One-pot synthesis of N, S co-doped photoluminescent carbon quantum dots for Hg2+ ion detection
2018-08-30WEIJumengLIUBitaoZHANGXinSONGChangchun
WEI Ju-meng , LIU Bi-tao , ZHANG Xin, SONG Chang-chun
(1. College of Chemistry and Materials Engineering, Anhui Science and Technology of University, Fengyang233100, China;2. College of Materials and Chemical Engineering, Chongqing University of Arts and Sciences, Chongqing402160, China;3. College of Science, Xi’an University of Science and Technology, Xi’an710054, China)
Abstract: N and S co-doped carbon quantum dots (N, S-CQDs) with a high fluorescence quantum yield (12.6%) were synthesized by a one-pot hydrothermal method. Results indicate that the N, S-CQDs have a small particle size and an amorphous structure, exhibiting unique surface states and excitation wavelength-independent fluorescent properties. Co-doping of N and S increases the electron-transfer rate and improves the coordination interaction between the N, S-CQDs and Hg2+ ions. The N, S-CQDs show a high sensitivity and selectivity in detecting Hg2+ ions even for a lake water sample. They are promising fluorescence probes for environmental monitoring.
Key words: Carbon quantum dots; L-cysteine; Co-doped; Photoluminescent; Hg2+ ions detection
1 Introduction
Heavy-metal ions are greatly hazardous to human health and ecological environments[1-4].During the past few decades, industrial and other anthropogenic processes have been constantly releasing heavy-metal ions into the environment. Among them, the Hg2+ions are never away from our sight due to its extreme toxicity, wide distribution and high industrial value[5,6]. According to research data, approximate 940 tons of Hg2+ions are released to lithosphere and hydrosphere each year, which is seriously harmful to human and other organisms due to the hydrologic cycle and accumulation[7].Conventional analytical techniques for the determination of Hg2+ions have been successfully developed such as chromatography, spectrofluorimetry, and atomic absorption spectrometry[5,8]. However, most of these methods are not convenient due to the requirement of expensive equipment and complicated sample pretreatment. Accordingly, the development of advanced analytical techniques for Hg2+ion detection is highly desired.
Compared with conventional analytical techniques, the fluorescent probe method possess a series of merits such as high sensitivity, high selectivity, and easy operation[9-11]. As an outstanding fluorescence probe material, carbon quantum dots (CQDs) have attracted growing interest owing to their distinct advantages such as low cost, simple synthesis route, good biocompatibility, low cytotoxicity, high photo and chemical stability, no blinking fluorescence, and tunable excitation and emission spectra. Up to date, the CQDs have been developed for the detection of Hg2+, Cu2+, Cr4+, Fe3+, et al. by monitoring the changes of their fluorescence intensities[12-15].Among them, the doped, especially co-doped CQDs with different heteroatoms such as nitrogen and sulfur introduced more active sites and improved the fluorescent quantum yield (FLQY), leading to outstanding sensing performance[16-20]. Therefore, the investigation on co-doped CQDs with peculiar properties for use as fluorescent probes have never been stopped. Wang et al. used citric acid and dithiooxamide to fabricate N, S-CQDs and investigated the Hg2+ion detection properties[21]. Xu’s group employed heparin sodium to obtain N, S-CQDs and studied the performance for Fe3+detection[22].Nevertheless, it is still a challenge to prepare N, S-CQDs with novel properties via a facile and effective route.
In this work, a facile and simple strategy is developed for the hydrothermal synthesis of N, S-CQDs by using L-cysteine as the single precursor. It is found that both N and S atoms are doped in the CQDs. As-prepared N, S-CQDs exhibit a small particle size, good fluorescence performance, relatively high quantum yield up to 12.6%, and have been successfully applied in Hg2+ion detection.
2 Experimental
2.1 Chemicals and reagents
L-cysteine (L-cys) with a purity of 99% was purchased from Aladdin Ltd. (Shanghai, China). All the other chemicals were purchased from Aladdin Ltd. (Shanghai, China) and used as received without further purification. All solutions were prepared using Milli-Q deionized water (18.2 MΩ cm-1, Millipore) as the solvent throughout the experiments.
