Protective effect of Ocimum sanctum Linn. leaf extract on ethanol withdrawal syndrome in Wistar rats
2018-09-08LalitSharmaAditiSharmaGuptaGopalSinghBisht
Lalit Sharma, Aditi Sharma, G.L. Gupta, Gopal Singh Bisht
1Department of Pharmacy, Jaypee University of Information Technology, Waknaghat, Solan, Himachal Pradesh India, Pin code 173234, India
2School of Pharmaceutical Sciences, Sholini University, Solan, Himachal Pradesh India, Pin Code 173229, India
3Department of Pharmacology, SPPSPTM, SVKM’S NMIMS University, Mumbai, Maharashtra, Pin code 400 056, India
4Department of BT and BI, Jaypee University of Information Technology, Waknaghat, Solan, Himachal Pradesh India, Pin code 173234, India
Keywords:Oscimum sanctum Ethanol withdrawal syndrome Anxiety Hyperlocomotor activity Histopathology
ABSTRACT Objective: To evaluate the effects of Oscimum sanctum L (O. sanctum), an important medicinal herb, on alcohol withdrawal syndrome in Wistar rats. Methods: Liquid diet with 7.2%, v/v ethanol was administered to the rats for 21 d. Control group animals received sucrose as an isocaloric liquid diet. After alcohol withdrawal, rats were examined at 6th and 24th hour for major withdrawal signs that included anxiety and hyper locomotor activity.Ethanol withdrawal anxiety was tested using elevated plus maze, light and dark model; the hyper locomotor activity using actophotometer. O. sanctum leaf extract (100, 200 and 300 mg/kg, oral) and diazepam (2 mg/kg, i.p) were administered to the treatment group animals 30 min before alcohol withdrawal estimation. Drug treatment was also given 30 min before the second observation at 24th hour. On the last day of the protocol, rats were sacrificed by cervical dislocation liver, kidney and brain were isolated and preserved in formalin for further histopathological examination. Results: Findings from the present study revealed that O. Sanctum leaf extract treatment at doses 100, 200 and 300 mg/kg, oral had a significant protective effect on signs and symptoms of ethanol withdrawal in alcohol-dependent rats. However, no remarkable pathological and microscopic alterations were observed in histopathological examination. Conclusions: O. sanctum seems to be an active drug for the treatment of alcohol abstinence syndrome.
1. Introduction
Alcohol abuse and dependenceimpose worldwide major public health and is the most important public health problem. Alcohol dependence is a long-term, relapsing brain disorder which results due to excessive ethanol intake. Abruption in the chronic alcohol intake leads to the ethanol withdrawal syndrome, which is mainly characterized by anxiety and various other signs and symptoms such as agitation, elevated blood pressure, pulse, nausea or vomiting,hallucinations, insomnia, delirium, sweating, tachycardia, tremors,convulsions and hypertension[1]. Symptoms may lead to severe forms marked by seizures, cardiac arrest and death occur in 5% to 10% of patients. Withdrawal symptoms generally begin after 6-24 h of the last ethanol consumption[2,3]. One of the factors in human alcoholics which lead them to heavy drinking is the use of alcohol to get relieved of the withdrawal symptoms or to re-experience the same level of the rewarding effects previously experienced with ethanol[4]. Negative emotional responses coming from ethanol withdrawal symptoms are the reported reasons that lead to relapse to alcohol use and anxiety is the most important negative motivation among them[5]. Among the various limbic structures, the amygdala and hypothalamus activation are believed to have an important role in modulating ethanol withdrawal anxiety-like behaviors[6].
