Synthesis of porous graphene-like carbon materials for high-performance supercapacitors from petroleum pitch using nano-CaCO3 as a template
2018-08-30LIUMingjieWEIFengYANGXuemeiDONGShianLIYingjieHEXiaojun
LIU Ming-jie, WEI Feng, YANG Xue-mei, DONG Shi-an, LI Ying-jie, HE Xiao-jun
(School of Chemistry and Chemical Engineering, Anhui University of Technology, Maanshan243002, China)
Abstract: Porous graphene-like carbon materials (PGCMs) for supercapacitors were synthesized from petroleum pitch by a nano-CaCO3 template strategy combined with KOH activation. The PGCMs were characterized by TEM, XPS, Raman spectroscopy and N2 adsorption. Results show that their specific surface areas are 1 542-2 305 m2 g-1, depending on the template/pitch ratio, KOH/pitch ratio and activation temperature. The PGCMs feature interconnected graphene-like carbon layers with abundant small pores. The optimum supercapacitor electrode is produced when the PGCM is synthesized with a template/pitch ratio of 1.5, a KOH/pitch ratio of 1.5 at 850 ℃ for 1h. It has a specific capacitance of 293 F g-1 at a current density of 0.05 A g-1, an excellent rate capability with a capacitance of 231 F g-1 at a current density of 20 A g-1 and an outstanding cycling stability with a 97.4% capacitance retention after 7 000 cycles in a 6 M KOH aqueous electrolyte. It also exhibits a high specific capacitance of 267 F g-1 at a current density of 0.05 A g-1, and a high energy density of 148.3 Wh kg-1 at a power density of 204.2 W kg-1 in a BMIMPF6 ionic liquid electrolyte. This is a possible method for the synthesis of PGCMs for high-performance supercapacitors.
Key words: Petroleum pitch; Nano-CaCO3 template; Porous graphene-like carbon material; Supercapacitor
1 Introduction
Given the growing depletion of fossil fuels and the increasing issues of environmental pollution, it is urgent to develop renewable and sustainable energy sources and design novel energy storage devices. Nowadays, electrical double layer capacitors, namely supercapacitors, are identified as promising energy storage devices owing to their high power density, long cycle life, and fast charge rate[1-5]. Generally, the electrochemical performance of supercapacitor mainly depends on the electrode materials[6].To date, many kinds of carbon materials have been investigated as prospective electrode materials for supercapacitors because of their high specific surface area, good electronic conductivity, and high chemical stability[7-10]. Of which, graphene is considered as one of the most promising materials for supercapacitor owing to its high theoretical surface area and conductivity[11]. However, the restacking of graphene reduces its surface area, leading to the reduced capacitance.
Porous graphene-like carbon materials (PGCMs) have attracted considerable attention owing to their graphene-like morphology and hierarchical porous structure[12-15]. However, the synthesis of PGCMs on a large scale is still a challenge due to its high production cost. Therefore, many attempts have been made to synthesize low-cost PGCMs from different carbon sources with the aid of templates[16-19]. PGCMs are expected to be made from petroleum pitch, a cheap and abundant carbon source from the distillation of crude, to realize its high value-added application[20-22]. In comparison to MgO, not only can nano-CaCO3act as cheap template, but also offer an activation agent, e.g. CO2from the decomposition of nano-CaCO3during the carbonization-activation process[23].
Herein, nano-CaCO3template coupled with in-situ KOH activation technique is reported for the first time to synthesize PGCMs from petroleum pitch. The as-prepared PGCMs feature interconnected graphene-like carbon layers with high specific surface area and abundant hierarchical short pores. The PGCM electrodes show excellent rate capability, high capacitance and superior cycle stability in 6 M KOH aqueous electrolyte. To the best of our knowledge, there are no reports about the synthesis of PGCMs using cheap nano-CaCO3as template from petroleum pitch for supercapacitors.
2 Experimental
2.1 Materials
Petroleum pitch (87.39 wt% C, 7.93 wt% H, 3.30 wt% S, 1.30 wt% N, and 0.07 wt% O) was provided by China National Offshore Oil Corporation. Nano-CaCO3particles were obtained from Ruicheng Xintai NanoMaterials Technology Co. Ltd. Other chemicals were supplied by Aladdin Co. Ltd. and used without further treatment.