2.2 Synthesis of the N, S-CQDs
The N, S-CQDs were prepared by hydrothermal treatment of L-cys. The typical experimental procedure is shown in Scheme 1. Typically, 1.0 g L-cys was dissolved in 30 mL deionized (DI) water under agitation at room temperature, and then the solution was transferred to a stainless steel autoclave with a 50 mL Teflon liner and heated at 180 ℃ for 12 h. After cooled to room temperature naturally, the resulting yellow aqueous solution was centrifuged at 12 000 rpm for 20 min to remove the non-fluorescent deposit. The resultant supernate containing fluorescent N, S-CQDs was dialyzed (MWCO: 3500) against DI water for two days to remove inorganic ions and molecules. Finally, a clear and transparent N, S-CQD solution without any precipitation was obtained. On exposure to UV light (365 nm), the obtained solution shows a bright blue color.

Scheme 1 Schematic of the preparation procedure of N, S-CQDs and the photograph of the N, S-CQDs solution excited by daylight and a 365 nm UV lamp.
2.3 Characterization
Transmission electron microscopy (TEM) images, high resolution TEM (HRTEM) images and selected area electron diffraction (SAED) were acquired by using a Tecnai-G2F30 transmission electron microscope operating at an acceleration voltage of 300 kV. X-Ray diffraction (XRD) measurements were performed on a Rigaku D/Max-2400 X-ray diffractometer using CuKαradiation. The Fourier transform infrared (FTIR) spectra were measured by a Thermo Nicolet Nexus FTIR model 670 spectrometer. X-ray photoelectron spectroscopic (XPS) analysis was carried on an ESCALAB 250xi photoelectron spectrometer. UV-Vis spectroscopic studies were performed with a TU-1901 dual beam UV-Vis spectrophotometer. Photoluminescent (PL) measurements were carried out with a FLs920 steadystate/transientstate spectro xsort.
2.4 Metal ion detection
The obtained N, S-CQD solution was diluted 20 times to be the fluorescent probe. For the detection of various metal ions, MnCl2, CaCl2, ZnCl2, MgSO4, NaCl, NiCl2, AlCl3, CdCl2, CuCl2, Pb(NO3)2, Hg(NO3)2were used as various ion sources. In a typical detection experiment, the solutions containing a calculated amount of ions were added into the N, S-CQDs solution. After mixing evenly, the PL spectra were detected at 365 nm excitation. All of the experiments were performed in phosphate buffer saline (PBS) unless stated otherwise.
3 Results and discussion
Fig. 1 represents a typical XRD profile of the N, S-CQDs. The (002) peak centers at 19.1° and the corresponding interlayer spacing isd= 0.425 nm, which is larger than that of graphite (0.34 nm)[23]. The increase in this value indicates an increase in amorphous nature, and is probably ascribed to the introduction of nitrogen-, sulfur- and oxygen-containing groups[24]. The poor crystalline nature of the N, S-CQDs is further confirmed by HRTEM image (in the set of Fig. 1b), from which no clear lattice fringes can be seen. Fig. 1b shows the TEM image of the N, S-CQDs, revealing that the N, S-CQDs have a spherical morphology and a narrow size distribution from 2.0 to 6.5 nm (inset of Fig.1a).
