Antihypertensive efficacy of extract of Hedera helix in high salt-induced hypertensive Sprague-Dawley rats
2018-09-08UmmeSalmaTaousKhanAbdulJabbarShah
Umme Salma, Taous Khan, Abdul Jabbar Shah
Cardiovascular Research Group, Department of Pharmacy, COMSATS University Islamabad, Abbottabad campus, KPK, Pakistan
Keywords:Hedera helix Antihypertensive Vasorelaxant Endothelium-dependent Nitric oxide Calcium channel blocker
ABSTRACT Objective: To explore the antihypertensive effect of extracts from the leaves of Hedera helix (H.helix) on normotensive and hypertensive rats in-vivo followed by vasodilatory studiesin-vitro.Methods: The crude methanolic extract was prepared and the activity directed fractionation was carried out. Spectrophotometric analysis of total phenolic and flavonoid content was also done. HPLC analysis was performed for the detection of hederacoside C.In-vivo blood pressure study was carried out in normotensive and high salt-induced hypertensive Sprague-Dawley rats. Isolated aortic tissues from rat and rabbit were used for in-vitro studies. The effects were recorded and analyzed through PowerLab data acquisition system. Results: Crude extract of H. helix (1-30 mg/kg) decreased blood pressure to greater extent in high salt-induced hypertensive rats in-vivo compared to the normotensive [Max. fall (58.59±0.02) mmHg vs.(67.53±3.07) mmHg]. The n-hexane, chloroform, ethyl acetate and aqueous fractions were also checked. These fractions were more effective in hypertensive rats. Aqueous fraction was more potent and n-hexane the least. In isolated rat aortic rings precontracted with phenylephrine,crude extract induced endothelium-dependent effect. The endothelium-dependent component of vasodilatory effect was ablated with L -NAME, and denudation of endothelium. The aqueous fraction was most potent vasodilator. In aortic rings from hypertensive rats, extract and fractions produced partial endothelium-independent effect which was not affected by pretreatment with L-NAME, indicating endothelium dysfunction in the hypertensive rats and suggesting additional vasodilatory mechanisms. In rabbit aorta, the extract and fractions also inhibited phenylephrine and high K+-induced precontractions, and shifted Ca++concentration–response curves. Conclusions: Our findings indicate that extract and fractions of H. helix are antihypertensive remedies, which is the outcome of vasodilatory effect. This vasodilatory effect is mediated through nitric oxide and Ca++ antagonism.
1. Introduction
Hypertension is a chronic disorder, which causes damage to major organs and results in increased morbidity and mortality[1,2].Multiple drugs with synergistic efficacy are required to have optimal control on blood pressure[3]. Alternatively, herbal drugs contain multiple constituents which can neutralize side effects, and these herbal drugs are considered to be safe and efficacious[4,5]. This is the reason why WHO recommends utilization of herbal drugs in the healthcare system, particularly in developing countries where more than 80% of the population relies on herbal drugs[6].Hedera helixL. (Araliaceae)(H. helix)or English Ivy has global distribution including Pakistan. The leaves ofH. helixare medicinally used for cardiovascular diseases especially hypertension[7]. According to a clinical study, daily intake of 15-50 drops of a tincture prepared from theH. helixleaves restores blood pressure to normal level within few days[8]. Additionally, theH. helixleaves have also been used in gout, cancer and parasitic infections[7]. The most effective biological constituents ofH. helixare triterpene saponins such as hederacoside C, hederacoside D, hederacoside B andα-hederin[9].Flavonoids such as rutin, isoquercitin, astragalin and kaempferol 3-O-rutinoside are also present inH. helix[10].
Accumulating evidence indicated that extract ofH. helixpossesses a wide variety of biological activities like antimicrobial[11],antimutagenic[12,13], hepatoprotective[14], antioxidant[15] and antiinflammatory[16]. Additionally, the efficacy of dry extract leaves for treatment of lung diseases has been validated through clinical trials and post marketing study[17]. The extract ofH. helixhas shown bronchodilatory effect through β2adrenergic receptors[18,19]. The extract ofH. helixhas also produced relaxant effect onin-vitrogut smooth muscles preparations[10]. Despite the fact thatH. helixhas been investigated preclinically and clinically, literature still lacks studies on its antihypertensive mechanisms. This study was aimed to evaluate the blood pressure lowering effect on normotensive and hypertensive rats. High salt-induced hypertension rat model was used because it was easiest and similar to human hypertension[20,21].The antihypertensive effect ofH. helixwas determined usinginvivoblood pressure experiments and underlying mechanisms were confirmed using isolated tissue preparations.
