Chemical compositions and biological activities of essential oils obtained from some Apiaceous and Lamiaceous plants collected in Thailand
2018-09-08NawanitThanaseelungkoonJakaphunJulsrigivalKulwadeePhannachetSuneeChansakaow
Nawanit Thanaseelungkoon, Jakaphun Julsrigival, Kulwadee Phannachet, Sunee Chansakaow✉
1Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand
2Department of Microbiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
Keywords:Essential oil Apiaceae Lamiaceae Antioxidant activity Antityrosinase activity Antibacterial activity
ABSTRACT Objective: To determine the chemical composition, as well as the antioxidant, antityrosinase and antibacterial activities of essential oils obtained from some Apiaceous and Lamiaceous plants collected in Thailand. Methods: The essential oils of the specified spices and aromatic herbs were obtained by hydro-distillation, and their chemical constituents were analyzed by gas chromatography/mass spectrometry. Antioxidant assays were based on the scavenging effects of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) free radicals as well as the lipid oxidation inhibition of β-carotene bleaching by linoleic acid. Tyrosinase enzyme inhibition was evaluated by the dopachrome method. Broth microdilution technique was performed for the purposes of studying microbial growth inhibition against the isolated bacterial strains. Results: The essential oils of Elsholtzia stachyodes, Coleus amboinicus (I) and Trachyspermum ammi presented a high degree of potency in DPPH, ABTS and β-carotene bleaching assays. The Trachyspermum ammi oil, which mainly contained thymol (49.04%) and p-cymene (22.06%), proved to be the most effective in terms of antibacterial activity. The major compositions of Coleus amboinicus (I) were carvacrol(51.57%), γ-terpinene (18.04%) and p-cymene (7.81%); while thymol (43.76%) and γ-terpinene(24.61%) were identified as the major components of Elsholtzia stachyodes oil, with p-cymene(6.73%) being identified as a minor constituent. Moreover, Cuminum cyminum oil containing cuminaldehyde (49.07%) and Elsholtzia communis oil composed with geranial (44.74%) and neral (35.27%) as the major components displayed a specific ability for the inhibition of the mushroom tyrosinase enzyme. Conclusions: The results indicated that these bioactive essential oils obtained from indigenous herbs are of significant interest as alternative raw materials in food, cosmetic and medicinal products.
1. Introduction
In the food industry, preservatives are used to protect against oxidants and foodborne microorganisms and to promote shelf-life stability. With regard to cosmetic products, the conditions associated with skin-aging and tanning have been treated with antioxidants and tyrosinase inhibitors. The natural bioactive compounds are now considered as an alternative material in both food and cosmetic industries due to the perception on their safety. Essential oils are the natural products, which feature with solvent-free distillation process and may apply as the bioactive ingredient or flavoring agent in the product formulation. According to the literature review, the essential oils that are composed of phenolic compounds seem to display a potential for the antioxidant, antityrosinase and antibacterial activities[1-11], because the percentage of major component in the essential oils affected individual odor and biological activities[2].The spices and aromatic plants within the Apiaceae and Lamiaceae families frequently produce essential oils with phenylpropanoids that are of significant interest for their potential bioactivities[2], because the molecular structure of phenylpropanoids is relative to phenolic compounds. There have been many reports on phytochemicals and the biological activities of essential oils obtained from both these families in different countries[2,9-11]. However, only a few studies have been conducted on antityrosinase enzyme activity[12,13]. We intend to perform and record the steps involved with providing scientific information on certain regional spices and herbs for further selection of effective raw materials to be used in the food, cosmetics,and medical industries. This study aims to determine the chemical composition and antityrosinase enzymes, as well as the antioxidant and antibacterial activities of the essential oils obtained from someApiaceousandLamiaceousplants collected in Thailand, particularly with regard to the indigenous spices and aromatic plant species that are widely used in northern Thailand.