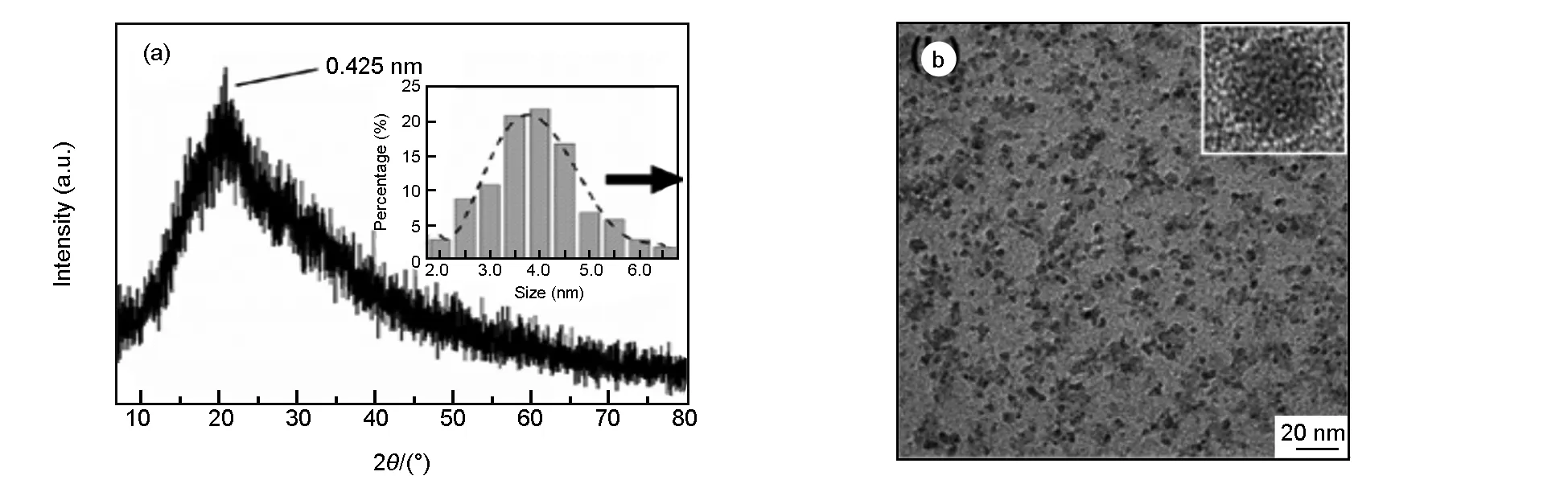
Fig. 1 (a) XRD pattern of the N, S-CQDs, inset: size distribution bar graph; (b) TEM image of the N, S-CQDs, inset: HRTEM image of one particle.

Fig. 2 Surface state and component characterization of N, S-CQDs: (a) FTIR spectrum; (b) XPS full scan spectrum; (c-f) high resolution scan XPS spectrum of C 1s, O 1s, S 2p and N 1s, respectively.

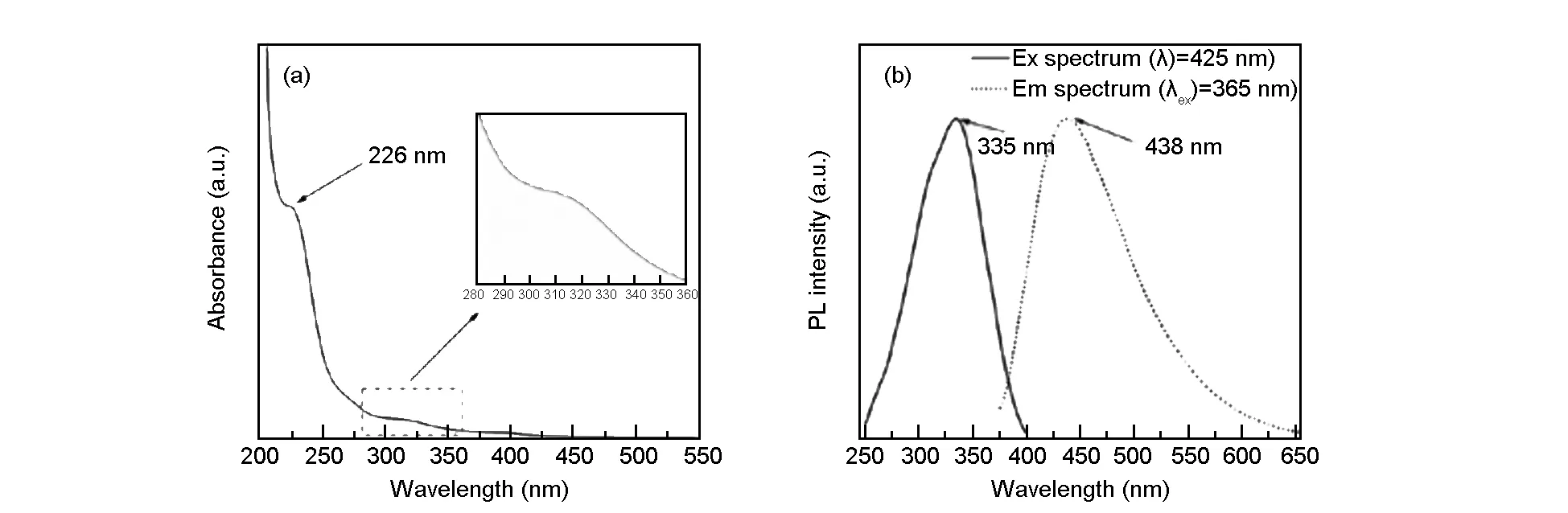
Fig. 3 (a) Absorbance spectrum and (b) emission and excitation spectra of N, S-CQDs.