2.2 Synthesis of PGCMs
In a typical synthesis, the mass of petroleum pitch and the calcination temperature were variable while that of KOH (6 g) and CaCO3(6 g) being constant. First, nano-CaCO3, KOH, and petroleum pitch were mixed homogeneously in a mortar. Second, the mixture was put into a corundum boat and heated to 850-950 ℃ at 5 ℃ min-1in a horizontal tubular furnace, and held for 1 h in flowing Ar (60 mL min-1). After being cooled to room temperature, the samples were then washed with 2 M HCl and distilled water to remove the template and inorganic impurities. Last, the PGCMs were obtained after being dried at 110 ℃ for 12 h. The as-prepared sample is named as PGCMm-t, where the subscript m and t refer to the mass of petroleum pitch and the calcination temperature, respectively.
2.3 Characterization
The PGCMs were characterized by transmission electron microscopy (TEM), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), and N2adsorption. Please see Supporting Information for details.
2.4 Electrochemical measurements
The electrochemical performances of PGCMs were evaluted by using cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charge-discharge (GCD). CV tests were carried out on an electrochemical workstation (CHI760C Instrument, China) at scan rates from 2 to 500 mV s-1. EIS was obtained on an impedance analyzer (SI1260, Solartron Analytical, UK) in the frequency range from 0.001 Hz to 100 kHz with an amplitude of 5 mV. GCD performance was evaluated by using an Arbin supercapacitance test system (SCTs, USA ). Please see Experimental Section in Supporting Information for details related to the fabrication of PGCM electrodes and the calculation methods for specific capacitance, energy density, and average power density.
3 Results and discussion
The TEM image of nano-CaCO3particles (Fig. 1a) exhibits a granular morphology with a size concentrated at ca. 65 nm. Fig. 1b is the TEM image of PGCM4-950, showing obvious mesopores and sheet-like structures. For PGCM4-850in Fig. 1c, abundant capsules are observed, whose sizes are similar to that of nano-CaCO3particles due to the template effect of nano-CaCO3. The capsule is ultrathin, ca. 2 nm in thickness (Fig. 1d), and the thin edge of capsule is composed of interconnected graphene-like layers, indicating a certain graphitization degree of PGCMs. The TEM image in Fig. 1e indicates the presence of obvious corrugated sheets in PGCM6-850, which is expected to prevent the restacking of PGCMs and is beneficial for the ion transport[20]. Furthermore, the corrugated sheets contains many graphene-like layers (ca. 3 nm in thickness) (Fig. 1f), indicating the good conductivity of as-prepared PGCMs.

Fig. 1 TEM images of (a) nano-CaCO3, (b) PGCM4-950, (c, d) PGCM4-850 and (e, f) PGCM6-850.
As shown in Fig. 2a, PGCMs exhibit a type IV isotherm with an obvious H4 hysteresis loop at a relative pressure (p/p0) of 0.40-0.99, indicating the presence of mesopores and macropores[24,25]. In addition, pronounced N2uptake at lowp/p0is observed, demonstrating the considerable amount of micropores in PGCMs. The micropores, mesopores and macropores are mainly ascribed to the activation of KOH, the template effect nano-CaCO3and the collapse of some mesopores, respectively[19,23,26]. The pore structure parameters obtained from the N2adsorption of PGCMs are listed in Table 1. The results show that theSBETof PGCM4-850is 1 922 m2g-1, which is higher than that of PGCM6-850(1 542 m2g-1) due to a higher mass ratio of KOH to petroleum pitch. In addition, theSBETof PGCM4-950increases to 2 305 m2g-1in comparison to that of PGCM4-850, which is ascribed to the further activation of KOH at a higher temperature. Simultaneously, the PGCMs possess hierarchical pores, e.g. micropores at 0.6-1.3 nm, mesopores at 2-6 nm, macropores beyond 50 nm (Fig. 2b), which is expected to enhance their capacitance and rate capability in supercapacitors.

Fig. 2 (a) Nitrogen adsorption-desorption isotherms and (b) pore size distribution of PGCMs.