2. Materials and methods
2.1. Plant materials
The dried fruits of fiveApiaceousplants comprised ofAnethum graveolens(A. graveolens) L.,Cuminum cyminum(C. cyminum)L.,Foeniculum vulgare(F. vulgare) Miller subsp. var. vulgare,Heracleum siamicum(H. siamicum) Craib. andTrachyspermum ammi(T. ammi) L. Sprague were purchased from herbal drug stores located in Bangkok (forA. graveolens,C. cyminum, andF. vulgare), Nakhon Pathom (T. ammi), and Chiang Mai (H. siamicum) Provinces,Thailand. The identification process was performed by taxonomists and cross-referenced with the relevant Thai herbal standardization references[14]. Additionally, aerial parts of sevenLamiaceousplants were collected from Mae Hong Son and Chiang Mai Provinces,Thailand. Identification ofColeus amboinicus(C. amboinicus) Lour. (Ⅰ),C. amboinicusLour. (Ⅱ) andHyptis suaveolens(H. suaveolens)(L.) Poit. References voucher number 004138 were compared with the voucher specimens in the Museum of the Faculty of Pharmacy,Chiang Mai University, Thailand. The remainingLamiaceousplants includedElsholtzia communis(E. communis) (voucher number WP5172),Elsholtzia griffithii(E. griffithii) (WP3707),Elsholtziakachinensis(E. kachinensis) (WP3723) andElsholtzia stachyodes(E.stachyodes) (WP552). These were identified by taxonomists and the voucher specimens were deposited at the Herbarium of the Faculty of Sciences, Chiang Mai University, Thailand.
2.2. Essential oil extraction
The selected plant parts were extracted by hydro-distillation using Clevenger-type apparatus for 3-5 h. The essential oils were dried with anhydrous sodium sulfate and kept in well-closed containers with light resistance at 4 ℃ for further use.
2.3. Essential oils chemical composition
A total of 0.5% v/v sample of essential oil suspended in absolute ethanol 1 µL (split ratio 1:20) was injected into the gas chromatography/mass spectrometry (GC/MS) equipped with electron impact ionization and mass-selective detector (Shimadzu Model QP 2010 Plus, Japan). The Agilent DB-5MS capillary column (5% phenylmethyl polysiloxane, 30 m × 0.25 mm, 0.25µm film thickness) was used with helium as a carrier gas (1.0 mL/min). The injector temperature was set at 250 ℃ and 230 ℃ for theApiaceousandLamiaceoussamples, respectively; whereas the detector temperature was set at 250 ℃. The ionization energy was 70 eV in EI mode with ion source temperature 200 ℃, scan mass mode and interface temperature 250 ℃. The temperature program forApiaceousanalyses was initiated at 70 ℃ and held for 1 min. The system was then programmed to 120 ℃ with a rate of 3 ℃/min and held for 2 min. After that, the system was programmed to 160 ℃ at 5 ℃/min and held for 1 min. Finally, the temperature was ramped up to 230 ℃ with 10 ℃/min rate and kept constant for 5 min. For theLamiaceoussamples, the analysis program was performed according to the method described by Moreiraet al.[15].
The volatile components were identified using the computer matching method and by comparisons of mass spectra with WILEY 7 and NIST 2005 Library. The Kovats retention indices were also calculated with a series ofn-alkanes (C8-C20) and compared with the relevant literature data-NIST Chemical Web Book and Adams[16].
