A dramatic improvement in the tensile strength of fullerene needle-like crystals
2018-08-30ToshioKonnoTakatsuguWakaharaKunichiMiyazawaKazuhiroMarumoto
Toshio Konno, Takatsugu Wakahara, Kun′ichi Miyazawa, Kazuhiro Marumoto,4
(1. Division of Materials Science, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki305-8573, Japan; 2. National Institute for Materials Science, 1-1 Namiki, Tsukuba, Ibaraki305-0044, Japan; 3. Imaging Section (IMG), Okinawa Institute of Science and Technology Graduate University (OIST), 1919-1 Tancha, Onna-son, Kunigami-gun, Okinawa904-0495, Japan; 4. Tsukuba Research Center for Interdisciplinary Materials Science (TIMS), University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki305-8571, Japan)
Abstract: Needle-like crystals consisting of a solid solution of C60-C70 fullerene were synthesized by a liquid-liquid interfacial precipitation method using toluene and 2-propanol as solvents. A machined crystal with a V-notch was placed in a focused ion beam scanning electron microscope where it was bent until fracture by pushing it with a molybdenum probe to measure its mechanical properties and its fracture surface was examined. The crystals had a tensile strength of 58-71 MPa, which is much higher than C60 fullerene needle-like crystals and slightly larger than alumina, and a fracture toughness of 1.1-1.3 MPa m1/2. Moreover, it is possible to change the plasticity and fracture toughness of the C60-C70 crystals by solvation of different numbers of fullerene molecules. The C60-C70 fullerene crystals have the potential for use as electrodes, anchors for brittle materials, and ductile wires to carry electricity.
Key words: Fullerene nanowhisker; Solid solution; Liquid-liquid interfacial precipitation; Tensile strength; Fracture toughness
1 Introduction
Carbon atoms can be bonded together by covalent binding to form some carbon allotropes such as fullerenes. A C60fullerene, which is truncated icosahedron molecule, was first observed by Kroto et al.[1]. Fullerenes have been well characterized[2]and reported to exhibit a low toxicity[3]with a low cost fabrication[4]to be applied in multiple ways.
One species of the fullerene compounds, molecular fullerene needle-like crystals can be dissolved in organic solvents to recover fullerene molecules[5,6]. The electrical conductivity of the fullerene needle-like crystals is affected by their diameters, exposure to oxygen, and solvents[7-10]. Thus, these crystals show a potential for the applications of field-effect transistors, solar cells, fuel cells, and surface enhanced Raman scattering sensors (SERS)[11-15]. As for the mechanical properties, it has been reported that the C60needle-like crystals have a density of 1.73 g cm-3[16]and a Young′s moduli ranging from 32 to 54 GPa[17]which decreases with increasing the crystal diameter and depends on the synthesis conditions[18,19]. Also, the C60needle-like crystals in air have been reported to be underwent brittle fracture during the bending and the buckling[19-21].
Fullerene needle-like crystals contain two species of fullerene molecules to form a solid solution with solution hardening. Since substitutional solid solutions can be formed from two species of atoms or molecules which are close in size, we use C60and C70molecules in order to obtain a solid solution in this study. C60-C70needle-like crystals consisting of a C60mother phase doped with 11 wt% C70molecules have been analyzed to exhibit the light absorption band of both C60and C70and a Young′s modulus of 60-100 GPa even when the crystal diameter exceeds 1 000 nm[22,23]. Although the C60-C70needle-like crystals are expected to be useful with a low density, a high Young′s modulus, a wide absorption band, and various electrical conductivities, the mechanical properties of such crystals have not yet been clarified. The understanding of the mechanical properties of the C60-C70needle-like crystals is indispensable to their applications as electric feeder lines, electrode materials, and anchor materials. In this paper, C60-C70needle-like crystals with a diameter of 20-40 μm were synthesized using toluene and 2-propanol as solvents. The crystals were fractured by bending using focused ion beam-scanning electron microscopy (FIB-SEM) to determine their tensile strength, fracture toughness, plastic deformation, and inner structure. The C60-C70
fullerene needle-like crystals were found to be brittle materials with the tensile strength much higher than that of C60fullerene needle-like crystals and the specific strength larger than that of alumina.
2 Experimental
The C60-C70fullerene needle-like crystals were synthesized by a liquid-liquid interfacial precipitation method[24]. Fullerene powders (C6099.5% and C7098%, both from MTR Ltd.) were dissolved in toluene (JIS special grade; Wako Pure Chemical Industries Ltd.) using an ultrasonic agitator (Iuchi VS-150; As-One Ltd.). The saturated solutions of C60and C70in toluene were respectively filtered using 450-nm pore filters (Whatman 25 mm GD/X; GE Healthcare UK Ltd.) and mixed using an agitator to give a C60-C70solution containing C70with 45 wt% in toluene as the mother solution.
