Electroless Ag deposition on the cell walls of carbon foam by a displacement method
2018-08-30GUOCongcongLIUXiujunLITongqiLEIYanZHAILili
GUO Cong-cong, LIU Xiu-jun, LI Tong-qi, LEI Yan, ZHAI Li-li
(1.School of Environmental and Chemical Engineering, Tianjin Polytechnic University, Tianjin300387, China; 2.National Key Laboratory of Advanced Functional Composite Materials, Aerospace Research Institute of Materials and Processing Technology, Beijing100076, China)
Abstract: To obtain efficient metalization of the surface and cell walls of carbon foam, an electroless silver displacement deposition method was investigated, in which a copper layer was first electroless-deposited and then displaced by silver. The purity of the silver coating, and the morphology, microstructure, mechanical strength and electrical conductivity of the resulting carbon foams were characterized by SEM, EDS, XRD, mechanical and conductivity tests. Results indicate that a uniform and continuous silver film is formed on the cell walls of the carbon foam with a better adhesion to the carbon compared to that obtained using the traditional one-step silver plating method. The coating is crystalline silver without copper or silver oxide. The conductivity and mechanical strength of these carbon foams increase from 700 to 2 055 S/cm and 0.54 to 1.05 MPa, respectively when the silver content is increased from 0 to 15.5 wt%.
Keywords: Bulk carbon foam; Electroless copper plating; Silver electroless replacement deposition
1 Introduction
Carbon foam is a kind of carbon material with certain features, whose microstructure looks like sponge. It was firstly made to obtain reticulated vitreous carbon (RVC) or carbonaceous skeleton by the pyrolysis of thermosetting polymer foam. With the development of technology, a series of carbon foams was developed by using different precursors, including coal tar pitch, coal and petroleum pitch. The dilatation and fluidity of precursors affect significantly the foaming performance and foam structure[1, 2]. Most of them have various properties such as high thermal conductivity[3, 4], large surface area, low thermal expansion coefficient, light weight and vibration damping. Their properties made them enable to use as sandwich material, heat conduction material, energy storge electrode and so on. However, the low thermal conductivity and mechanical properties of carbon foams limited their applications to a certain degree. So it is the key to study on the modification of carbon foam to improve their properties. With the carbon foam composited with metal, integrated property could be improved by making full use of advantages of each component materials.
Metal coating has been utilized commercially to enhance mechanical properties of composites. Nonmetal materials are exploited as substrates, and they are coated with metals or non-metallic materials via electroless plating, sputtering deposition[5-7], chemical vapor deposition[8]. Several metals such as copper, silver, nickel have been deposited on these materials[9-11]. Controlling factors related to the stability and rate of electroless copper plating has been investigated by Hanna[12]. Moreover, Yoshio Kobayashi et al. reported that a homogeneous membrane of silver nanoparticles was deposited on the surface of colloid silica spheres through pretreatment in electroless plating[13]. Yu et al. developed a method to prepare silver plating on aramid fiber with a good adhesion strength and conductivity by using crosslinked chitosan (CS) with —NH2and —OH functionalities as a chelator. When silver was deposited on carbon fibres, the fibres with a higher degree of graphitization was coated faster[14]. Warrier[15]designed a physical model to analysis the nucleation and growth of the coating.
The technology of electroless plating on nonmetal surface has been well developed. But in this report, we want to deposit silver on the inner wall of bulk carbon foam. That’s hard to pull off, because of the influence of pressure difference, flow rate, reaction rate and other factors. Traditional electroless silver plating always used methanol, glucose, tartarate or dimethylamine broane as a reducing agent[15]. Firstly, silver nitrate reacted with ammonia to form a complex ion. Next, the solvent was exchanged by reduction reaction, followed by depositing silver on substrate. In this work, a new method was developed including mainly two steps. First, Cu particles were reduced on the inner surface of carbon foams. Second, Cu particles were substituted by Ag by the reactive replacement. Under the assistance of vacuum and ultrasonic, the inner pore of bulk carbon foams can also be coated with uniform and continuous silver-coating by this method. The effective availability of silver was greatly increased, conductivity and compression strength of bulk carbon foams were improved simultaneously.