2.4. Antioxidation activity assays
2.4.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The method that was employed was modified from Sritularaket al.[17]. Briefly, the essential oils were prepared and tested at 100 mg/mL in absolute ethanol. The samples that showed more than 50% inhibition will be determined for their concentration at 50% inhibition (IC50) value. The test was performed in a 96-well microtiter plate by the sample addition (50 µL) to about 150µM DPPH in absolute ethanol (180 µL) (DPPH, Aldrich). The measurement of absorbance at 510 nm was completed using a microplate reader (BMG LABTECH Model SPECTROstar Nano,Germany) after incubation of the reaction mixture was done in the dark at room temperature for 30 min. The percentage of DPPH scavenging activity was calculated using the following equation:
Scavenging activity (%) = [(Acontrol– Asample/ Acontrol) × 100]
where Acontrolrepresents the absorbance of the control that contained all reagents except the test sample, and Asamplerepresents the absorbance of a test sample. Butylated hydroxytoluene (BHT, Fluka)and 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid(Trolox, Aldrich) were used as the positive control.
2.4.2. 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging assay
The test was previously performed by Liet al.[18]. Following a similar process that was used to determine DPPH assay, 100 mg/mL of a sample in absolute ethanol was tested and calculated for ABTS scavenging activity percent (ABTS, Sigma) using the same equation mentioned in 2.4.1. The samples that showed more than 50%inhibition would be determined for the IC50value. BHT and Trolox were also used as the positive control.
2.4.3.β-carotene-linoleic acid bleaching assay
Some modifications were made to the method described by Wanget al.and the procedure was performed[19]. Briefly, 0.5 mg/mL of β-carotene (Sigma) in chloroform was mixed with 25 µL linoleic acid (Sigma) and 200 mg Tween 40. After that, chloroform was removed using nitrogen gas. And the remaining part was mixed with 50 mL of oxygenated distilled water. The mixture emulsion (180 µL)and test sample (20 µL) were placed in a 96-well microtiter plate and incubated at 50 ℃ for 2 h. A level of absorbance at 460 nm was measured at the times of 0 h and 2 h. The percentage of antioxidant activity (AA) was calculated in terms of the bleaching of β-carotene using the following equation[18]:
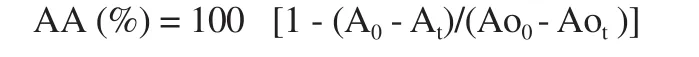
where Ao0and Aotrepresent the levels of absorbance of the control at 0 h and 2 h, and A0and Atrepresent the levels of absorbance of the test sample at 0 h and 2 h, respectively. Screening of the samples was done at 10 mg/mL in ethanol. The samples that showed more than 50% AA would be determined for the AA50value. BHT and Trolox were also used as the positive control.
2.5. Antityrosinase enzyme activity assay
The dopachrome method that was modified from Solimineet al.was used[20]. The screening test of a sample was performed at a concentration of about 3 mg/mL of the samples in 10% of Tween 20 in 10 mM phosphate buffer pH 6.8 (PBS). The test sample(60 µL), PBS (70µL) and tyrosine solution (0.4 mg/mL in PBS)(70 µL) (mushroom tyrosinase, Sigma) were added to a 96-well microtiter plate. The reaction was then initiated with tyrosinase enzyme solution (250 units/mL in PBS) (10 µL) and the sample was incubated at 35 ℃ for 20 min. Absorbance was measured at 480 nm using a microplate reader. Percentage inhibition was calculated using the following equation:

where A represents the absorbance of the control that contained all reagents except the test sample, and B represents the absorbance of the test sample. The samples indicated that about 50% inhibition could be used to determine the IC50value. Arbutin (Alfa Aesar) and Kojic acid (Sigma) were used as the positive control.
2.6. Antibacterial activity assay
2.6.1. Bacterial culture
The isolated bacterial species used in this study are listed as follows: gram-positive bacteria [SAU25923:Staphylococcus aureus(S.aureus) ATCC 25923, R34: methicillin-resistantS. aureus(MRSA)and S156: methicillin-sensitiveS. aureus(MSSA)], gram-negative bacteria [ECO25922:Escherichia coli(E. coli) ATCC 25922, CREN27: carbapenemase-producing E. coli, KPN-P-16: carbapenemsensitiveKlebsiella pneumoniae(K. pneumoniae) P-16 and CRE-105:carbapenemase-producingK. pneumoniae].
