Potential applications of lactic acid bacteria and bacteriocins in antimycobacterial therapy
2018-09-08AnbarasuSivarajRevathySundarRadhakrishnanManikkamKrupakarParthasarathyUmaRaniVanajaKumar
Anbarasu Sivaraj, Revathy Sundar, Radhakrishnan Manikkam, Krupakar Parthasarathy, Uma Rani, Vanaja Kumar
Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai-600119. Tamil Nadu, India
Keywords:Bacteriocin Lactic acid bacteria Antimycobacterial peptides Tuberculosis Immunomodulation Hybrid bacteriocin
ABSTRACT Tuberculosis (TB) is a communicable disease caused by Mycobacterium tuberculosis (M.tuberculosis). WHO estimated that 10.4 million new (incident) TB cases worldwide in year 2016. The increased prevalence of drug resistant strains and side effects associated with the current anti-tubercular drugs make the treatment options more complicated. Hence, there are necessities to identify new drug candidates to fight against various sub-populations of M. tuberculosis with less or no toxicity/side effects and shorter treatment duration. Bacteriocins produced by lactic acid bacteria (LAB) attract attention of researchers because of its “Generally recognized as safe” status. LAB and its bacteriocins possess an effective antimicrobial activity against various bacteria and fungi. Interestingly bacteriocins such as nisin and lacticin 3147 have shown antimycobacterial activity in vitro. As probiotics, LAB plays a vital role in promoting various health benefits including ability to modulate immune response against various infectious diseases. LAB and its metabolic products activate immune system and thereby limiting the M. tuberculosis pathogenesis. The protein and peptide engineering techniques paved the ways to obtain hybrid bacteriocin derivatives from the known peptide sequence of existing bacteriocin. In this review, we focus on the antimycobacterial property and immunomodulatory role of LAB and its metabolic products. Techniques for large scale synthesis of potential bacteriocin with multifunctional activity and enhanced stability are also discussed.
1. Introduction
Tuberculosis (TB) is known as one of the oldest communicable diseases in human and still a foremost cause of high death in the world. The etiological agent of tuberculosis,Mycobacterium tuberculosis(M. tuberculosis), which multiplies within macrophages.TB tends to impact more on poorest, migrant communities,young and weak children, immunocompromised people (HIV and aged) and people who have diabetes and cancer. World Health Organization (WHO) has estimated that over 10.4 million people have fallen ill with TB in which around 1.7 million people died in 2016. Further WHO estimates around 600 000 new cases with resistance to rifampicin, of which 490 000 had multiple drug resistant tuberculosis (MDR-TB) (WHO global tuberculosis report-2017). Therefore TB poses serious health problem around the world by way of increase in the rate of MDR- TB, extensive drug resistance (XDR-TB), HIV-TB, paediatric TB and latent TB. The latent tuberculosis infection is asymptomatic and not infectious,but it is at risk of progression to active disease at any point of time.TB treatment requires 6 to 8 months for newly diagnosed patients and 18 to 24 months for MDRTB patients. However, the treatment is ineffective for XDR-TB which complicates the treatment options with adverse side effects such as hepato toxicity that discourages both patients and providers.
Antimicrobial peptides such as bacteriocins have many advantages including less immunogenicity, specific affinity to bind on negatively charged prokaryotic cell envelope, and various modes of action[1].Studies reported that the immunomodulation potential of lactic acid bacteria (LAB) and its metabolites show immune response towards macrophage enhancement by up-regulation and down-regulation of Th1 and Th2 cytokines respectively[2]. Antimicrobial peptides found in most living organisms usually consist of 20 to 60 amino acid residues, which are cationic, amphipathic and have a wide range of activity against microbes[3]. Antimicrobial peptides produced by bacteria are classified into two different types as ribosomally synthesized peptides or bacteriocins and non-ribosomally synthesized peptides which exhibit relatively narrow range of antimicrobial activity and broader antimicrobial activity respectively[4]. Marret al.[5] reported that antimicrobial peptides are mainly bactericidal in nature which induce rapid killing of microbial pathogens and also reveal that an increased concentration is not required to fight against drug resistant strains, as compared to antibiotics. According to Riley and Wertz[6], most of bacteria (> 99%) produce at least one bacteriocin. Bacteriocins derived from LAB, are likely to enter into the pharmacopeia as oral or gastrointestinal antibiotics[7]. There are many reports on LAB producing bacteriocins which show prominent antimicrobial activity against wide range of microbial pathogens and also have strong probiotic potential. Hence, bacteriocin can act either as potent alternative or in synergy with antibiotics to enhance the therapeutic effects and also to decrease the prevalence of resistant strains[8]. Bacteriocins of LAB have all the advantages to be developed as peptide based drugs for multidrug resistant pathogens. Although the advantages of bacteriocins with respect to antimicrobial properties are enormous, the peptide can be hindered by high production costs and potency. Owing to the heterogeneous nature of bacteriocins, unique purification procedures have been considered for each producer strains[9,10].