The mother solution (10 mL) was added to a glass bottle (30 mL, No. 6; As-One Ltd.) and held at 15 ℃ in a water bath. Then, 2-propanol (10 mL) was slowly added along the inside wall of the bottle to form a liquid-liquid interface. After mixing the solution by shaking 30 times to promote a homogeneous reaction, the bottle was stored at 15 ℃ for 5 days to grow needle-like crystals. After the supernatant was replaced with 2-propanol, filtration (5B-21; Kiriyama Glass Inc.) under reduced pressure and vacuum drying (VO-300; As-One Ltd.) at room temperature for 120 min were conducted to obtain C60-C70fullerene needle-like crystals.
To fracture the C60-C70fullerene needle-like crystals, the crystals were fixed to a silicon substrate by tungsten deposition, cleaved by gallium ion sputtering, and then bent using a molybdenum probe until fracture, as outlined in Fig. 1. The fracture surfaces were observed using a FIB-SEM (NB5000; Hitachi High-Technologies).
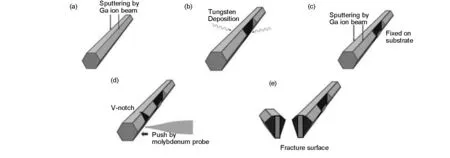
Fig. 1 Outline of the process to fracture a C60-C70 fullerene needle-like crystal. (a) Side surface formation, (b) fixture to a substrate, (c) slit formation, (d) bending and (e) fracture.
3 Results and discussion
Fig. 2 shows a SEM image of a representative C60-C70fullerene needle-like crystal. The mean length and mean diameter of the C60-C70fullerene needle-like crystals were obtained to be 168±43 μm and 21±7 μm, respectively.
Figs. 3(a) and (b) depict the SEM images of the fracture surfaces of the C60-C70fullerene needle-like crystals. The fracture surfaces were mostly smooth with some porous structures and a small fraction of particles and fibrous surfaces. The magnified SEM images of the smooth surfaces with porous structure are presented in Figs. 3(c) and (d). Fullerene needle-like crystals with a porous structure have been reported previously[25]. The porous structure was attributed to rapid evaporation of solvent molecules. The smooth fracture surfaces of the C60-C70fullerene needle-like crystals have been considered to result from brittle fracture[26,27]because the smooth fracture surfaces have been observed for C60fullerene needle-like crystals[19-21]. The brittle fracture of fullerene needle-like crystals has been considered to originate from a stress concentration at the end of the cracks composed of continuous pores[28]. The stress distribution around the end of a crack is evaluated by the stress intensity factor (K) of three-point bending, schematic of which is shown in Fig. 4, using the following equations[29]:
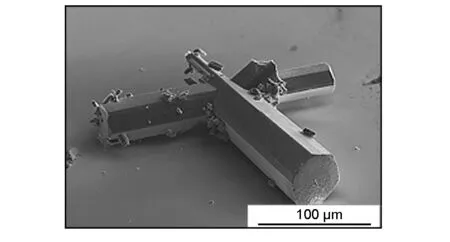
Fig. 2 SEM image of a C60-C70 fullerene needle-like crystal.

Fig. 3 (a,b) SEM images of the fracture surfaces of the C60-C70 fullerene needle-like crystals. (c,d) SEM images of the smooth fracture surfaces of the C60-C70 fullerene needle-like crystals.
K=F(
F(
(1)
whereσbenis the bending stress,ais the length of crack on one side, andWis the thickness of bending samples.σbenis calculated using the following equations when a cantilever is applied to a load[30]:
(2)
whereMis the bending moment,Zis the section modulus of a rectangular cross section,Pis the load,bis the length of the cross section perpendicular toP,his the length of the cross section parallel toP, andxis the distance between the points of the effort and the load. The flexureδis expressed using thePas follows[31]:
(3)
whereEis the Young′s modulus,Iis the second moment of the area on a rectangular cross section, andlis the distance between the points of effort and fulcrum. Eq. (4) is derived from Eqs. (2) and (3):
(4)
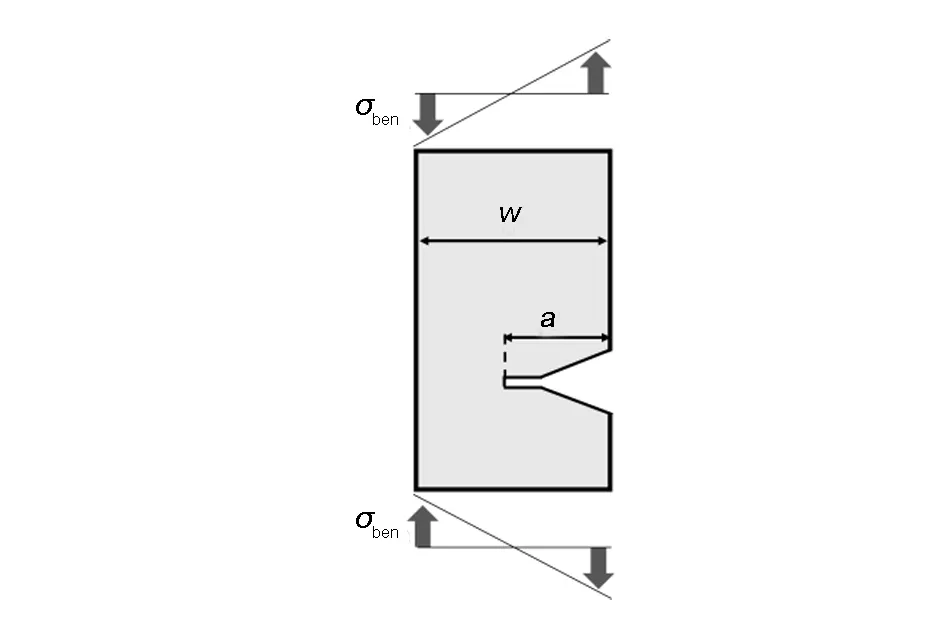
Fig. 4 Schematic of bending in this study. Here, a is the length of the crack on one side, W is the thickness of bending samples and σben is the bending stress.