2. Materials and methods
2.1. Crude extract
Fresh leaves ofH. helixwere collected from Abbottabad, KPK,Pakistan in August, 2014 identified and authenticated by Dr.Hassan Sher, Director Institute of Plant Sciences and Biodiversity,University of Swat, Swat Pakistan. A voucher specimen was placed in a code Hh-L-08/14 in the Graduate Lab of Pharmacology and Pharmacognosy, Department of Pharmacy, COMSATS University Islamabad, Abbottabad campus, KPK, Pakistan.
The dried leaves powder (2.0 kg) ofH. helixwas macerated in methanol for 15 d, and filtered through eightfolds of muslin cloth.After that this filtrate was passed through Whatman-1 qualitative grade 1 filter paper. The collected filtrate was then evaporated in rotary evaporator under reduced pressure (-760 mm Hg) and 37 ℃temperature[22]. The approximate yield of crude extract ofH. helix(Hh.Cr) was 12% (w/w).
2.2. Fractionation of crude extract
The fractionation was performed using methods described earlier[22,23]. The Hh.Cr (80 g) was dissolved in 10% tween-80 and distilled water. Then-hexane (300 mL) was also added. This solution was agitated vigorously and then left for about 30 min so that the two layers got separated. Then-hexane layer was collected and the procedure was repeated. Then-hexane layers were evaporated to obtain then-hexane fraction, yielding 10%. The recovered aqueous layer was treated with chloroform and then with ethyl acetate in order to obtain chloroform and ethyl acetate fractions yielding 7.5%and 5.0%, respectively. The yield of aqueous fractions (Hh.Aq) was 55.5%. Hh.Cr and all the fractions were soluble in combination of 10% tween-80 and distilled water.
2.3. Determination of total phenolic content
Phenolic contents were analyzed in adherence to the Folin-Ciocalteu method[24] with some modifications. In this method,0.5 mL of each extract (dissolved in 50% methanol) or gallic acid was mixed with 1.5 mL of the Folin-Ciocalteu reagent and 20%(w/ v) sodium carbonate. After 30 min, absorbance was measured at 765 nm using a UV/VIS spectrophotometer.
2.4. Determination of total flavonoid content
The total flavonoid contents of each extract was investigated using the aluminium chloride colorimetry method[25] with slight modifications. In brief, the extract sample was diluted with 80%methanol. The diluted extract or quercetin (0.5 mL) was mixed with 1.5 mL methanol, 0.1 mL of aluminium chloride solution, potassium acetate solution and 2.8 mL distilled water. The solution was stored at room temperature. After 30 min, the absorbance was measured at 415 nm using a UV/VIS spectrophotometer.
2.5. HPLC analysis
The analysis of Hh.Cr and hedercoside C was carried out using HPLC [Perkin Elmer (Series 200 Auto Sampler)] attached with UV/Vis detector (200-700 nm). Gradient elution was performed on reverse phase column (Shim-pack GIST C18 column, 150 mm×4.6 mm, 5 μm). Hederacoside C standard solution was made by dissolving 5 mg in methanol and final volume was made 10 mL with further addition of methanol. The extract was dissolved in methanol (100 mg/mL). The stock solutions were filtered and degassed ultrasonically before analysis. Composition of mobile phase was water: acetonitrile: 85% H3PO4(90:10:0.5) (solvent A)and acetonitrile (solvent B). The samples were eluted by gradient elution by linear increase from 20% to 100% of solvent B in 30 min,started with 80% of solvent A. Monitoring wavelength for eluent was 210 nm[26].
2.6. Drugs and standards
Drugs and standards were purchsed from the source specified:acetylcholine chloride, atropine phosphate, potassium chloride,phenylephrine hydrochloride, norepinephrine bitartrate, Nω-nitro-L-arginine methyl ester (L-NAME) hydrochloride, indomethacin,verapamil hydrochloride (Sigma Chemical Company, St. Louis,MO), sodium thiopental (Abbott Laboratories Ltd, Karachi,Pakistan).