Recently, the focus has been shifted to immunological functions of LAB with considerable attention on a promising strategy for healthpromoting effects[11]. Probiotic LAB has been shown to have the capacity to boost the immunity against infections. According to WHO, the probiotics are described as, “Live microorganisms when administrated in adequate amounts, confer a health benefit on the host”[12]. The proteins secreted and released into the gastrointestinal environment by probiotics might mediate interactions with epithelial cells and immune cells[13]. In this article, research works pertaining to antimycobacterial activity and immunomodulatory property of LAB and its bacteriocins are reviewed. The protein and peptide engineering approaches for the preparation of bacteriocin derivatives with improved activity and stability are also discussed.
2. LAB and characteristics of bacteriocins
LAB possess various industrial applications in the dairy industry,pharmaceutical and special dietary applications[14]. LAB produces various compounds including organic acids, diacetyl hydrogen peroxide, bacteriocins,etc[15]. They also play a key role in maintaining healthy microbiota and have many benefits including managing diarrhoea, food allergies, inflammatory bowel diseases,gastrointestinal disorders and also possess the potential in the prevention of colon cancer[16-19]. Lactobacilli are known to be highly suitable vehicles for the delivery of compounds to the mucosa homeostasis[20].
Bacteriocins are extracellularly released peptides, which are produced by Gram positive (+) and Gram negative (-) bacterial species. Gram (+) bacteria, particularly LAB, produce bacteriocins in different sizes, structures and inhibitory spectra[21]. Bacteriocins of LAB are categorized into class I, class II, class III based on physicochemical properties. The class I bacteriocins are small peptides (<5 kDa) and also known as lantibiotics (lanthionine containing antibiotics), possess unusual post-translationally modified lanthionine or 3-methyllanthionine[22]. Class II bacteriocins are non-lantibiotics, which are relatively small (<10 kDa), heat stable and have fewer post-translational. They are subdivided into class IIa, class IIb, class IIc and class IId[23]. Class III bacteriocins are large molecular weight (>30 kDa), heat labile proteins. Since this class of bacteriocins are lytic enzymes rather than peptides, it was suggested to be excluded from group of bacteriocins and renamed as bacteriolysins. In contrast to antibiotics, bacteriocins from LAB are believed more natural and safe because of their presence in food items[24]. In recent years, bacteriocins of LAB have potential application in both food and pharmaceutical industries[25]. Nisin,produced fromLactococcus lactissubsp.Lactococcus lactisis the first bacteriocin that obtained regulatory approval by FDA for use in certain foods in 2005. They are also known for its ability to enhance food safety and increase health benefits[26]. Another bacteriocin,pediocin produced byPediococcus pentosaceusalso got approved later for their use in food industry[27].
Typically, bacteriocins form pores on cell wall of target pathogens,especifically in Gram (+) bacteria as they possess high anionic lipid contents in the membrane. The formation of pores in the membrane causes small intracellular components leakage which leads to cell death and the debauchery of the proton motive force[28]. Perezet al.[29] reported that the general cationic nature of bacteriocins plays a very important role in their initial interaction with the cell membrane of target strains. The negative charge of bacterial cell membranes and the positive charge of bacteriocin create an electrostatic attraction between them thereby facilitating the interaction of the molecules to the membranes. Due to the cationic nature of bacteriocin, the anionic lipids role in membrane binding has been emphasized. The binding of nisin (class I bacteriocin) to lipid II,which is necessary for bacterial cell-wall synthesis, results in the prevention of proper cell wall synthesis, thereby causing cell death.The nisin-lipid II molecule complex initiates membrane insertion at higher concentrations forming pores in the bacterial cell membrane.Thus, the binding of nisin to lipid II facilitates the preventive action involving cell wall synthesis and membrane pore formation[30,31].Corret al[32], demonstrated thatLactobacillus salivariusUCC118 produced bacteriocinin vivo, which protected mice againstListeria monocytogenesinfection. The possible bactericidal mechanism of nisin on Gram (+) bacterial cell wall including mycobacteria is illustrated (Figure 1).
