results have been elucidated by Khanet al[8] who reported that these animals are not reservoirs of leishmanial parasite, but they can increase the contiguity of sand fly and humans. Dogs have been found infected withL. tropicain Morroco[29], but are not potential reservoirs. A study conducted on animal reservoirs in Addis Ababa, Ethopia, showed rodentsRattus ratuswere negative forLeishmania[30]. These rodents may not be the preferable source for a blood meal. Therefore, detail study of sand fly species, their identification, feeding preferences and habitats in this part of FATA are needed to be investigated in future. The role of animal reservoirs need detail investigation even during the studyL. tropicawas found to be the causative agent in different districts of KP. In previous studies,P. sergentiregarded as the susceptible vector of ACL in Afghanistan and Pakistan, but further work is needed to isolate and typeL. tropicafrom wild sand fly females[12].Phlebotomus paptasiis also susceptible to carryL. tropicaand is widely distributed in different parts of Pakistan including KP[31]. In addition, the vector sand fliesPhlebotomus perniciosusandP. sergentihave been found engorged with rodent blood in southern Portugal[32]but also in central Morocco[33] where the sand fly composition is well established[34]. Svobodovàet al[35] reported the transmission ofL. tropicato mice by the bite ofP. sergenti, a species widespread in our study area[34].
The endemic areas acknowledged during this study present a risk for nearby CL free areas.L. tropicaandL. majorare the main causative agents of CL in Waziristan. More molecular epidemiological studies in the endemic areas should be conducted properly with the help of local and provincial government authorities to identify leishmania outbreaks on time. It was concluded that there is a polymorphism inL. tropicaand a dimorphism inL.majortherefore the clinical aspect and the microscopic diagnosis are insufficient to identify the species of Leishmania, therefore,the molecular biology highly recommended for the diagnosis and identification of the causative agent. These results suggest the possible involvement of rodent species inL. majorcycles in study area. The present findings should be taken into consideration when developing control programs to combat this disease in CL endemic areas of Pakistan.
Conflict of interest statement
The authors declare no conflict of interests.
Acknowledgments
We are thankful to Department of State, USA for establishment of vector borne diseases management center at Kohat University of Science and Technology, Pakistan (DOSFY15) through US Naval Medical Research Unit 3 (NAMRU3), Cairo, Egypt. We also acknowledge Relief International, Pakistan for provision of funds for molecular studies on leishmaniasis in Waziristan, Pakistan. We thank Department of Zoology, Kohat University for their help in identification of rodents. Last but not the least, special thanks to the patients for providing samples and staff of Basic health Units located in different parts of Waziristan for their help in our research project.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Potential applications of lactic acid bacteria and bacteriocins in antimycobacterial therapy
- Protective effect of Ocimum sanctum Linn. leaf extract on ethanol withdrawal syndrome in Wistar rats
- Antihypertensive efficacy of extract of Hedera helix in high salt-induced hypertensive Sprague-Dawley rats
- Comparative phytochemical analysis of Coffea benghalensis Roxb. Ex Schult, Coffea arabica L. and Coffea liberica Hiern.
- Chemical compositions and biological activities of essential oils obtained from some Apiaceous and Lamiaceous plants collected in Thailand