2 Experimental
The substrate used in this study is carbon foam (average porosity of 70%, average pore size of 100-200 μm) with a size of 30.00×30.30×50.00 mm3. The carbon foam was prepared from mesophase pitch and graphitized. Before replacement deposition, the carbon foam was pretreated by degreasing, roughening, sensitization and activation. The pretreatment conditions are listed in Table 1.
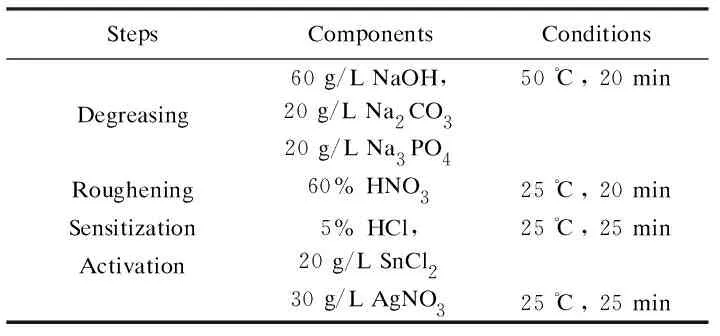
Table 1 Compositions and conditions of each step during the pretreatment.
There are two steps of silver electroless replacement deposition. First, copper was deposited on the inner pore surface of carbon foam by electroless plating. The thickness of coating was controlled by appropriate reaction conditions. And then copper was replaced with silver by replacement deposition. The detailed parameters are summarized in Table 2.
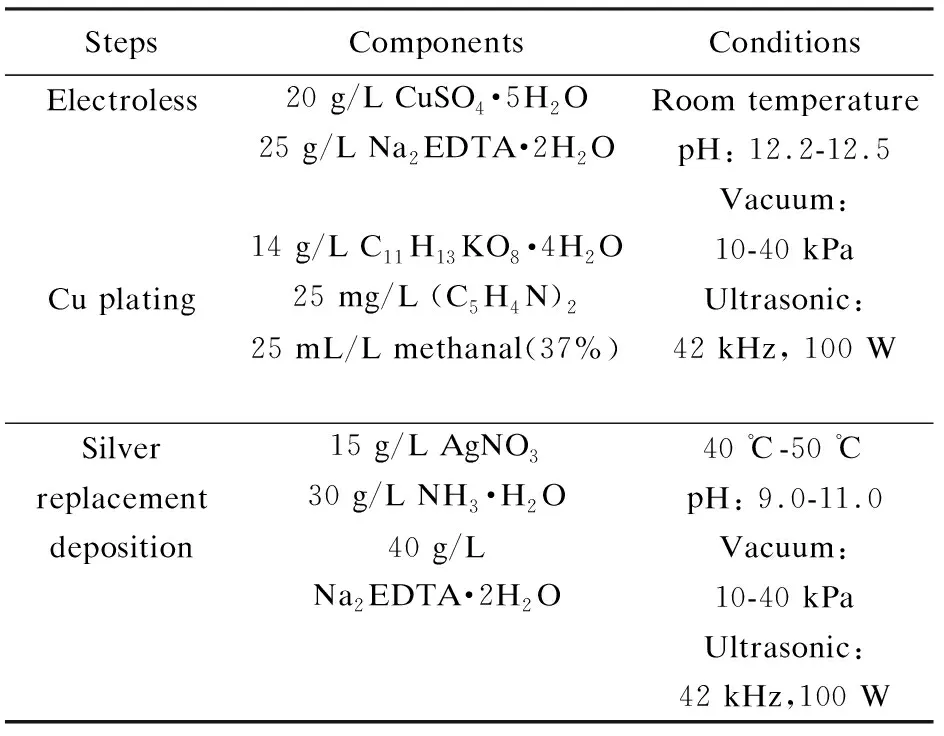
Table 2 Compositions and conditions during the silver electroless replacement deposition.