2.7. Experimental animals
All the experiments were performed according to the rules and regulations of Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council[27] and approved by the Ethical Committee of Pharmacy Department, COMSATS University Islamabad, Abbottabad campus, KPK, Pakistan in its meeting held on 17-06-2013 vide Notification EC/PHM/07-2013/CIIT/ATD.Following animals were used: Sprague-Dawley (SD) rats (200-250 g) and adult rabbits (1-1.5 kg). All animals were housed at 23-25 ℃ in the animal house of the Pharmacy Department, COMSATS University Islamabad, Abbottabad campus, KPK, Pakistan. The animals were allowed water and foodad libitum.
2.8. Pharmacological experimentations
2.8.1. In-vivo blood pressure study in normotensive and high salt-induced hypertensive anaesthetized SD rats
This study was carried out by using normotensive and hypertensive rats, following protocols as described earlier[28-30]. Male SD rats were caged in uniform hygienic conditions and kept on 8% sodium chloride in diet and drinking water for 8 weeks. Anesthesia was induced in rats using sodium thiopental (Pentothal, 50-90 mg/kgi.p). The trachea was cannulated by polyethylene tubing (PE-20)to facilitate spontaneous respiration and the right carotid artery by polyethylene tubing (PE-50), connected to pressure transducers coupled with bridge amplifier and PowerLab Data Acquisition System (ADInstruments, Australia). In addition, the left jugular vein was cannulated for injecting standard drugs, crude extract and fractions. An overhead lamp was used to maintain the temperature.After 20 min period of stabilization, 0.1 mL normal saline or test material was injected intravenously. Arterial blood pressure was stabilized for 10-15 min. Standard drugs, crude extract and fractions were administered by intravenous injections and immediately by a 0.1 mL normal saline flush. Acetylcholine (1 μg/kg) and norepinephrine (1 μg/kg) responses were observed before injection of test materials. Rats having blood pressure 150-190 mmHg were considered hypertensive.
2.8.2. Vascular reactivity studies in isolated rat aorta of normotensive and hypertensive rats
The descending thoracic aortae were isolated from normotensive and hypertensive rats, transferred into normal Kreb’s solution,cleaned and prepared rings (3 mm). The aortic rings preprations were carefully hanged in tissue bath containing Kreb’s solution,aerated with carbogen (5% CO2in O2) and attached to a force transducer which was coupled to PowerLab Data Acquisition System (ADInstruments, Sydney, Australia). Rings were stabilized for 60-90 min at preload of 2 g by changing buffer for every 15 min.In some aortic rings, endothelium was deliberately damaged.After the isometric tension stabilization, the inhibitory response of acetylcholine (1 μM) was observed against contractions induced with phenylephrine (1 μM) to determine the endothelium integrity. The tissues were pre-incubated withL-NAME (10 μM) for 20-30 min.The vasorelaxant effect of crude extract and fractions were compared with and withoutL-NAME. Extract and fractions were parallel tested in removed endothelium aortic rings and rings from hypertensive rats[30,31].
2.8.3. Calcium channel blocking activity in isolated rabbit aorta
Thoracic aortae were isolated and made aortic rings preparations(3 mm length). The aortic rings were hanged in Kreb’s solution,and aerated with 5% CO2in O2(carbogen) at 37 ℃. At pre load of 2 g, tissues were stabilized for 1 h. The effect of all the extracts was observed against phenylephrine (1 μM) and high K+(80 mM). The calcium channel blocking activity was checked by preparing Ca++CRCs in Ca++-free/EGTA solution[30].
2.9. Statistic analysis
Data were expressed in mean, standard errorof the mean (SEM),and the median effective concentrations (EC50values) with 95%confidence interval (CI). The difference in fall between mean arterial pressure (MAP) and pretreated values was determined by one-way analysis of variance (followed bypost hocTukey HSD test) using SPSS 21 software (USA). The results were considered significant atP<0.05.