2.6.2. Determination of minimum inhibitory concentration
Broth microdilution technique was performed for minimum inhibitory concentration according to the procedure described by the Clinical and Laboratory Standard Institute, the National Committee for Clinical Laboratory Standards and Eloff[21-23]. The stock solutions of essential oil (1 g/mL) were dissolved in dimethyl sulfoxide and five-fold serial dilution (0.8-500 mg/mL) of each sample was prepared. The suspension of microbial strains was standardized with 0.5 McFarland (1.0×108CFU/mL) and diluted to 2.0×105CFU/mL with Mueller Hinton broth (MHB, Labscan Asia). Into a 96-well microtiter plate, 45 µL of MHB, 5 µL of a test sample and 50 µL of microbial suspension (final concentration 1.0×105CFU/mL) were added. The turbidity of microbial growth was determined after being incubated at 37 ℃ for 16-20 h and the minimum inhibitory concentration was recorded. In order to confirm that there was no bacterial growth in the wells with the clear solution,a confirmation test was conducted. Briefly, MHB with glucose and phenol red were added into each well and they were incubated at 37℃ for 4 h. If bacterial growth was present that could not be detected by the naked eye, the acid metabolic products of glucose metabolism produced from the bacteria will change the color of the phenol red from orange to yellow[24]. The experiments for each test sample were done in duplicate. Sterility control, growth control and effect of DMSO against bacterial strains were tested in this experiment as well.
2.7. Statistical analysis
The screening results were expressed as the average ± standard deviation (SD) of three or five replicates. One-way-ANOVA was performed for analysis of variance and Duncan’s test was used to determine the essential oil effects at the significant level ofP<0.05.The SPSS program was used for the statistical analysis. The IC50value of each sample was determined by the linearity plotted (R2≥0.990) between the average of percentage inhibition from five replicates versus the sample concentrations (at least five points), the process was done on the Microsoft Excel program.
3. Results
The percentage of essential oil yields fromApiaceousdried fruits ranged from 1.03 to 2.07 and 0.03 to 0.63 for the aerial parts ofLamiaceousplants. GC-MS analysis of the essential oils determined that the major components of both families were monoterpenes.Sesquiterpenes were found at high quantities in the Lamiaceae family as well. Whereas phenylpropanoids (e.g.estragole,trans-anethole,dillapiole, myristicin and elemicin) were higher inApiaceousoils than in theLamiaceoussamples. The acylfuran derivatives were only found in twoElsholtziaspecies(E. griffithiiandE. kachinensis). The percentage of major compounds and Kovats retention indices related ton-alkanes (C8-C20) on DB-5MS column in this experiment are presented in Table 1 and Table 2.
Figure 1 and Table 3 present antioxidant activities of the essential oils measured by DPPH, ABTS and β-carotene bleaching assays.The screening results revealed that the antioxidant abilities ofT.ammi,C. amboinicus(Ⅰ),C. amboinicus(Ⅱ) andE. stachyodesessential oils were found in both radical scavenging assay and β-carotene bleaching method. The inhibition of β-carotene bleaching resulted from the free radical scavenging effect on peroxide radicals,which was self-generated by linoleic acid at high temperatures[18].Among these essential oils,E. stachyodesrepresents the lowest IC50values in DPPH assay, whileC. amboinicus(Ⅰ) andT. ammiare the most potent in the ABTS method and β-carotene bleaching assay,respectively. Although the screening results of antityrosinase enzyme activity in this experiment were rather low (less than 10% inhibition),the essential oils ofC. cyminum(58.91% ± 2.90% inhibition) andE.communis(47.44% ± 3.56% inhibition) at the tested concentrations were significantly in the enzyme inhibition with an IC50value of 2.239 mg/mL and 3.236 mg/mL while the IC50values of Arbutin and Kojic acid were 0.852 mg/mL and 0.013 mg/mL, respectively.