According to Figs. 5 (b) and (c), the parametersh= 6.5 μm,l= 46 μm andx= 18 μm or h = 2.1 μm,l= 46 μm andx= 20 μm were obtained for the 1stor 2ndC60-C70fullerene needle-like crystal using the experimental setup in the present study, respectively. It was assumed that the C60-C70
fullerene needle-like crystals were flexed before fracture as shown in Figs. 6(a)-(c), and the just before fracture was estimated to be 0.50 μm for the 1stcrystal or 1.1 μm for the 2ndcrystal using Figs. 6(d) and (e), respectively. When theEwas supposed to be 80 GPa, as in the case for the C60-C70fullerene needle-like crystals with a diameter of more than 1 000 nm[23], the maximum value of theσben, that is the tensile strength, was calculated to be 71 MPa for the 1stcrystal or 58 MPa for the 2ndcrystal. These values are approximately six times larger than the tensile strength of the C60fullerene needle-like crystals (11.5 MPa) calculated using the maximum buckling force of 230 nN and the diameter of 160 nm[17]. Therefore, we demonstrate that the C60-C70fullerene needle-like crystals have larger specific strength, which means the tensile strength per unit density, than that of alumina using the density of the C60-C70fullerene needle-like crystals of 1.7 g cm-3and alumina of 3.95 g cm-3.
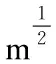

Fig. 5 (a) The definitions for the respective lengths. (b,c) SEM images of the C60-C70 fullerene needle-like crystal with the respective lengths. Here, l is the distance between the points of effort and fulcrum, h is the length of the cross section parallel to the load, x is the distance between the points of the effort and the load, a is the length of crack on one side, and W is the thickness of bending samples.

Fig. 6 Schematics of a C60-C70 fullerene needle-like crystal (a) with no load, (b) just before fracture, and (c) just after fracture. (d,e) SEM images of C60-C70 fullerene needle-like crystals just after fracture.
The magnified SEM images of the particle structures are depicted in Figs. 7(a) and (b). The particles are thought to be composed of the solvated C60molecules. It has been reported that the C60fullerene needle-like crystals with a diameter of less than 1 μm contain toluene with 1 wt% even after vacuum drying at room temperature for 120 min[33]. Therefore, it is possible that the C60-C70fullerene needle-like crystals in this work, which had a diameter of 20-40 μm, contain toluene with more than 1 wt%. The magnified SEM images of the fibrous surfaces are presented in Figs. 7(c) and (d). The fibrous surfaces have been reported to result from ductile fractures[26,27]. Thus, the fibrous surfaces are thought to occur when solvated fullerene molecules expanded and were subjected to strain in the present study. We can obtain ductile solvated C60-C70fullerene needle-like crystals which exhibit lower tensile strength and higher fracture toughness than the C60-C70fullerene needle-like crystals in order to apply them for strained materials such as electric feeder lines.

Fig. 7 (a,b) SEM images of the particle structures at the fracture surfaces of a C60-C70 fullerene needle-like crystal. (c,d) SEM images of the fibrous structure at the fracture surfaces of a C60-C70 fullerene needle-like crystal.
4 Conclusion
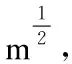
The C60-C70fullerene needle-like crystals contain a small amount of solvent, which led to partial ductility. Thus, we can improve the fracture toughness of the C60-C70fullerene needle-like crystals and change their plasticity from brittle to ductile by solvent in order to apply them for strained materials such as electric feeder lines.
Acknowledgements
This work was partly supported by the Center of Materials Research for Low Carbon Emission of National Institute for Materials Science and by the Japan Society for the Promotion of Science KAKENHI (No. 26600007).
杂志排行
新型炭材料的其它文章
- “碳”无止境
—— 记2018世界炭会议 - 炭纤维毛丝评价表征研究
- Electroless Ag deposition on the cell walls of carbon foam by a displacement method
- Microstructure and thermophysical properties of graphite foam/Sn-Bi alloy composites for use as a thermal sink for electronics
- 纤维素纳米纤丝-碳纳米管/天然橡胶柔性导电弹性体的合成与性能
- Fabrication of magnetic activated carbons from corn cobs using the pickle liquor from the surface treatment of iron and steel