According to the electroless plating mechanism, the reaction of Cu plating could be expressed as follow:

+2H2O+2HCOO-
(1)
Replacement deposition on the inner wall of bulk carbon foam could be shown by:

(2)
Considering the effect of the complexing agent, the overall reaction in replacement deposition could be represented by:

(3)
Where, L denotes the complexing agent, Na2EDTA·2H2O or NH3·H2O.
Surface morphology of sample was observed by a scanning electron microscope of Quanta 200 manufactured by FEI. Chemical compositions on the surface and inner wall of bulk carbon foam was examined by energy dispersive spectroscopy. The analysis of crystal structure was carried out by X-ray diffraction. The conductivity was evaluated by a SZ85-digital four-probe conductivity meter. The compression tests were measured by a CMT4502-Electronic universal testing machine.
3 Results and discussion
From the view point of thermodynamic, the reducing power of a reactant is determined by its standard potential. Silver has a high standard potential, which produces a large potential difference between silver and a reducing agent (eg. methanol, glucose, tartarate). It makes the redox reaction drastic. In the traditional electroless silver plating, a thin silver film appears almost instantly on the surface of carbon foam at the beginning of deposition. Most of the silver ions take part in the silver mirror reaction, and are deposited on the inner wall of the beaker. The rapid deposit and reaction in solution may result in a severity of skip plating, causing a plating solution decomposition and forming black heart on the surface of carbon foam. Because copper has a lower standard potential than silver, the reduction reaction happens smoothly, which can be easily controlled by adjusting the temperature, pH, concentration of alkali solution or the other reaction conditions. Therefore, the inner pore of bulk carbon foam is first deposited with a compact, homogeneous copper film. Then, a uniform silver layer is formed on the inner surface of the carbon foam by a silver electroless replacement deposition. In this two-step method, the reduction reaction can be easily controlled, a uniform silver film is formed on carbon foam and the effective availability of silver can be greatly improved.
The surface morphology of silver-coated bulk carbon foam obtained by the direct electroless silver plating is shown in Fig. 1a,c,e. Fig. 1a shows the existing skip plate problem. Fig. 1c shows the rough surface of coated carbon foam. Fig. 1e shows the weak binding force between silver particles and carbon foam. In Fig. 1b,d,f, the morphology of silver-coated carbon foam obtained by the replacement deposition is shown. It can be seen from Fig. 1b that a layer of uniform film is covered on the surface, and there is no skip plate existed. As show in Fig. 1d the homogeneous silver layer is coated on the carbon surface. Fig. 1f illustrates the well adhesion between silver and carbon foam. In replacement deposition, the effective availability of silver exceeds sixty percent.
Fig. 2a shows the internal morphology of bulk carbon foams obtained by the direct vacuum and ultrasonic co-assisted electroless silver plating method. Fig. 2b shows the morphology by the replacement deposition method. The thickness of the sediment is about 3.4 μm for the replacement deposition as shown in Fig. 2c. It is clearly demonstrated in Fig. 2b that the silver layer is overspread completely on the inner pores. In contrast, there are few silvers to scatter on the wall of foam cells as shown in Fig. 2a.