Antibacterial activity with minimum inhibitory concentration values of the essential oils fromApiaceousandLamiaceousplants were revealed in Table 4. The results indicated that essential oils fromF. vulgare,T. ammi,C. amboinicus(Ⅰ),C. amboinicus(II),E. stachyodes, andE. communiswere more effective than theother oil samples.T. ammioil was found to be the most effective antibacterial.C. amboinicus(Ⅰ) andE. stachyodesoils were able to inhibit the same bacterial strains at equal minimum inhibitory concentration values. The bacterial strains used in this experiment can be grouped into carbapenem and methicillin-sensitive strains(ECO25922, KPN-P-16, SAU25923 and S156), carbapenemaseproducing strains (CRE-N27 and CRE-105) and methicillinresistant strains (R34). Based on the antibacterial activity against different bacterial groups, essential oils fromT. ammi,C.amboinicus(Ⅰ),C. amboinicus(II), andE. stachyodesreported low minimum inhibitory concentration values and displayed activity against not only carbapenem and methicillin-sensitive strains, but also carbapenemase-producingK. pneumoniae(CRE-105) and methicillin-resistantS. aureus(R34) as well.

Table 1 The percentage area of the major compounds with the experimental Kovats retention indices (KIE) in the essential oils from Apiaceous plants.
4. Discussion
C. amboinicus(Ⅰ) andC. amboinicus(Ⅱ) are aromatic plants and are usually used as side dishes in northern Thailand.C.amboinicus(Ⅰ) leaves are green whileC. amboinicus(Ⅱ) leaves are both green and white. This study shows the differences between theC. amboinicus(Ⅰ) andC. amboinicus(Ⅱ) essential oils in both biological activities and compositions. The antioxidant and antibacterial activities were found to be different due to their chemical profiles.C. amboinicus(Ⅰ) oil contained carvacrol(51.57%), γ-terpinene (18.04%) andp-cymene (7.81%), whereasC.amboinicus(Ⅱ) oil components were 3-carene (19.75%), carvacrol(17.78%), camphor (15.29%), γ-terpinene (8.54%) andp-cymene(4.59%). A higher amount of phenolic compound (carvacrol)increased the potential of antioxidation capacity. In addition, the synergistic effect of carvacrol andp-cymene also increased the level of inhibition of microbial growth. The hydrophobicity ofp-cymene clearly incorporated the lipid bilayer and the transportation of carvacrol into the cell[25]. Disturbances of the cell membrane’s physical structure lead to destabilization of the membrane’s permeability[9].

Table 4 Antibacterial activity with minimum inhibitory concentration (MIC) of the essential oils from Apiaceous and Lamiaceous plants (mg/mL).
Acetophenone, caryophyllene oxide, and β-caryophyllene were reported as being widely distributed in the essential oil ofElsholtziaspecies[26]. According to the previous study, carvacrol was identified as the main compound ofE. stachyodesoil obtained from Mae Hong Son and Chiang Mai Provinces, at 55.81% and 43.75%respectively[27]. In contrast to the findings of this study, thymol(43.76%) and γ-terpinene (24.61%) have been identified as the major components ofE. stachyodesoil, whilep-cymene (6.73%), α α-terpinene (6.15%) and Z-ocimene (3.84%) were considered the minor constituents. Similarly to that which was reported by Phetsanget al., the oxygenated monoterpenes that consisted of geranial(44.74%) and neral (35.27%) were the most abundant in theE.communisoil[27]. In the present report,E. griffithiiandE. kachinensisoils mostly composed of acylfuran derivatives, β-dehydroelsholtzia ketone (79.96% and 85.24%) and elsholtzia ketone (10.66%and 1.78%). Phetsanget al.reported that elsholtzia ketone was represented as dominant at 83.87%, however β-dehydroelsholtzia ketone and β-caryophyllene were also found in theirE. griffithiioil samples in significant amounts[27]. This study has identified the major compounds ofH. suaveolensoil, which are sabinene (19.30%),1,8-cineole (16.28%) and β-caryophyllene (11.81%). This was found to be similar to NigerialH. suaveolensoil which contained sabinene(16.5%) and β-caryophyllene (19.8%) as major compounds[28],while spathulenol (7.7%) and bicyclogermacrene (6.5%) that were present in TanzanialH. suaveolensoil, were absent in this study[29].The effects related to plant source, environmental factors, harvesting time, extraction methods and analysis techniques are remarkably important factors and play an important role in the differentiation of the chemical profile of plants[26].