The results of PL spectra of the N,S-CQDs (Fig. 4a) indicate that their emission wavelength is excitation-independent under the excitation wavelength ranges from 280 to 500 nm and the intensity of the emission peaks are relatively strong along with excitation wavelength ranging from 280 to 360 nm. Such excitation-dependent emission property has been reported as an important criterion for CQD materials. This is attributed to the inhomogenous carbogenic core emission in the sample, which results in the distribution of different emissive sites on each nanoparticles[33-35]. The PLQY of the CQDs was obtained by referencing to a standard (quinine sulphate in 0.1 M H2SO4) following the previous reports[36,37]according to the equation (1):
(1)
Whereφandφ′ (54%) is the PLQY of sample and standard,m(sample, 3.61×109) andm′ (standard, 1.546×1010) is the slope with the plot of integrated fluorescence intensity vs. absorbance,η(sample) andη′ (standard) is the refractive index of the solvent (for the aqueous solutionsη/η′ =1). The FLQY of the N, S-CQDs is calculated to be 12.6%, which is acceptable for their use as fluorescent probes. It is worth mentioning that reducing reaction temperature from 200 to 120 ℃ leads to a decrease in the PLQY from 13.9% to 8.4%. Fig. 4b shows the PL decay curve and the instrument response function, the observed lifetimes of the CQDs areτ1= 0.64 ns,τ2= 3.05 ns andτ3= 10.11 ns, which reveals the radiative recombination nature of excitations. The calculated average lifetime is 4.25 ns. This lifetime located in the magnitude of nanosecond suggests that the N, S-CQDs are most suitable for optoelectronic applications[24,38].
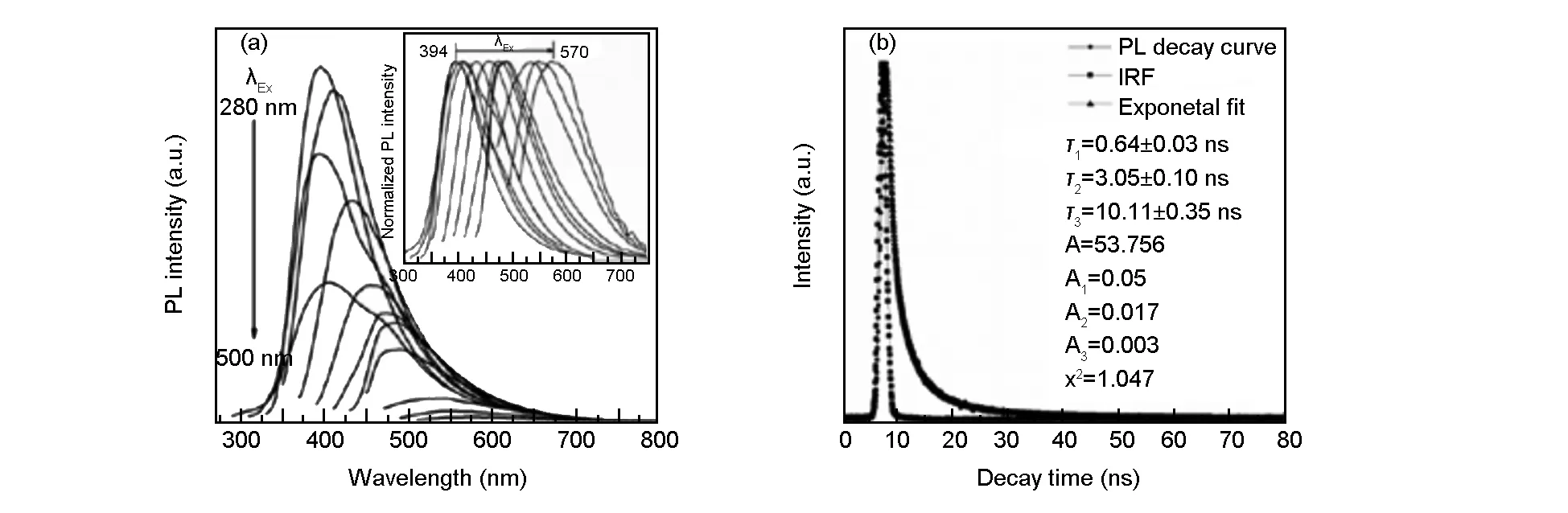
Fig. 4 PL emission spectra of N, S-CQDs with progressively longer excitation wavelengths from 280 to 500 nm, inset: normalized PL emission spectra; (b) PL decay curve of N, S-CQDs, inset: instrument response function.