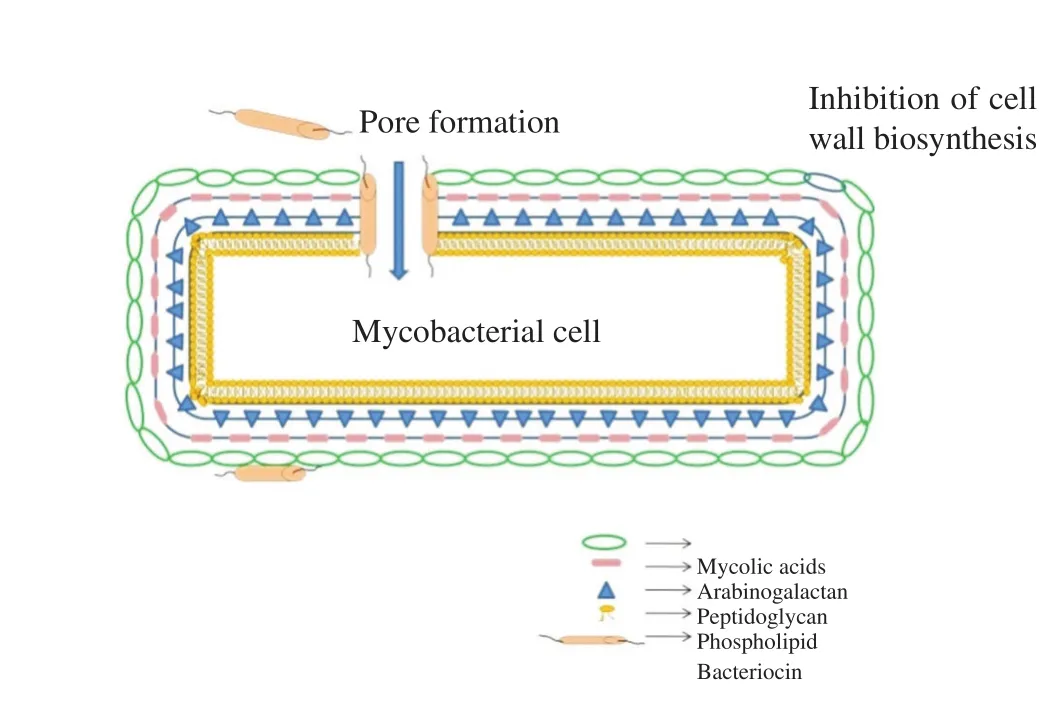
Figure 1. Bactericidal mechanism of nisin on cell wall of Gram positive bacteria including Mycobacteria.