Among theElsholtziaspecies,E. communisandE. stachyodesoils were also tested for bacterial growth inhibition. Phenolic compounds such as thymol are able to present both antioxidative and antibacterial activities, a combination withp-cymene also presented the synergism of the antibacterial properties ofE. stachyodesoil.Generally, gram-positive strains are more susceptible to antibacterial agents than gram-negative bacteria, due to the production of lipopolysaccharides covering the cell wall of the gram-negative bacteria. This facilitates the restriction of hydrophobic compound diffusion. Citral isomers, such as neral and geranial and other related compounds have been reported for their effectiveness against postharvest pathogenesis fungal strains[30]. These aldehyde constituents are able to form a covalent bond to nucleophile substances and can cause a malfunction in the microorganism’s cells, in a similar way as geranial and neral of theE. communisoil affecting the gram-positive tested strains.
Moreover, the mixture of geranial and neral (citral) obtained from citrus essential oil has been identified as a noncompetitive tyrosinase inhibitor[31]. This result also confirmed the activity of antityrosinase enzymes ofE. communisoil. In 1998, cuminaldehyde,a benzaldehyde derivative, was investigated as a potent mushroom tyrosinase inhibitor obtained from cumin extract. The mechanism of competitive inhibition was described in greater detail 3 years later as being the most potent agent of inhibition among the other 4-substituted banzaldehydes[32,33]. Competitively, the aldehyde group of cuminaldehyde reacts directly to the primary amino group of the enzyme while a non-competitive inhibitor can react only to the enzyme-substrate complex[7].C. cyminumoil containing cuminaldehyde (49.07%) as the major compound, which is similar to Tunisian cumin oil[34], is also of interest as a tyrosinase inhibitor.There have been various studies on the inhibitory effects ofC.cyminumoil against different bacterial strains[10], which is in contrast to the low efficacy results in this study.
T. ammioil has been found to be the most effective in terms of its antioxidation and antibacterial activities when compared with otherApiaceousspecies. Its major components are the phenolic compound [thymol (49.04%)] and terpene hydrocarbon [p-cymene(22.06%) and γ-terpinene (21.23%)]. In previous reports, these three compounds were recognized as the main constituents of the seed samples[35,36]. The biological activities that were displayed against bacterial and fungal strains were also observed in the different fractions ofT. ammioil. An increase in the amount of thymol could promote a higher level of efficacy, likewise the synergism of thymol and carvacrol was also found to be present with a small amount of carvacrol[36]. The synergism of thymol andp-cymene is emphatically mentioned in these experimental results.
The oxygenated monoterpenes: carvone (32.70%),cisdihydrocarvone (20.55%) andtrans-dihydrocarvone (10.42%);phenylpropanoids: dillapiole (12.57%) and monoterpene: limonene(20.67%), have been identified as the major compounds ofA.graveolensoil. This is in agreement with previously published research reports. Additionally, carvone (55.2%), limonene (16.6%)and dillapiole (14.4%) were the major compounds of the oil, while linalool,trans-dihydrocarvone andcis-dihydrocarvone were the minor components[37]. Carvone was reported to be ineffective to the outer membrane ofE. coli, whereas only a few studies have described the disturbance of the metabolic energy state of the bacterial cells[9].Thus, the antibacterial effect of this oil was considered to be quite low, except against the R34 strain.