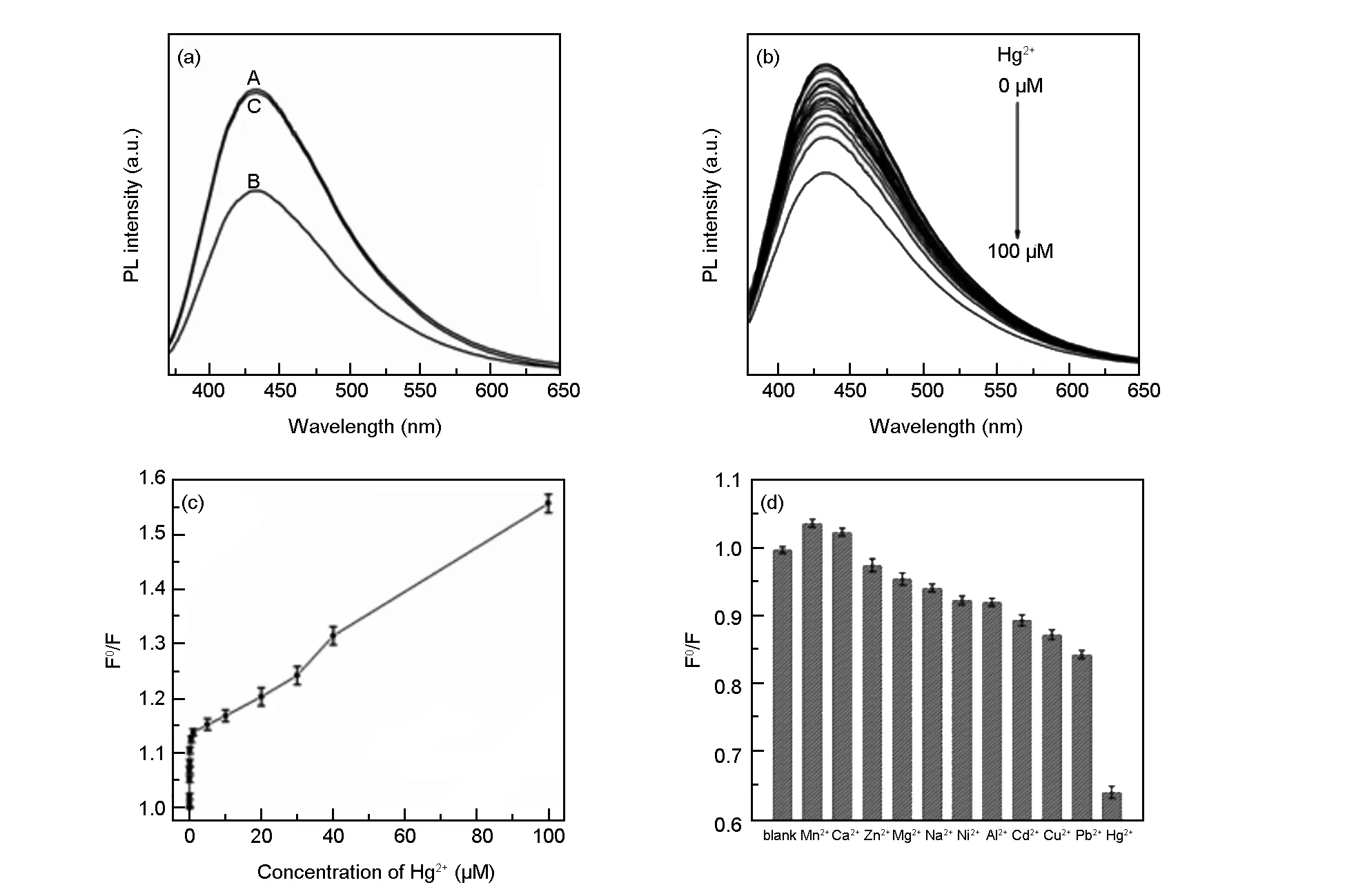
Fig. 5 (a) PL spectra of N, S-CQDs in the absence-(A), presence-(B) of Hg2+ ions and N, S-CQDs-Hg2+-EDTA mixture-(C); (b) PL spectra of the CD dispersion in the presence of different Hg2+ ion concentrations(0, 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 50 and 100 μM); (c) the dependence of F0/F on the concentrations of Hg2+ ions; (d) the dependence of F0/F on the blank and solutions containing different metal ions (λex= 360 nm,[ions] = 100 μM).