3. Results
3.1.Determination of total phenolic content and total flavonoid content
Hh.EtAc produced the highest yield of total phenolic content[(74.44±2.65) mg GAE/g], followed by chloroform, Hh.Aq, Hh.Cr and Hh.n-hexane fraction in the decreasing order. Moreover,Hh.EtAc produced (13.61±0.08) mg QCE/g of total flavonoid content which was the highest yield, followed by chloroform, Hh.Cr,Hh.Aq and Hh.n-hexane (Table 1).

Table 1 Total phenolic content and flavonoid contents., Hh.Chlor, Hh.EtAc, and Hh.Aq (mean±SEM, n=3).
3.2. HPLC analysis
In the present study, HPLC analysis of Hh.Cr was carried out to determine the presence of hederacoside C (major saponin). A major peak was obtained at a retention time of 22 min and was identified as hederacoside C by comparing the retention time with the standard hederacoside C sample (Figure 1).
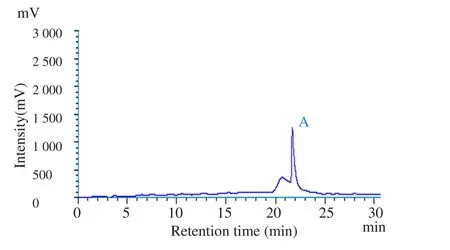
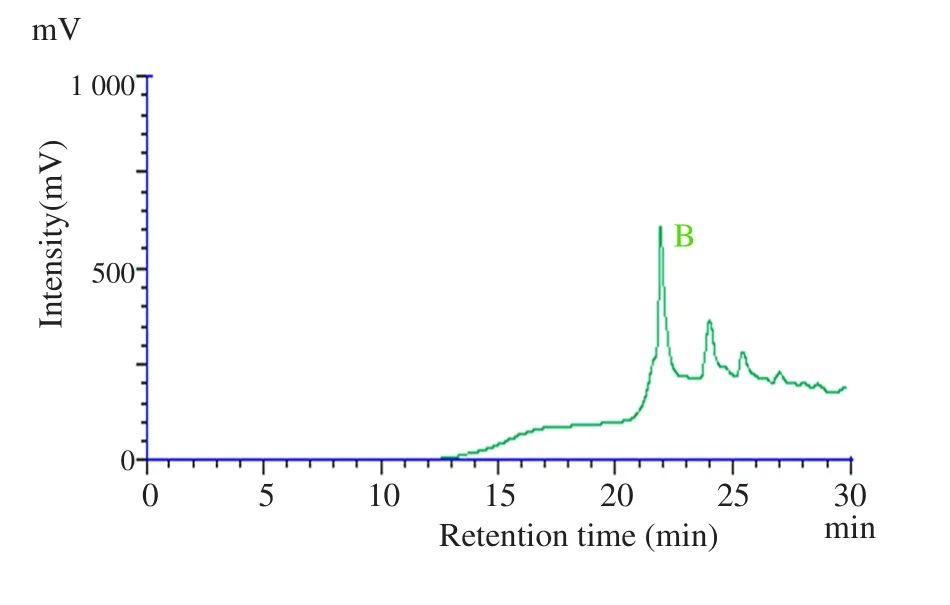
Figure 1. HPLC chromatogram of (A) standard hederacoside C and (B)Hh.Cr at wavelength of 210 nm.
3.3. In-vivo blood pressure study in normotensive and high salt-induced hypertensive anaesthetized SD rats
Hh.Cr resulted in a decrease in MAP in normotensive rats at all doses (1, 3, 10 and 30 mg/kg) with maximum fall of (58.59±0.02)mmHg. All fractions decreased MAP, Hh.Aq was the most potent fraction with maximum fall of (62.11±4.45) mmHg (Table 2).

Table 2 Fall in MAP in normotensive rats (mmHg)(mean±SEM, n=5).
Compared with the results of normotensive rats, the extracts and fractions are more effective in hypertensive rats. Hh.Cr was more effective antihypertensive in the high salt-induced hypertensive rats at all four doses tested (P<0.001). All fractions such as Hh.n-hexane,chloroform, Hh.EtAc and Hh.Aq, like the parent crude extract were more effective in the hypertensive rats than that in the normotensive rats, particularly at dose of 30 mg/kg. Hh.Aq was the most potent(P<0.001) with fall of (71.93±1.21) mmHg in hypertensive rats,respectively at dose of 30 mg/kg (Table 3).