From the view point of dynamics, the diffusion rate of silver should be larger than its deposition rate to get a uniform film over pore. We use ultrasonic stirring to achieve smooth exchange across the inner pore in the plating[16]. Cavitation effect, heating effect and mechanical effect are main functions of ultrasonic. Its cavitation effect could break the bubbles on the surface and inner wall of bulk carbon foam. The agglomeration of metal particles could dramatically be decreased by mechanical effect. Accordingly, the dispersion of particles in plating solution can be improved. In addition, the disturbance and friction of ultrasonic have a positive effect on cleaning the surface, which are able to remove the plating solution in foam pores. In fact, the ultrasonic do not play an effective role in improving the deposition rate in the traditional electroless plating. Through analyzing, the main reason is that the disturbance could not remove the plating solution timely, which results in a negative effect on its stability. A large amount of silver particles is separated out into plating solution while the deposition occurring inside carbon foam is slowed down. It’s difficult to uniformly coat the wall of foam cells with a silver layer by electroless plating. But, in the replacement deposition, the ultrasonic stirring is helpful. In copper deposition, the ultrasonic could break the hydrogen produced by reaction and remove them from the inner. After a compact and uniform copper layer is obtained, a high quality silver layer could be much more easily to get by replacement reaction.
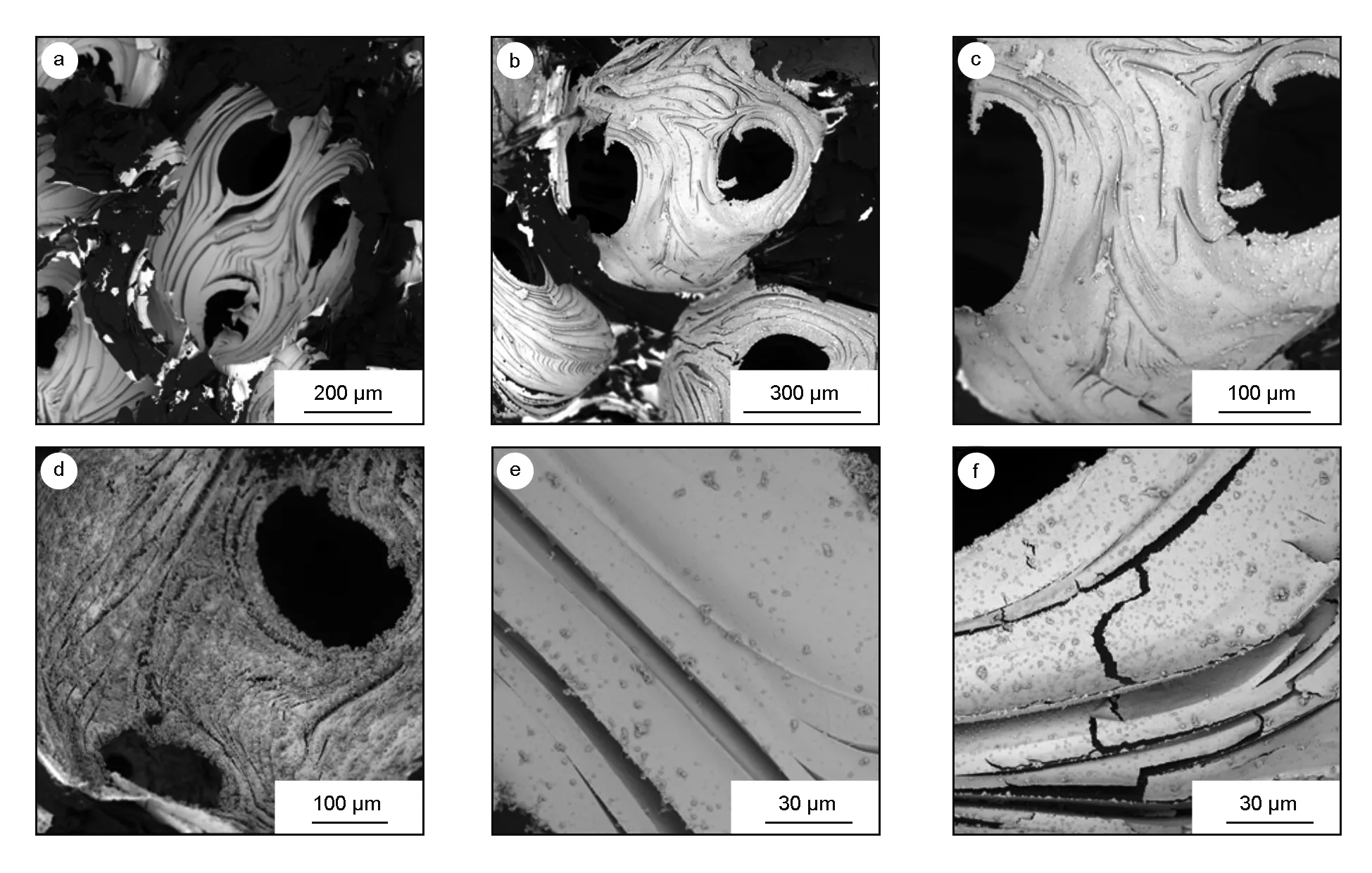
Fig. 1 SEM images of morphology of silver coatings: (a, c, e) obtained by the direct electroless plating and (b, d, f) obtained by the replacement deposition.