For qualitative detection of Hg2+ions, as shown in Fig. 5a, the N, S-CQD solution in the absence of Hg2+ions exhibits a strong PL peak at 433 nm (λex= 360 nm). In contrast, the presence of Hg2+ions leads to an obvious intensity decrease of fluorescence at the same test conditions, indicating that Hg2+ions can effectively quench the fluorescence of the N, S-CQDs. This observation is attributed to the electron transfer from excited N, S-CQDs to Hg2+ions, leading to substantial fluorescence quenching[39,40]. The possible quenching mechanism of the N, S-CQDs fluorescent probe is presented in Scheme 2. When ethylene diamine tetraacetic acid (EDTA) was employed as a strong Hg2+ion chelator, the intensity of the PL emission peak was almost completely restored, which verifies the above theoretical explanation. In the following experiments, the selectivity of this detection system for Hg2+ions was also carried out. Different concentrations of Hg2+ions in the range of 0-100 μM (0, 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 50 and 100 μM) in the blank N, S-CQDs solution were tested. Fig. 4b shows the PL spectra of N, S-CQDs in the presence Hg2+ionswith different concentrations, revealing that the PL intensity of the mixture is sensitive to Hg2+concentration and decreases with the increase of Hg2+concentration. The sensitivity limit can be as high as 1 nM, which is higher than those of traditional detection methods and is comparable to the previously reported works[18,40-42]. This accuracy exceed the maximum allowable Hg2+level (10 nM) in drinking water as established by the Environmental Protection Agency (USA)[12]. To get insight into the fluorescence quenching mechanism involved, the fluorescence quenching data were analyzed by the Stern-Volmer equation (2),
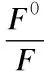
(2)
whereF0andFare the N, S-CQDs fluorescence intensities at 433 nm in the absence and presence of Hg2+ions, respectively.[Q] is the concentration of the quencher (i.e. Hg2+ions), andKSVis the Stern-Volmer constant. As shown in Fig. 5c, the Stern-Volmer plot does not fit a conventional linear Stern-Volmer equation, indicating both dynamic and static quenching processes occur in this sensing system[43-45].

Scheme 2 The proposed FL quenching mechanism for N, S-CQDs system.
To evaluate the selectivity of the proposed Hg2+sensor, the detection of Hg2+was carried out in the presence of various representative metal ions under identical conditions with the same concentration of 100 μM. As shown in Fig. 5d, a much low PL intensity can be observed for the N, S-CQDs due to the addition of Hg2+, while no obvious decrease is seen after adding other metal ions into the N, S-CQDs solution. The results reveal that the N, S-CQDs possess excellent ion selectivity, and the other metal ions have an ignorable interference on the sensing system. The high selectivity is probably assigned to the high affinity between Hg2+and the functional groups on the surface of the N, S-CQDs.
As shown in Fig. 6a, an anti-interference Cd2+, Cu2+, or Pb2+ions only have a slight influence on the fluorescence of the detection system. Furthermore, we also performed Hg2+detection to evaluate this sensor in real water system, the analysis was challenged by lake water samples obtained from the local Longzi Lake of Bengbu, Anhui province, China. The lake water was centrifuged at 12 000 rpm for 20 min and filtered through membrane to remove the solid impurities and impurity inorganic ions. Subsequently, the Hg2+ions with different concentrations were added into the resultant water samples and then analyzed with the proposed method. It is seen that the PL intensity decreases with increasing the concentration of Hg2+ions from 0.5 to 20 μM (Fig. 6b), and the sensitivity limit is about 500 nM in spite of the interference from numerous minerals and organics existing in the lake water. These results imply the practical applicability for Hg2+ion detection in environment monitoring.