Table 3 Fall in MAP induced in high salt-induced hypertensive rats (mmHg)(mean±SEM, n=5).
3.4. Vascular reactivity studies in isolated rat aorta of normotensive and hypertensive rats
In intact rat aortic rings preparations, Hh.Cr inhibited the phenylephrine precontractions having EC50value of 0.2 mg/ mL(0.1-0.3). Preincubation of the aortic rings withL-NAME (10 μM)attenuated the vasorelaxant effect of Hh.Cr (Figure 2A). In endothelium removed rings, Hh.Cr reduced the vasorelaxation. In aortic rings from hypertensive rats precontracted with phenylephrine(1 μM), Hh.Cr inhibited vasorelaxant effect superimposable to that withL-NAME and aorta with removed endothelium.

Figure 2. Vasorelaxant effect of (A) Hh.Cr and its fractions: (B) Hh.nhexane, (C) Hh.Chlor, (D) Hh.EtAc, and (E) Hh.Aq in rat aortic rings precontracted with phenylephrine (1 μM).
Hh.n-hexane and chloroform produced endothelium-dependent response which decreased by pre-incubation withL-NAME in intact aortic rings, denuded aortic rings and rings from hypertensive rats(Figure 2B & C), similar to Hh.Cr. Hh.EtAc induced an endothelium independent vasorelaxation (Figure 2D).
Hh.Aq also induced vasodilatory effect (0.01-0.03 mg/mL). Hh.Aq induced relaxation at higher concentration withL-NAME (10 μM),aortic rings with removed endothelium and aortic rings from hypertensive rats (Figure 2E).
3.5. Calcium channel blocking activity in isolated rabbit aorta
Hh.Cr produced vasodilator effect against high K+and phenylephrine precontractions with EC50values of 2.5 (1.5-3.5)and 6.6 mg/mL (3.5-9.7) (Figure 3A). Hh.Cr (1.0-5.0) mg/mL pretreatment caused a rightward shift in Ca++CRCs (Figure 3C) as verapamil (Figure 3B & D).

Figure 3. The vasorelaxant effect of (A) Hh.Cr; (B) verapamil on phenylephrine (PE 1 μM) and K+ (80 mM) precontractions, respectively (C& D) and their effect on the Ca++ concentration–response curves in isolated rabbit aorta preparations.Values shown are mean ± SEM (n=5-7).
Hh.n-hexane, chloroform, Hh.EtAc, and Hh.Aq also produced inhibitory effect against the precontractions of K+(80 mM) and phenylephrine (1 μM) with no prominent dominancy like crude extract, and shifted the Ca++CRCs rightward (Figure 4).
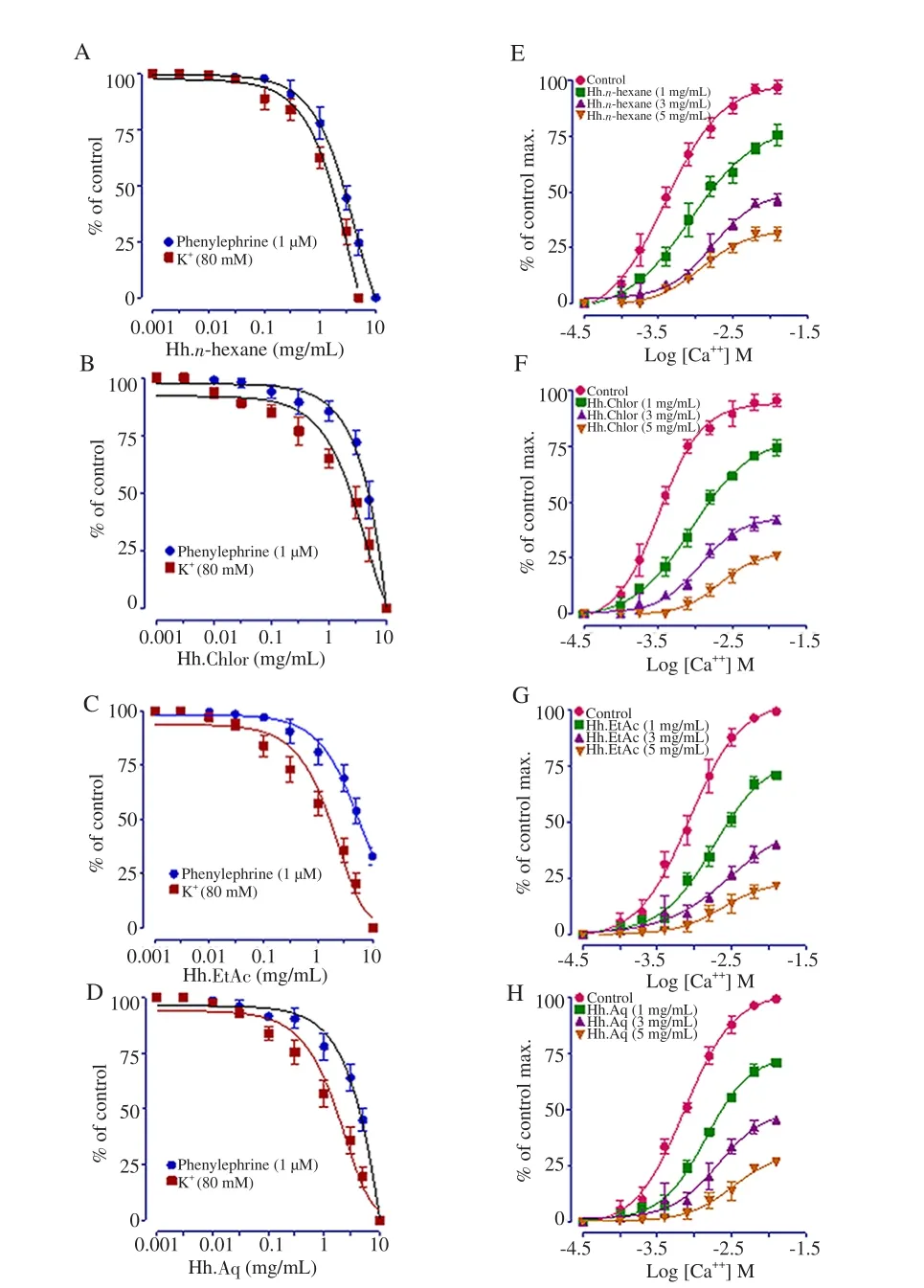