In this study,F. vulgareoil presented a moderate effect in terms of its antibacterial properties. It is composed of the following major constituents;trans-anethole (54.78%), estragole (16.40%) and α-fenchone (13.60%). A previous study onF. vulgareoil fraction demonstrated that the oil fraction is with higher percentages of anethole and estragole, but lower percentages of fenchone, was found to display the most effective antifungal activity. Anethole was identified as the major compound with the most significant antifungal effect. Even though fenchone showed a moderate level of antifungal activity, it did not respond to the total oil potential effect.Moreover, the synergism of the total oil may be present in the active compound even at small concentrations[38].
H. siamicumis also known as Malabe and is considered a local spice in northern Thailand. The major compounds ofH. siamicumoil are octyl acetate (46.55%), β-pinene (16.31%), γ-terpinene (10.75%)andp-cymene (6.67%). These compounds can be found in some IranianHeracleumspecies as minor constituents[39]. Similarly toH.siamicumthat was collected from a market in Chiang Mai Province,Thailand in January 2008, octyl acetate (65.30%) was reported as the major compound of this oil[40].
In conclusion, the essential oils with a higher distribution of the phenolic compounds thymol, carvacrol and terpene hydrocarbonp-cymene in the samples displayed greater potential in terms of both their antioxidative and antibacterial activities. The beneficial findings in terms of the antioxidative effects of these substances are related to their active anti-aging properties as well as their antibacterial affects. These antibacterial effects can preserve the stability of their formulation against bacterial strains and reduce the number of synthetic preservatives that need to be added to the products. The essential oils ofT. ammi,C. amboinicus(Ⅰ),C. amboinicus(Ⅱ) andE. stachyodesdemonstrated their antioxidant ability through the free radical scavenging effect.T. ammioil was also identified as possessing the most active antibacterial agent against both gram-negative/gram-positive and sensitive/resistance pathogens, subordinated byC. amboinicus(Ⅰ) and E. stachyodes oils. Although the species of theC. amboinicusplant was the same, the appearance of the plant,chemical profile, odor and also the biological activities of theC.amboinicus(Ⅰ) andC. amboinicus(Ⅱ) oils demonstrated different results. The plant that will be used as a source of an essential oil should be carefully selected, identified and documented. Besides,C. cyminumoil containing cuminaldehyde andE. communisoil with citral (geranial and neral) is of significant interest as they are considered the major components and have been as a source of the moderated mushroom tyrosinase inhibitor. Finally, the results of this study can significantly support the existing scientific data as well as add to the value of these bioactive essential oils that have been obtained from indigenous plants. The essential oils can be seen as promising alternative raw materials for use in foods, cosmetics and medicinal products.
Acknowledgments
The research project was supported by the grants from the Utilization and Genetic Conservation of Local Plants in Complementation to the Plant Germplasm Conservation Project of H.R.H. Princess Maha Chakri Sirindhornd (No.270363) and the general supports from Department of Pharmaceutical Sciences,Faculty of Pharmacy and Graduate School, Chiang Mai University,Chiang Mai, Thailand. Grateful appreciation is also to Asst. Prof.Dr.Angkhana Inta; Faculty of Science Chiang Mai University, for providing the plant samples and identification.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Potential applications of lactic acid bacteria and bacteriocins in antimycobacterial therapy
- Protective effect of Ocimum sanctum Linn. leaf extract on ethanol withdrawal syndrome in Wistar rats
- Antihypertensive efficacy of extract of Hedera helix in high salt-induced hypertensive Sprague-Dawley rats
- Comparative phytochemical analysis of Coffea benghalensis Roxb. Ex Schult, Coffea arabica L. and Coffea liberica Hiern.
- Molecular detection of Leishmania species in human and animals from cutaneous leishmaniasis endemic areas of Waziristan, Khyber Pakhtunkhwa, Pakistan