3. Antimycobacterial activity of bacteriocins and LAB
The bacteriocins from LAB have potent activity against variousMycobacteriumspecies. The LAB bacteriocin, nisin was tested againstMycobacterium smegmatis(M. smegmatis) at 10 µg/mL and the results showed that (97.7±2.0)% reduction in internal ATP and leakage of intracellular ATP[33]. Mota-Meiraet al[34], have shown that nisin A and mutacin B-Ny266 (type A lantibiotics), have ability to kill a broad range of bacteria includingM. smegmatis. Donaghyet al[35], reported that the cell free supernatant ofLactobacillus paracaseiisolated from cheese has strongly inhibited the growth ofMycobacterium aviumsubsp.paratuberculosis(MAP)in vitro. On treating sterile milk with this strain, MAP growth was completely undetectable up to 50 d. Bacteriocin of LAB isolated from Boza(Turkish beverage) was tested for antimycobacterial activity. Among the isolates, bacteriocin produced byLactobacillus plantarum(L.plantarum) ST194BZ have shown activity againstM. tuberculosisand growth was repressed up to 69% whereasLactobacillus paracaseiST242BZ,L. plantarumST414BZ and ST664BZ showed 50%of growth repression. In another study,L. plantarumST202Ch,L.plantarumST216Ch,Lactobacillus sakeiST153Ch,Lactobacillus sakeiST154Ch andEnterococcus faeciumST211Ch were isolated from Portuguese fermented meat products and bacteriocins produced from the isolates have significantly reduced the growth ofM. tuberculosisby 38.3%, 48.6%,16.2%, 16.1% and 21.7%respectively[36,37]. Sosunovet al[38], reported that bacteriocin isolated fromLactobacillus salivarius,Streptococcus cricetusandEnterococcus faecalis, shown to have more promising antimycobacterial activity than equal rifampicin concentrations in anin vitromodel. These bacteriocins were non-toxic for mouse macrophages with activity of >90 MIC at a concentration of 0.1 mg/L. They administered the bacteriocins as a complex with phosphatidylcholine-cardiolipin liposomes in TB infected mice model and have demonstrated its capacity to inhibit intracellularM. tuberculosisand to extend the survival of mice. James Carrollet al[39], showed that antimycobacterial activity of lacticin 3147 againstMycobacterium kansasii, MAP andM. tuberculosisH37Ra at MIC90values of 60.0 mg/L, 15.0 mg/L and 7.5 mg/L respectively. Whereas,nisin showed MIC90values of 60 mg/L forMycobacterium kansasiiand >60 mg/L for MAPandM. tuberculosisH37Ra. Hence, lacticin 3147 found as a more effective antimycobacterial