Figure 4. The vasorelaxant effect of all the fractions on rabbit aortic tissues,and their respective Ca++ curves constructed in Ca++-free medium; (A & E)Hh.n-hexane, (B & F) Hh.Chlor, (C & G) Hh.EtAc, and (D & H) Hh.Aq fractions.Values shown are mean ± SEM (n=5-7).
4. Discussion
H. helixis medicinally used for cardiovascular diseases including hypertension[7,8]. The present investigation for the first time demonstrates the antihypertensive property of Hh.Cr and its fractions when intravenously injected in normotensive and hypertensive SD rats. Feeding of 8% NaCl in the diet and drinking water produced hypertension in SD rats[32,33]. Hh.Cr, in both normotensive and hypertensive rats, produced a decrease in MAP compared to pretreatment values. All extracts were more effective antihypertensive in high salt-induced hypertensive rats at all four doses tested, particularly at dose of 30 mg/kg, with Hh.Aq being the most potent (P<0.001).
Vascular resistance and cardiac output are the important determinants of blood pressure[34]. We tested the vascular aspect of all extracts to probe the underlying mechanisms. The isolated thoracic aortas from the normotensive rats were used to test the effect on phenylephrine precontractions in the denuded rings and intact rings pretreated with different inhibitors. The finding in the aorta from normotensive rats was compared with that in the hypertensive rat aorta. Crude extract induced a vasorelaxant effect against phenylephrine precontractions. This effect was endotheliumdependent as denudation of the vascular endothelium ablated (>80%)it. This finding suggests that the extract possesses vasorelaxant constituents which act predominately through vascular endothelium.Vascular endothelium regulates vascular tone and blood pressure[35].It synthesizes and releases nitric oxide (NO), prostacyclin and many others[36]. NO is a major regulator of vascular tone in the endothelium[37], which is produced in the presence of NO synthase(eNOS). NO is also involved in the production of cyclic-guanosine 3, 5-monophosphate which further induces vascular relaxation[38].The involvement of the nitric oxide pathway was also investigated by pre-incubation withL-NAME, a nitric oxide synthase inhibitor[39].