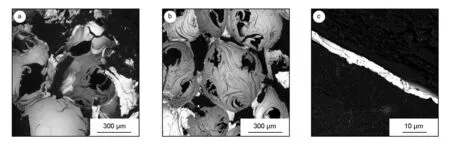
Fig. 2 SEM images of morphology of bulk carbon foams: (a) direct elecroless plating, (b) replacement deposition and (c) the thickness of silver coating obtained by the replacement deposition.
Fig. 3 shows the XRD patterns of the carbon foams obtained before (Fig. 3b) and after silver electroless replacement deposition (Fig. 3a). The peaks at 38.12°, 44.28°, 64.43° and 77.47° are matched with the crystal faces of (111), (200), (220), (311) planes of silver. Meanwhile the other diffraction peaks represent for C phase with the standard data of JCPDS. To be noticed, the copper and silver oxide phase were not detected, which reveals that there is only a pure silver layer produced by replacement. The EDS image shown in Fig. 4 illustrates that the deposited particles and coating is crystalline silver without impurities.
The binding force of silver plating layers to carbon foam was investigated by cold-heat circulation method. After the samples were placed in boiling water for 30 min, samples were soaked in cold water at the temperature of 0-5 ℃. These steps were repeated for three times. The coating on samples obtained by replacement deposition has no phenomenon of spall, peel and blister while these phenomena are quite obvious on the samples obtained by the direct electroless plating. These results indicate that the samples prepared by the replacement deposition have better coating binding force than that by the direct electroless plating.
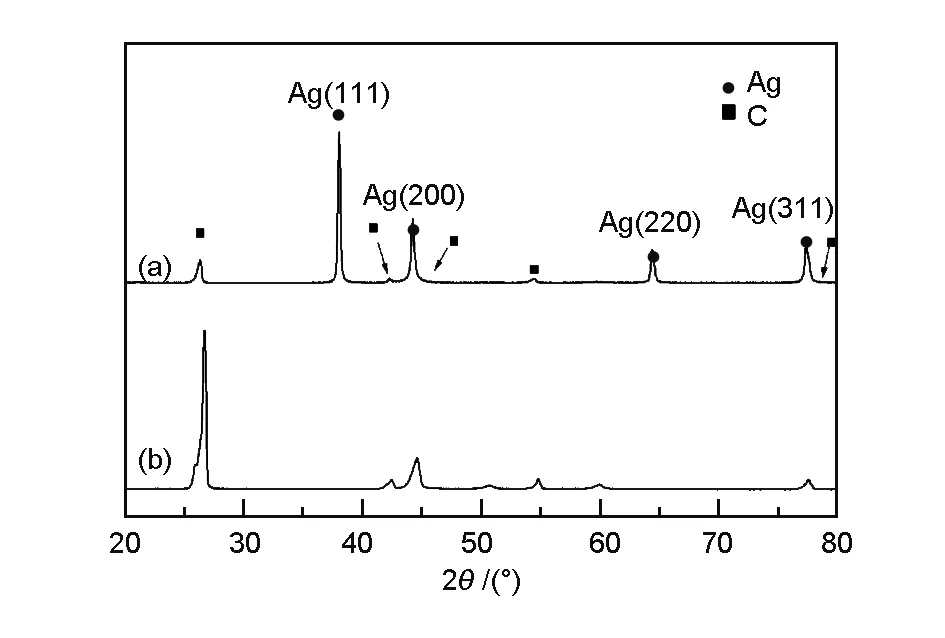
Fig. 3 XRD patterns of (a) Ag-coated carbon foam and (b) uncoated carbon foam.