peptide than nisin.Lantibiotics certainly possess sufficient potential for future therapies treating tuberculosis. A study demonstrated that nisin and lacticin 3147 arrest the mycobacterial lipid II moiety and suggest that inherent cell wall modifications do not provide lantibiotic resistance toMycobacteria[40]. Bacteriocins ofPediococcus pentosaceusVJ13 exhibited activity against various pathogens includingM.smegmatis. Zahiret al[41], reported thatAerococcussp. ZI1 produces proteinaceous inhibitory substances which showed antagonistic effect againstM. smegmatis. The process of developing a potential bacteriocin peptide library active against different mycobacteria and its characterization are illustrated in Figure 2. Breifly, the partially purified bacteriocins of LAB are screened for antimycobacterial activity againstM. tuberculosisH37Rv, MDRM. tuberculosisand drug sensitiveM. tuberculosisusing Luciferase reporter phage assay.The active bacteriocin are further subjected to purification by HPLC methods. The lyophilized purified bacteriocins are subjected for anti-TB activity against theM. tuberculosisstrains. The potential bacteriocin are characterized by LC-MS, peptide mass finger printing. Peptide library is created for each potential bacteriocin showing activity against different mycobacterial strains.
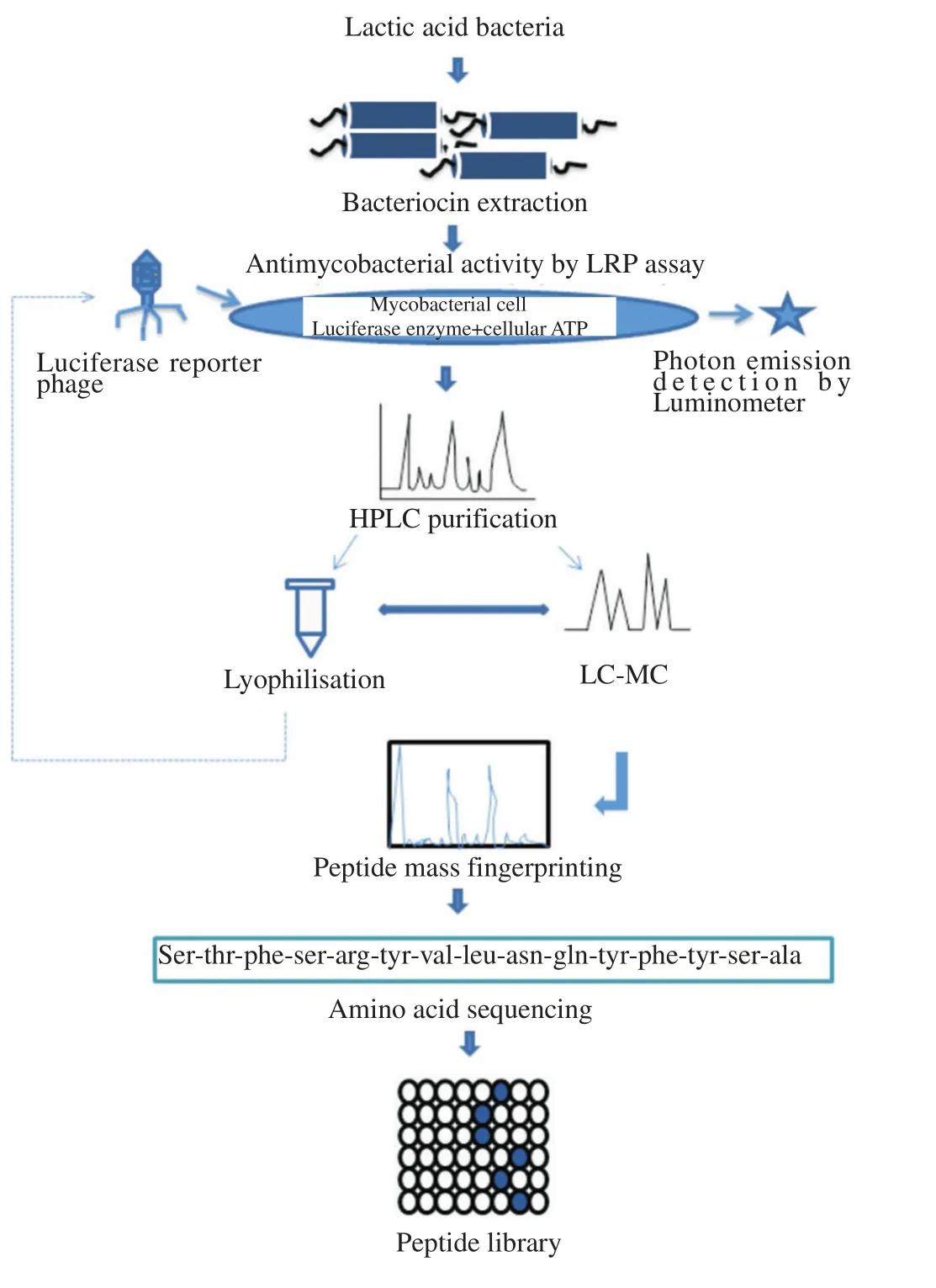
Figure 2. Schematic representation of characterization of bacteriocin screening for activity against M. tuberculosis, bacteriocin purification and proteomics analysis.
LAB have shown to be a natural effective antimicrobials in food industries that exert inhibitory activity against various microorganisms that cause food spoilage. Studies by Mariam[42,43],have reported that milk fermented withLactobacillusstarters has a pronounced antagonistic effect on theMycobacterium bovis(M. bovis)BCG and also found undetectable growth ofM. tuberculosisin the milk by day 7. It is believed that when theMycobacteriumcontaminated milk is fermented, the indigenous LAB confer protective effect. The study suggested that selected LAB may have potential applications as antimycobacterial agents. Macuamuleet al[44], reported that long term fermentation of raw milk with LAB may inactivateM. bovisBCG present in milk. It was shown that during fermentation of milk, factors such as non-bacterial and heatstable components as well as the LAB populations have played a major role in the bactericidal effect againstM. bovisBCG.