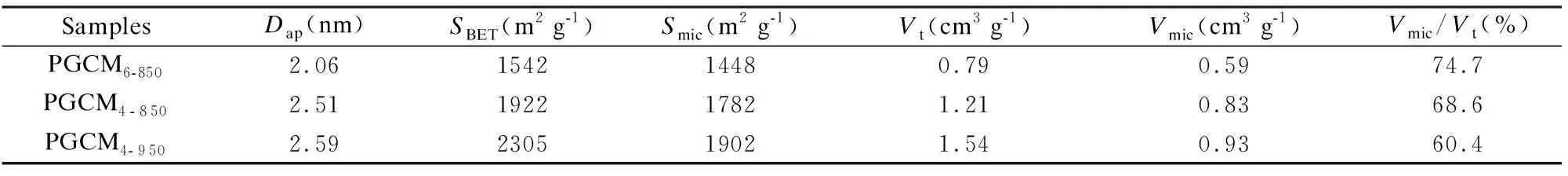
SamplesDap(nm)SBET(m2 g-1)Smic(m2 g-1)Vt(cm3 g-1)Vmic(cm3 g-1)Vmic/Vt(%)PGCM6-8502.06154214480.790.5974.7PGCM4-8502.51192217821.210.8368.6PGCM4-9502.59230519021.540.9360.4

Fig. 3 The characterizations of PGCM4-850: (a) Raman spectrum, (b) XPS spectrum, (c) C1s spectrum and (d) O1s spectrum.

SamplesC1s (%)O1s (%)O1sC=O (%)C-O (%)O-H (%)PGCM6-85080.7419.2610.608.650.01PGCM4-85086.4913.515.437.011.07PGCM4-95090.947.283.643.630.01
The pathway for the synthesis of PGCMs is proposed (Fig. 4). First, the petroleum pitch is liquefied and coated onto the surface of KOH and nano-CaCO3after being ground and heated, and then the capsule-like films are formed. Second, these films are translated into porous graphene-like carbon at elevated temperature due to the in-situ activation of KOH. At the same time, the CO2generated from decomposition of nano-CaCO3is also helpful for the formation of the micropores. Last, the PGCMs are obtained after the template and impurities are removed by washing with HCl solution and distilled water.

Fig. 4 Schematic for the preparation process of PGCMs.
Two-electrode supercapacitor was assembled to evaluate the electrochemical performances of PGCMs in the 6 M KOH aqueous electrolyte and BMIMPF6ionic liquid electrolyte. As shown in Fig. 5a, the CV curves of PGCM4-850and PGCM6-850in the 6 M KOH aqueous electrolyte show rectangular-like shapes with redox peaks due to Faradic redox reaction caused by oxygen-containing species, indicating the combined electrical double-layer capacitance and Faradic pseudocapacitance[30,31]. In addition, the CV curves of PGCM4-850in the BMIMPF6ionic liquid electrolyte show rectangular-like shape (Fig. S1a, Supporting Information). The GCD curves of PGCM electrodes exhibit a symmetrical triangular shape (Fig. 5b), indicating their outstanding electrochemical reversibility. Moreover, negligible IR drop of the PGCM4-850is observed (ca. 0.00015 V), demonstrating a small internal resistance. Additionally, the GCD curves of PGCM4-850in the BMIMPF6ionic liquid electrolyte show a highly symmetrical triangle and a small IR drop of 0.0404 V(Fig. S1b, Supporting Information). Fig. 5c shows the relationships between specific capacitance and current densities for PGCM electrodes. Over the current density from 0.05 to 20 A g-1in the 6 M KOH aqueous electrolyte, the specific capacitance of PGCM4-850at the current density of 0.05 A g-1is 293 F g-1, which is bigger than other two samples. Furthermore, the specific capacitance of PGCM4-850remains at 231 F g-1at 20 A g-1, of which the high capacitance and good rate capability are ascribed to the short pore length on thin carbons. Not only so, the supercapacitor electrode fabricated from PGCMs in the BMIMPF6 ionic liquid electrolyte also exhibits a high specific capacitance of 267 F g-1at a current density of 0.05 A g-1(Fig. S1c, Supporting Information). The capacitance of PGCM4-850is bigger than that of PGCM6-850though the specific surface area of PGCM4-850is smaller than that of PGCM6-850, which is ascribed to the higher content of oxygen-containing groups in PGCM4-850(Table 2). These oxygen-containing groups are expected to improve the surface polarity of PGCM4-850and increase its affinity in aqueous electrolyte[32], which are beneficial to promoting the capacitive performance of PGCM4-850.