Ethanol consumption leads to neurobiological and behavioral alterations which are mediated by N-methyl-D-aspartic acid(NMDA) excitatory and γ-aminobutyric acid (GABA)Ainhibitory receptor systems. Ethanol dependence results in a decreased GABAAneuroreceptor response and up-regulation in excitatory NMDA receptors, this modulate ethanol drinking reinforcement reward, drug discrimination, tolerance, dependence, and withdrawal. GABAergic and NMDA/glutamatergic systems can be the important drug targets in achieving a long-term abstinence from alcohol[7,8]. In present treatments for the ethanol withdrawal syndrome are very few such as benzodiazepines, acamprosate, naltrexone and disulfiram, but these medications can only debilitate ethanol craving and have no effect on withdrawal symptoms. Among all benzodiazepines is the only drug for treatment of alcohol abstinence syndrome, but are associated with side effects that mimic the symptoms of alcohol withdrawal includes anxiety, emotional stress, trembling, sweating, headache,sleep disturbances, irritability, nausea, weight loss and myalgia[9].New drug choices and an effective drug treatment for the ethanol withdrawal syndrome are necessary.
Plants are the important sources of medicine and about a large number of drugs (25%–30%) in use are derived from plants[10-12]. Among plants of high therapeutic value, the plants of the genusOscimumfamily Lamiaceae are very important due to their diverse medicinal properties.Oscimum sanctum(O. sanctum) (Tulsi)has been used traditionally for thousands of years for its varying healing properties and has been well documented for its medicinal properties[13,14]. Chemical compounds isolated from various parts of the plant include eugenol, cubenol, rosmarinic acid, β-sitosterol,apigenin, luteolin, borneol, cardinene, linolenic acid, gallic acid,palmitic acid, oleic acid, stearic acid, carnosic acid, vallinin, vitexin,vicenin, rientin, vallinin acid, circineol, vitamin C, vitamin A, iron and phosphorous. Animal studies showed that chronic use ofO.sanctumin large doses may cause infertility in both male and females.These anti-fertility effects are though reversible, and may not cause permanent damage. The major constituent which is reported for the anti-fertility effects is ursolic acid[15,16]. Another component estragol present in theO. sanctumleaf extract may cause uterine contractions[17]. A study reported thatO. sanctumleaves may lower blood sugar level[18]. Apart from the few side effectsO. sanctumis also reported to possess analgesic, anti-inflammatory, antidiabetic,antistress, immunomodulatory, antipyretic, antiasthmatic,anticancer, hepatoprotective, anticonvulsant, antihyperlipidemic,neuroprotective and memory enhancer activities. Moreover,in-vitroresearch documented the affinity ofO. sanctumextract for GABAA,GABAB, benzodiazepine, adenosine, inositol triphosphate and 5-Hydroxytryptamine receptors[19]. Hence, the present investigation was designed to explore the protective properties of theO. sanctumagainst the ethanol withdrawal syndrome in rats.
2. Materials and methods
2.1. Plant material
Plant material (Dried leaves) of theO. sanctum(Bath number: RHD 283), has been procured from Natural Remedies, Bangalore. Dried leaves were then coarsely powdered with a mechanical grinder and passed from sieve number 60. Coarsely powdered plant material was stored in an airtight container and further defatted with petroleum ether (30–40 ℃). The air-dried defatted plant material was further extracted using soxhlet apparatus (ethanol 70% v/v, temperature 50 ℃for 48 h). The hydroalcoholic solvent was recovered by evaporation using a rotary evaporator (Heidolph, Germany). The semisolid mass has been further lyophilized (New Brunswick) at-40 ℃ for 24 h. The obtained dry powder of the plant was stored at 2–8 ℃ till further use.
2.2. Phytochemical screening
The phytochemical screening tests of the hydroalcoholic extract ofO. sanctumwere performed for the detection of phytoconstituents like alkaloids (Wagner’s test), flavonoids (Shinoda’s test), glycosides(Legal’s test), fixed oils (Oil Stain test), phenolics (With 5% Ferric chloride solution), sugars (Molisch’s test), tannins (With 10% Lead acetate solution), saponin (Foam Test), steroids (Salkowski reaction)and terpenoids (Salkowaski Test)[20-22].