4. Immunomodulatory effects of probiotic LAB and their metabolic products
LAB offer attractive opportunities for infectious disease treatmentvis-à-vistheir immune modulating capabilities[45].M. tuberculosisreplicates within macrophage, thereby inhibiting the maturation of phagosome which is involved in the elimination ofM. tuberculosis.Autophagy is an immune response which targets bacteria thereby controlling the proliferation ofM. tuberculosisin macrophages following its infection[46]. Activation of autophagy may also control the inflammation enabling the host immune response againstM. tuberculosis. Hence, many tuberculosis therapies have been focused on the activation of autophagy with innovative approaches.TB infection itself relatively increases the level of Th2 cytokines and inhibits Th1 cytokines[2]. The interaction of LAB and their products with macrophages and T-cells can lead to the cytokines production[47]. A pilot study conducted by Suarez-Mendezet al.[48]for drug resistant therapy by administring IFN-γas an immune adjuvant. IFN-γactivates autophagy which stimulates the delivery of mycobacteria to lysosomes[49,50]. Katoet al[51], demonstrated that male BALB/c mice received intraperitoneal injection ofLactobacillus casei(LC 9018) have shown the activation of macrophages and natural killer cells. Some strains of LAB have increased the production of reactive oxygen, nitrogen radicals, monokines of phagocytic cells. Studies demonstrated that theLactobacillus acidophilusderived non-lipopolysaccharide component stimulates the IL-1αand TNF-αproduction[52,53]. LAB enhance the bactericidal ability of mononuclear phagocytes by increasing autophagy-inducing cytokine such as IFN-γlevels and by reducing IL-4 and IL-13 that is adequate to down-regulate the lung Th2 response, which is known to restrict autophagy[54]. The treatment with probiotic can modulate the immune responses in the lung which enhances the regulatory T cell response in the airway, emphasizing the potential therapeutics[55].Noverret al.[56], reported that cytokine profiles at the intestinal level and systemically were modulated by orally administered Lactobacilli. LAB can protect airway infection in host animals through an interaction of Peyer’s Patches in the gut and enhance respiratory immunity indirectly[57]. LAB probiotics play a key role as immunomodulatory substances and activators of host defence pathway. Increasing evidences suggest that delivered probiotics regulate the immune responses in the respiratory system[58]. The peptidoglycan, polysaccharide, and teichoic acid of LAB cellwall have shown to possess immune-stimulatory properties[59].
Antimicrobial peptide helps in stimulation of innate immune response while reducing associated harmful inflammatory responses[60]. Mitsumaet al.[61], reported that pentapeptide(CHWPR) produced byBifidobacterium animalissubsp.lactisBB-12 up-regulates thec-mycandIL-6genes in HL-60 cell line.Herawatiet al[62], also reported that the bacteriocins isolated fromLactobacillus acidophiluswere able to improve phagocytosis activity of macrophage. Chenet al.[63], showed that live LAB, heatinactivated LAB or LAB-SCS were able to induce macrophages and show immunopotentiating activities, including the induction of tumour necrosis factor-α, interleukin-6 and NO.
5. Improvement of efficiency of bacteriocins and synthesis of hybrid bacteriocins-protein and peptide engineering approach
Several natural antimicrobial peptides which are isolated from natural sources have common characters among their chemical features, which may be linked with their biological activities. Thus,the penetration of the molecule into the target cells can be increased through the modification of molecular structures[64]. Many different processes have been applied to produce antimicrobial peptides in a cost-effective manner through advanced approaches like chemical synthesis, r-DNA technology, cell-free expression systems and transgenic animals or plants. All the processes offer a large production of material required for therapeutic use[65]. Bacteriocins identified with functional activity and sequence have been chemically synthesized in order to increase the scale of production and also to improve the thermal and cleavage stability. Many of the bacteriocins were synthesized by using a Wang resin and by sequentially adding N-Fmoc-protected amino acids by manual or automated synthesis[66]. For instance, Samar Lastaet al.[67],have synthesized the bacteriocin J46 by FMOC peptide synthesis.NMR characterization and biophysical studies are carried out for the synthesized peptides to determine the structural confirmation of the peptide in lipid or polar environment for understanding the mechanism of action. The advantages of chemical synthesis of bacteriocins are bulk production, short duration, combinatorial synthesis and peptide back bone engineering for hybrid stable peptides[68,69]. Bacteriocins with engineered functions or increased stability can be produced by combination of chemoenzymatic approach. This integrates the chemical biology (synthesis) followed by molecular biology (r-DNA technology and use of enzymes for modifications) or vice versa. In the first case, the bacteriocins are synthesized by FMOC synthesis followed by enzyme mediated addition of specific functional groups or linkages. Xinyaet al.[70],described the total synthesis of a circular AS-48 bacteriocin with butelase 1 enzyme by the chemoenzymatic approach. Here the linear AS-48 peptide was synthesized using microwave stepwise synthesis followed by using an Aspargine specific butelase mediated cyclization. The advantage of this approach is that the circular bacteriocin produced, has the ability to withstand pasteurization and this has opened up an arena in the field of food preservation using bacteriocin. In the second case, the bacteriocins are produced using recombinant DNA technology inEscherichia colior other expression systems followed by the addition of specific functional groups by specific chemical reactions[71].