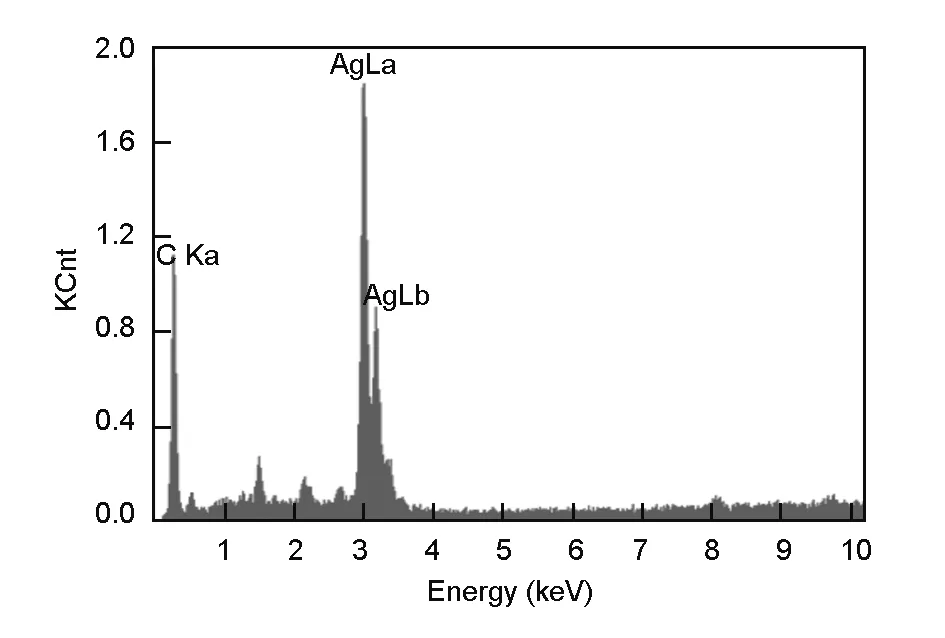
Fig. 4 EDS spectrum of silver-coated carbon foam.
Conductivity of the silver plated carbon foam is presented in Fig. 5. Its electrical conductivity increases with the silver content. When silver content is 15.5 wt%, the conductivity of sample is increased from 700 to 2 055 S/cm. The slope representing the rate of electrical conductivity increase with the silver content turns steep when silver content is larger than 11 wt%. The main reason is that the ligament layer and cracks are connected to form a continuous layer after the replacement plating, which offers a continuous path for the free electron transfer among the layers. Consequently, the electrical conductivity is greatly enhanced.
Fig. 6 shows the stress-strain curve of the silver-coated carbon foams with a silver content of 15.5 wt%. Because of a uniform silver film is deposited on the pore, the compressive strength is increased. The compression strength increases from 0.54 to 1.05 MPa with the silver content from 0 to 15.5 wt%, which is shown in Fig. 7. The stability of substrate skeleton structure is the main factor that influences on the compressive strength of materials. Silver is deposited among ligament and cracks, which could effectively prevent fracture and increase the compressive strength of carbon foams.