Fractions were tested parallel in aortic rings preparations from normotensive rats. The Hh.n-hexane produced further relaxation with next higher concentrations. Chloroform and Hh.Aq were similar to the crude extract in potency in response toL-NAME, while Hh.EtAc produced endothelium-independent response.
In aortic rings from the hypertensive rats, crude extract and its fractions inhibited the vasorelaxant effect against phenylephrine precontractions. These results further support our finding that high salt damages endothelium[32].
To have insight into the effect of extract and fractions on vascular smooth muscle tone, rabbit aortic preparations were precontracted with phenylephrine and high K+. Both these agents increase intracellular level of Ca++from membrane receptors-operated and voltage-dependent Ca++channels[40,41]. Interestingly, the extract produced relaxation of high K+with more potency than phenylephrine like verapamil, a calcium channel blocker[42] and suggesting antagonistic effect on Ca++movements. A rightward shift in the concentration response curves of Ca++further confirmed these results. These findings suggest that extract induces vascular relaxation in part due to decreased Ca++movement from voltage-dependent Ca++channels, similar to typical Ca++antagonist verapamil. However, results also indicate that it might interfere with Ca++movement through receptors-operated Ca++channels. All fractions were tested under similar experimental conditions. Our findings indicate that all four fractions exhibited similar pattern with no prominent dominancy in potency. The vasorelaxant effects are also in agreement with the previous studies[18,19], which might contribute to the overall effectiveness ofH. helixin hypertension.
Total phenolic and flavonoid content tests were performed to check the profile of the tested extracts. Several factors such as climate,geographical conditions and maturity stage of plants may affect the composition of bioactive products and biological properties[43].Crude extract and its fractions indicated the presence of total phenols and flavonoids. Hh.EtAc showed the highest yield while Hh.n-hexane the least. Previous studies have shown that flavonoids and phenols exhibit cardiovascular protection and improvement of endothelial function[44,45]. Total flavonoids also possess Ca++channel blocking activity[46]. Therefore the content of total phenols and flavonoid might be responsible for antihypertensive effects of Hh.Cr. Previous studies have also shown that saponins are the major components ofH. helixextract[9]. The HPLC analysis of the crude extract for the determination of hederacoside C (saponin) was also performed. The results confirmed the presence of hederacoside C in the crude extract ofH. helix. According to previous literature,hederacoside C has vasorelaxant effect on smooth muscles [18,19], so it might be possible that it also contributes to fall in MAP ofH. helix.In conclusion, these results suggest that the extract ofH. helixpossesses antihypertensive activity. The underlying antihypertensive mechanism is through vascular relaxation, which is mediated by endothelium-dependent and -independent pathways. The former pathway includes NO pathway. Additionally, Ca++antagonisms explain its effect on vascular smooth muscle and justify its endothelium-independent effect in high salt-induced hypertension.The endothelium-dependent and -independent effects indicate that extract ofH. helixis a potent vasodilator which rightly explains the antihypertensive efficacy of this important herbal drug. The phenols, flavonoids and saponin in extracts ofH. helixmay further strengthen our finding. Further studies would be interesting to isolate, differentiate and molecularly probe the underlying pathways and clinical potential of this important herbal drug.
Conflict of interest statement
The authors report no conflict of interest.
Acknowledgements
We would like to thank Dr. Muhammad Ihtisham Umar, Assistant Professor, COMSATS University Islamabad, Abbottabad campus,KPK, Pakistan for his support in statistical analysis.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Potential applications of lactic acid bacteria and bacteriocins in antimycobacterial therapy
- Protective effect of Ocimum sanctum Linn. leaf extract on ethanol withdrawal syndrome in Wistar rats
- Comparative phytochemical analysis of Coffea benghalensis Roxb. Ex Schult, Coffea arabica L. and Coffea liberica Hiern.
- Chemical compositions and biological activities of essential oils obtained from some Apiaceous and Lamiaceous plants collected in Thailand
- Molecular detection of Leishmania species in human and animals from cutaneous leishmaniasis endemic areas of Waziristan, Khyber Pakhtunkhwa, Pakistan