2.3. Animals
Wistar rats (220-250 g) were purchased from the National Institute of Pharmaceutical Education and Research, Mohali, Punjab, India and housed (two animals per cage) at Animal House, Jaypee University, Solan, H.P. They were acclimatized to laboratory conditions maintained at temperature (23±2) ℃, controlled humidity conditions, and light and dark cycles (12:12 h). Animals were fed with food pellets diet (Aashirwad Industries, Chandigarh, India) and waterad libitum. Experimental procedures were carried out after the approval from the Institutional Animal Ethics Committee (3/GLG/2014/JUIT/IAEC) and as per CPCSEA (Committee for the Purpose of Control And Supervision of Experiments on Animals)norms (1716/PO/a/13/CPCSEA).
2.4. Study design
Animals were divided into 6 groups (normal group, disease group,3 treatment groups, and standard control group) each comprising 6 animals (n=6) and housed individually. Alcohol treatment was given with a liquid diet as specified in earlier studies[23,24]. Liquid dietad libitumwith or without alcohol was given to the rats; no other chow or water was given. Liquid diet was prepared as per previous studies which contain cow milk 925 mL, alcohol 25–75 mL (ethyl alcohol 96.5%), sucrose 17 g and vitamin A 5 000 IU. Liquid diet(no alcohol) was administered to the animals at the beginning of the study for 7 d then 2.4% ethanol was given with the liquid diet for 3 d. The concentration of ethanol was then increased to 4.8% for the next 4 d then 7.2% for 21 d. Alcohol was withdrawn from the liquid diet after 21 d. Drug treatmentO. sanctumleaf extract (100, 200 and 300 mg/kg, oral), saline, diazepam (2 mg/kg, i.p) were given to the animals 30 min before alcohol withdrawal assessment. For evaluating alcohol withdrawal signs like anxiety and hyper locomotor activity were tested at 6th and 24th hour of the withdrawal period using elevated plus maze, light and dark model and actophotometer.Between evaluation period the animals were returned to their native cages. Each group received another dose of the test drug at 24 h after the first. Control group rats received a sucrose-containing liquid diet(a caloric substitute to alcohol) and were also evaluated parallel to disease control groups[24]. All observations were performed during the light period. Fresh liquid diet was presented at the same time(09:00 h) daily. Body weight and ethanol intake by the rats were observed daily and expressed as g/kg/day. On completion of the protocol animals were sacrificed by cervical dislocation and liver,kidney, brain were isolated and preserved in formalin for further histopathological studies[25-27].
2.5. Elevated plus maze model
The device consist of two open and two closed arms arranged opposite to one another and a central platform elevated at 50 cm.Animals were placed individually on the central platform facing towards the open arm. The number of entries in the open arms, total time spent and the number of entries in the closed arms and total time spent were recorded by the observer for 5 min. The observations were carried out under red dim light[28,29].
2.6. Light and dark model
The model consists of two compartments one light and one dark in a rectangular box connected by an opening of 7.5 cm× 7.5 cm in between the wall. Animals were placed individually in the center of the light chamber facing towards the opening of the dark chamber.The number of entries among the light and dark chamber and the time spent in the light and dark chamber was recorded by the observer for 5 min[30].
2.7. Locomotor activity
The spontaneous locomotor behavior of the animals was recorded individually for 5 min using a digital actophotometer. The count of the movement of the animal cutting a beam of light which falls on the photocell was recorded digitally[25].
2.8. Histopathological Studies
Rats were sacrificed by cervical dislocation and brain, liver and kidney were isolated. Isolated organs were fixed in neutral buffered formalin (10%). The 5 μm histological sections of the tissues were stained with hematoxylin and eosin method and were examined further under a microscope for determination of change or cellular damage[31].