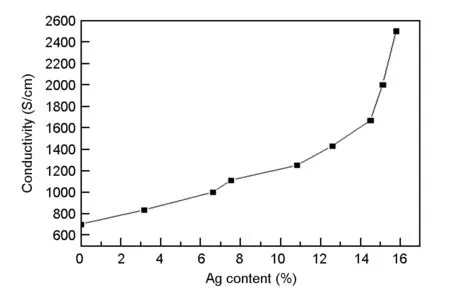
Fig. 5 Conductivity with Ag content for the Ag-coated carbon foam.
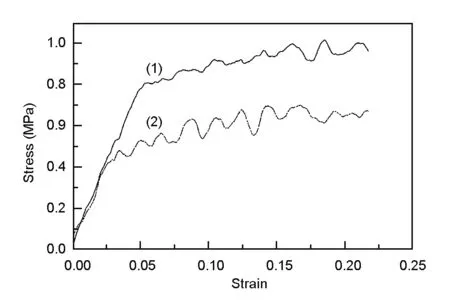
Fig. 6 Stress-strain curves for (1) the silver-coated carbon foam and (2) uncoated carbon foam.
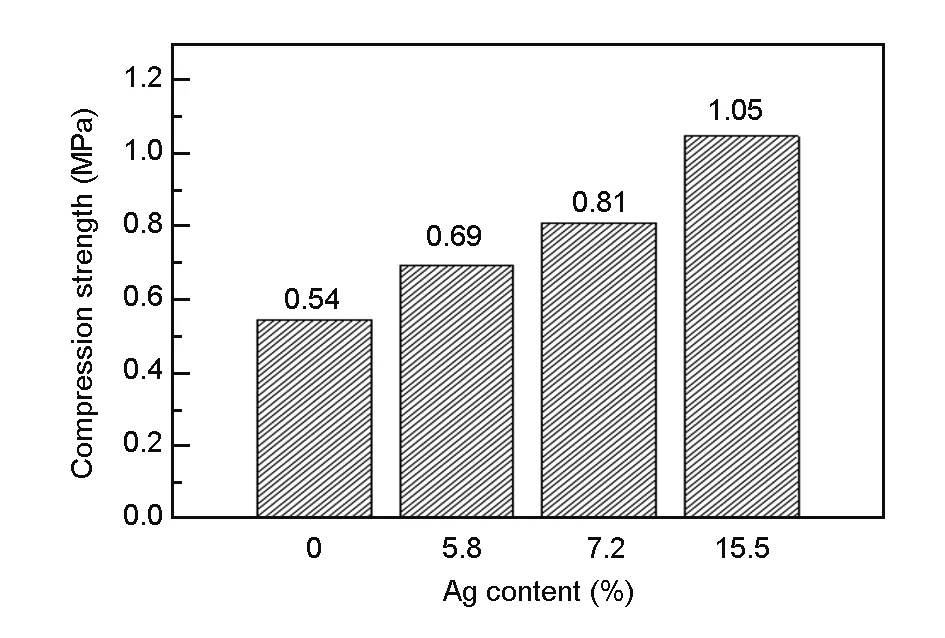
Fig. 7 Histograms of compression strength with the Ag content for the coated foam.
4 Conclusions
Ailver electroless replacement deposition could obtain a uniform and compact silver plating coating on the surface and inner wall of bulk carbon foams. The effective availability and binding force of silver are greatly increased by this method. When the silver content is 15.5 wt%, the conductivity of carbon foam is increased from 700 to 2 055 S/cm and the compressive strength from 0.54 to 1.05 MPa.
杂志排行
新型炭材料的其它文章
- 磷酸活化法活性炭孔隙结构的调控机制
- A dramatic improvement in the tensile strength of fullerene needle-like crystals
- Synthesis of porous graphene-like carbon materials for high-performance supercapacitors from petroleum pitch using nano-CaCO3 as a template
- 锂氟电池用高倍率氟化多壁碳纳米管正极材料
- One-pot synthesis of N, S co-doped photoluminescent carbon quantum dots for Hg2+ ion detection
- Numerical simulation of carrier gas effects on flow field, species concentration and deposition rate in the chemical vapor deposition of carbon