The synthetic bacteriocins have been shown to be more stable and absence of contaminating proteases than those which are produced from bacterial strains. Many studies have attempted to create bacteriocin variants with enhanced activity[72]. Fimlandet al[73], have constructed four new hybrid bacteriocins from various pediocin-like bacteriocins by interchanging corresponding modules which are biologically active. All hybrid bacteriocins had significant bactericidal activity. The peptide’s hinge region facilitates C-terminal of the bacteriocin insertion into membrane of cell, which leads to cell death through pore formation. James Carroll and Jim O’Mahony[74],have identified numbers of nisin variants with enhanced activity againstStreptococci,Staphylococci,Clostridium,Bacillusspp, MRSA.Previous study by Carrollet al[75], reported that nisin variants such as K22T N20P and M21V have improved antimycobacterial activity against pathogenic mycobacteria.
An improved bacteriocin activity could be obtained by addition of disulphide bridge which results in rigidifying a specific conformation. Moreover, it also enhances the net positive charge of a bacteriocin which promotes the initial electrostatic interaction with the outer cell membrane of target[76,77]. Derksenet al.[78],explored the essential of N-terminal disulfide bridge for class IIa bacteriocins activity. The replacements of allylglycine, norvaline,and phenylalanine resulted in retention of leucocin A activity.Oppegardet al.[79], synthesized analogues of class IIb bacteriocin such as lactococcin G by replacement of N- and C-terminal residues with D-amino acids. The resulted anologues were less susceptible to exopeptidases without compromising on the activity. Tominaga and Hatakeyama[80], constructed improved version of pediocin PA-1 (Chimera EP) by fusing C-terminal half of pediocin PA-1 and N-terminal half of enterocin A, which showed increased activity againstLeuconostoc lactis. Authors believed that the design of hybrid bacteriocins with broad spectrum of high specific antibacterial activity through fusing microcins (active on Gram-negative bacteria)and class IIa bacteriocins (Gram-positive bacteria). A novel recombinant hybrid peptide such as Ent35–MccV was designed by combining enterocin CRL35 and microcin V which displayed activity against entero-hemorrhagicEscherichia coliandLactobacillus monocytogenes[23,81].
6. Conclusions
Due to adverse side effects, long duration and emergence of MDRM. tuberculosisand XDRM. tuberculosis, the current antimycobacterial drugs still exhibit many barriers for effective treatment to cure the disease. Hence, novel TB drugs from natural sources with non-toxic and shorter treatment durations are needed to target all sub-populations ofM. tuberculosis. Bacteriocins of LAB exhibit broad spectrum of activity in targetingM. tuberculosisthat can be developed as a leading molecule for the treatment of tuberculosis.Increasing evidences suggest that enhancement of immune response especially autophagy can control the proliferation ofM. tuberculosisin macrophages following infection. In this regard, LAB and its metabolites have shown to impact on the immune system thereby enhancing macrophage activation. As LAB is considered “Generally recognized as safe”, the LAB can be developed as probiotic supplements for the enhancement of autophagy to kill intracellular pathogens likeM. tuberculosis. Synthesis and production of large quantity of bacteriocins with increased stability and enhanced activity from an identified peptide sequence of existing bacteriocin are possible with protein and peptide engineering techniques. It is known that multi-drug resistant variants ofM. tuberculosishave emerged during inadequate tuberculosis treatment. This may be overcome by fusing sequences of two or more known bacteriocins into a new hybrid bacteriocin.
Conflict of interest statement
The authors declare no conflict of interests.
Acknowledgments
We are thankful to the management of Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu, India and Indian Council of Medical Research (ICMR), New Delhi, India (Ref. No:5/8/5/19/2014-ECD-I) in the form of research grant.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Protective effect of Ocimum sanctum Linn. leaf extract on ethanol withdrawal syndrome in Wistar rats
- Antihypertensive efficacy of extract of Hedera helix in high salt-induced hypertensive Sprague-Dawley rats
- Comparative phytochemical analysis of Coffea benghalensis Roxb. Ex Schult, Coffea arabica L. and Coffea liberica Hiern.
- Chemical compositions and biological activities of essential oils obtained from some Apiaceous and Lamiaceous plants collected in Thailand
- Molecular detection of Leishmania species in human and animals from cutaneous leishmaniasis endemic areas of Waziristan, Khyber Pakhtunkhwa, Pakistan