2.9.Statistical analysis
The body weight variations of alcohol control rats in comparison to normal group animals were analyzed by Student’st-test. Twoway ANOVA (analysis of variance) followed by Bonferroni’s test was carried out for determination of theO. sanctumeffects in the elevated plus maze, light and dark model and locomotor activities.The significance level was set atP< 0.001 levels.
3. Results
3.1. Phytochemical screening of the plant extract
Phytochemical detection tests of theO. sanctumleaf extract revealed the presence of chemical constituents like alkaloids,phenolics, flavonoids, glycosides, fixed oils, sugars, tannins, saponin,steroids, and terpenoids.
3.2. Ethanol consumption and body weight changes of animals
Day to day alcohol consumption in ethanol-fed rats vary between(13.28±0.89) to (16.41±0.64) g/kg and there was no significant difference among the groups. The body weight was (225.71±4.56)g and (231.30±5.49) g at the beginning of the study in control and ethanol fed group, respectively; (262.34±5.06) g and (242.14±6.01) g at the end of the study in control and ethanol fed group, respectively.A gain of 4.6% body weight in ethanol control and 16.2% in control group rats were observed over the initial body weight at the end of the study.
3.3. Elevated plus maze test
In comparison to the normal control group, ethanol-fed rats showed a significant decrease into the time spend in open arms, and in the number of entries into the open arms and closed arm; while it showed a significant increase in the time spend into the closed arms on the 6th and 24th hour of the withdrawal period (Figure 1a-d). Treatment withO. sanctumleaf extract (100, 200 and 300 mg/kg, oral) at 6th and 24th hour of alcohol withdrawal produced a significant increase into the time spend in open arms, and in the number of entries into the open arms and closed arm; while it showed significant decrease in the time spend into the closed arms as compared to the disease control rats (Figure 1).
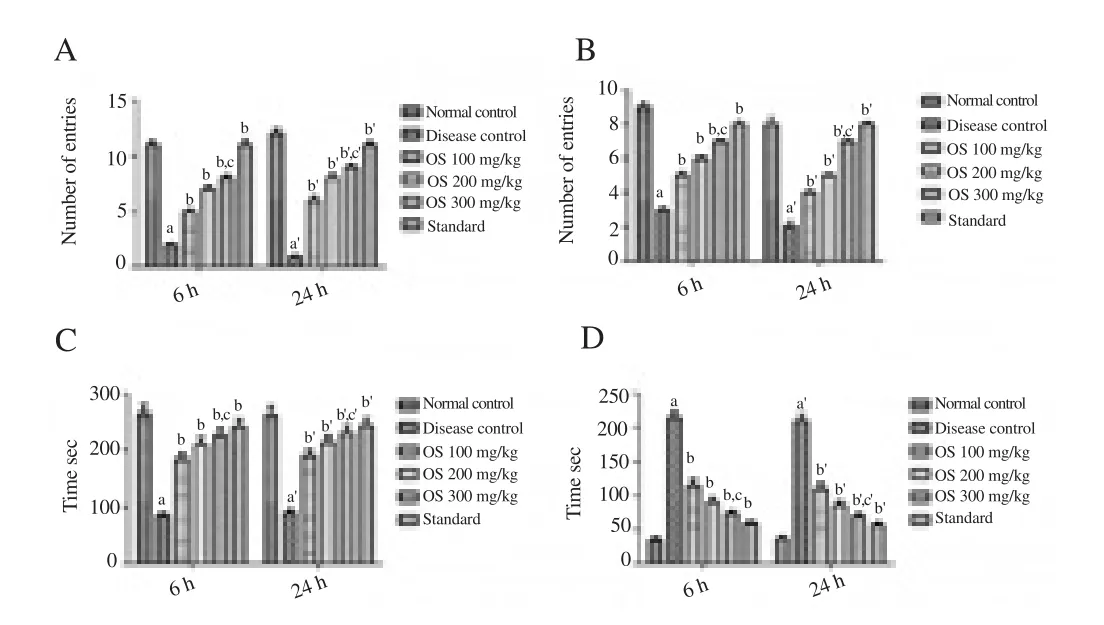
Figure 1. Effect of drug treatment on alcohol withdrawal anxiety when tested on elevated plus maze model.