Generally speaking, the specific capacitance of PGCMs in the BMIMPF6ionic liquid electrolyte are lower than that of PGCMs in the KOH aqueous electrolyte, which is attributed to the low utilization of micropores in the ionic liquid electrolyte due to the large ion sizes and low conductivity of the latter (Fig. S1c, Supporting Information)[33]. Interestingly, the energy density of PGCM electrodes in the BMIMPF6ionic liquid electrolyte is up to 148.3 Wh kg-1at 204.2 W kg-1(Fig. S1d, Supporting Information), while that of PGCM electrodes in the aqueous electrolyte is only 10.2 Wh kg-1at 25.6 W kg-1(Fig. 5d). For PGCM4-850, 97.4% of the initial capacitance is retained at 5 A g-1after 7000 cycles (Fig. 5e), indicating an excellent cycling stability. Nyquist plots of the PGCM4-850electrode exhibit a straight line and semicircle at the low and high frequency regions, respectively (Fig. 5f). High slope value at the low frequency region reflects superior pore accessibility for the electrolyte ions. The short x-intercept (0.53 Ohm) indicates a low internal resistance. The negligible diameter of semicircle in the high-frequency region indicates a low charge-transfer resistance[34-36].
Moreover, compared with other carbon electrode materials, the as-prepared PGCMs possess high capacitances along with excellent cycle stability (Table S1, Supporting Information), especially for PGCM4-850[37-45]. This is attributed to their well-balanced hierarchical short pores and graphene-like structure, which supply the crucial factors required for advanced supercapacitors. Considering the low cost of raw materials and templates, feasible preparation technique, and excellent electrochemical performances, the PGCMs are among the best carbon-based electrode materials for supercapacitors.
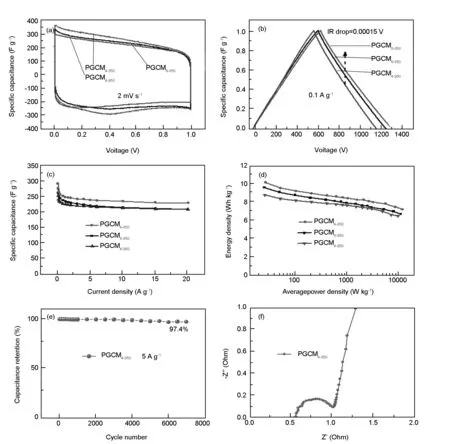
Fig. 5 Electrochemical characterizations of two-electrode supercapacitor made from PGCMs: (a) CV curves at 2 mV s-1, (b) GCD curves at 0.1 A g-1, (c) specific capacitance at different current densities, (d) Ragone plots of PGCMs, (e) capacitance retention of PGCM4-850 at 5 A g-1and (f) Nyquist plots of PGCM4-850.
4 Conclusions
PGCMs have been synthesized from petroleum pitch by using nano-CaCO3as template coupled with KOH activation. The interconnected graphene-like carbon layers and developed hierarchical short pores in PGCMs are beneficial to improve their electronic conductivity and shorten ion transfer distance. Because of these synergistic features, the PGCM-based electrodes exhibit high specific capacitance, excellent rate capability and outstanding cycle stability. This work provides a novel method for the preparation of low-cost PGCMs from aromatic hydrocarbon sources for energy storage.
杂志排行
新型炭材料的其它文章
- 磷酸活化法活性炭孔隙结构的调控机制
- A dramatic improvement in the tensile strength of fullerene needle-like crystals
- 锂氟电池用高倍率氟化多壁碳纳米管正极材料
- One-pot synthesis of N, S co-doped photoluminescent carbon quantum dots for Hg2+ ion detection
- Numerical simulation of carrier gas effects on flow field, species concentration and deposition rate in the chemical vapor deposition of carbon
- 酚醛气凝胶/炭纤维复合材料的结构与烧蚀性能