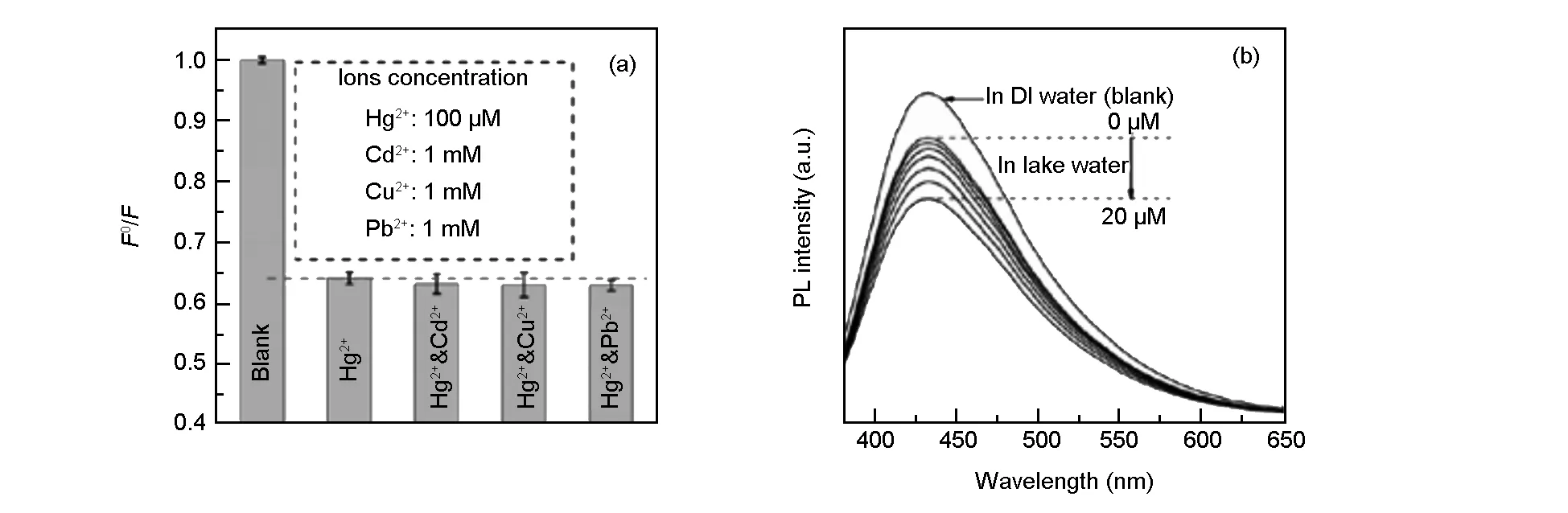
Fig. 6 (a) The dependence of F0/F on the blank solutions, and the solutions containing Hg2+ ions and different interfering ions (λex= 360 nm,[Hg2+] = 100 μM,[Cd2+] =[Cu2+] =[Pb2+] =1 mM); (b) PL spectra of N, S-CQD dispersion in the presence of different Hg2+ concentrations (from top to bottom: 0, 0.5, 1, 2, 5, 10 and 20 μM) in lake water.
4 Conclusion
One-pot hydrothermal treatment of L-cys has been performed for preparing N, S co-doped CQDs. The N, S-CQDs with a small size and higher FLQY have been further used as an outstanding sensing platform for detection of Hg2+ions in DI water and a real water sample. This fluorescent probe with a fairly high sensitivity and selectivity provides a simple and fast route for sensing heavy-metal ions.
杂志排行
新型炭材料的其它文章
- 磷酸活化法活性炭孔隙结构的调控机制
- A dramatic improvement in the tensile strength of fullerene needle-like crystals
- Synthesis of porous graphene-like carbon materials for high-performance supercapacitors from petroleum pitch using nano-CaCO3 as a template
- 锂氟电池用高倍率氟化多壁碳纳米管正极材料
- Numerical simulation of carrier gas effects on flow field, species concentration and deposition rate in the chemical vapor deposition of carbon
- 酚醛气凝胶/炭纤维复合材料的结构与烧蚀性能