3.4. Light and dark model
Ethanol-fed rats in comparison to the normal control group showed a significant decrease in the time spend into open arms, and in the number of entries into the open arms and closed arm; while it showed a significant increase in the time spend into the dark chamber at the 6th and 24th hour of the alcohol withdrawal (Figure 2a-d). Treatment withO. sanctumleaf extract (100, 200 and 300 mg/kg, oral) at 6th and 24th hour of alcohol withdrawal produced the significant increase in the time spend into open arms, and in the number of entries into the open arms and closed arm; while it showed a significant decrease in the time spend into the dark chamber when compared to the disease control rats (Figure 2).

Figure 2. Effect of drug treatment on alcohol withdrawal anxiety when tested on light and dark model.

Figure 4. Histology of brain, liver, and kidney (H&E, ×100).
3.5. Locomotor activity
Ethanol-fed rats showed a significant hyper locomotor activity in the 6th and 24th hour of the alcohol withdrawal when compared with the normal group animals (Figure 3).O. sanctumleaf extract (100,200 and 300 mg/kg, oral) produced significant suppressive effects on locomotor hyperactivity at 6th and 24th hour of alcohol withdrawal(Figure 3).
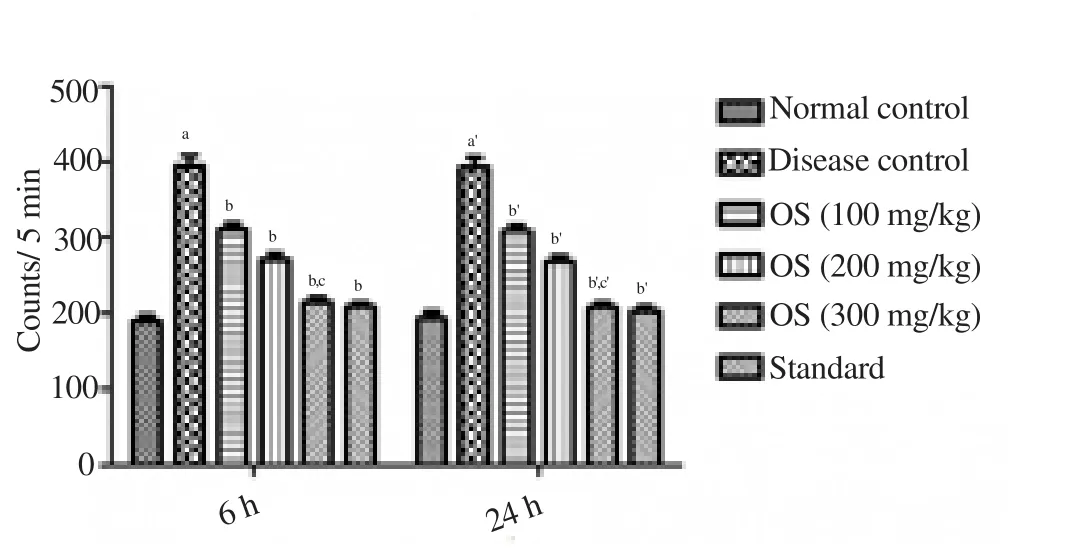
Figure 3. Effect of drug treatment on locomotor hyperactivity when tested on actophotometer.
3.6. Histopathology
Alcohol consumption and withdrawal did not produce any treatment-related pathological alterations in the alcohol-treated animals. Histopathological examination of the brain revealed the presence of normal glial cells (Figure 4) the liver showed normal hepatocytes with central and portal veins (Figure 4) and kidney displayed normal nephrons without any vacuolar degeneration of tubular cells, glomerular congestion and interstitial bleeding(Figure 4). No histological changes were seen in brain, kidney, and liver of the alcohol-treated animals.
4. Discussion
The findings from our study revealed the inhibitory effects of theO. sanctumleaf extract on ethanol withdrawal symptoms in alcohol-dependent rats. Ethanol administration with liquid diet is a relevant and well-established model for ethanol consumption in rats[23,32]. Previous studies reported that daily consumption of ethanol over 9 g/kg for 15 continuous days leads to dependence and abstinence leads to various signs and symptoms in rats[33]. In this study, we observed major signs of alcohol withdrawal such as withdrawal anxiety and hyper locomotor activity. The incidence of alcohol withdrawal anxiety observed in the alcohol-fed rats at 6th hour and 24th hour of abstinence were very similar to the previous studies[26,27,34]. The present study demonstrates the significant effect of theO. sanctumin reducing anxiety at 100, 200 and 300 mg/kg which is similar to some of the previous findings[35,36]. The earlier studies reported the anti-anxiety effects of theO. sanctumin normal rats while our study revealed the anti-anxiety effects ofO. sanctumin alcohol abstinence animals for the first time.O. sanctumat 100, 200 and 300 mg/kg showed a significant anti-anxiety effect in ethanolwithdrawn rats. Diazepam (2 mg/kg, i.p) was used as a standard drug. A significant hyperlocomotor activity was seen in the alcoholdependent rats at the 6th and 24th hour of the alcohol abstinence when compared to the normal group rats. The hyperlocomotor activity of the alcohol withdrawal rats is similar to some of the earlier findings[24,25].O. sanctumat 100, 200 and 300 mg/kg significantly normalized the locomotor hyperactivity in the actophotometer test.Body weight gain (6.2% approx.) was noted in control group rats,and body weights of the ethanol consuming rats changes lightly during the study as there was a 4.6% increase in body weight. This change in the body weight of the animals agrees with earlier findings as alcohol decreases the secretion of digestive enzymes and affects absorption, metabolism, and excretion of essential nutrients[37-39]. Brain, kidney, and liver are the main organs which are affected by the alcohol, however, alcohol dependence and withdrawal did not have any pathological changes in the treated animals which were confirmed through histopathological examination. NMDA receptors and the glutamatergic system are believed to have a critical role in the progress of the alcohol withdrawal signs[40]. The phytochemical study revealed that the hydroalcoholic leaf extract ofO. sanctumcontained constituents such as flavonoids, tannins,steroids, terpenoids, and glycosides. The literature reported that β-sitosterol, gallic acid, vallinin and luteolin have well documented anti-anxiety properties[41-44]. Hence, such phytoconstituents may have a protective role in the ethanol abstinence syndrome and these constituents may ameliorate the ethanol deprivation effects asO.sanctumis proved to have inhibitory effects on the reuptake of neurotransmitters such as noradrenaline, serotonin, dopamine and modifies neuronal excitability via GABAergic and glutamatergic and mechanisms. In conclusion,O. sanctumleaf extract seems to be pharmacologically active by suppressing ethanol withdrawal signs and symptoms and may have therapeutic potential in treating ethanol dependence.
Conflict of interest statement
The authors declare no conflict of interests.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Potential applications of lactic acid bacteria and bacteriocins in antimycobacterial therapy
- Antihypertensive efficacy of extract of Hedera helix in high salt-induced hypertensive Sprague-Dawley rats
- Comparative phytochemical analysis of Coffea benghalensis Roxb. Ex Schult, Coffea arabica L. and Coffea liberica Hiern.
- Chemical compositions and biological activities of essential oils obtained from some Apiaceous and Lamiaceous plants collected in Thailand
- Molecular detection of Leishmania species in human and animals from cutaneous leishmaniasis endemic areas of Waziristan, Khyber Pakhtunkhwa, Pakistan
