High-temperature oxidation behavior and analysis of impedance spectroscopy of 7YSZ thermal barrier coating prepared by plasma spray-physical vapor deposition
2018-08-21WenlongCHENMinLIUJifuZHANGZiqianDENGJieMAO
Wenlong CHEN ,Min LIU ,Jifu ZHANG ,Ziqian DENG ,Jie MAO
a School of Materials and Energy,Guangdong University of Technology,Guangzhou 510006,China
b National Engineering Laboratory for Modern Materials Surface Engineering Technology&the Key Lab of Guangdong for Modern Surface Engineering Technology,Guangdong Institute of New Materials,Guangzhou 510650,China
KEYWORDS
Abstract Quasi-columnar structure 7YSZ(yttria stabilized zirconia)thermal barrier coatings(TBCs)are prepared by plasma spray-physical vapor deposition(PS-PVD)onto pretreated and un-pretreated bond coating,respectively.An isothermal oxidation experiment of 7YSZ TBCs is carried out in the atmosphere of 950°C in order to simulate the high-temperature oxidation process of engine blades.The isothermal oxidation process of 7YSZ thermal barrier coatings is investigated systematically by impedance spectroscopy.The electrochemical physical model and equivalent circuit of columnar 7YSZ coatings are established.Results show that the isothermal oxidation kinetic curve of columnar 7YSZ thermal barrier coatingsappearsto follow theparabolic law.A pretreatment of bond coating can reduce the growth rate of the thermally grown oxide(TGO)layer,restraining the initiation and propagation of microcracks between YSZ and TGO layers.The oxidation rate constants of 7YSZ coatings with pretreated and un-pretreated bond coating are 0.101 × 10-12 cm2·s-1 and 0.115× 10-13 cm2·s-1,respectively.Impedance analysis shows that the content of oxygen vacancies decreases and the density increases after the TGO layer is oxidized for 150 h.In addition,shrinkage microcracksformed by sintering during theoxidation processisthemain reason for an increaseof the capacitance and a decrease of the resistance in the grain boundary of YSZ.
1.Introduction
Thermal barrier coatings(TBCs)are widely applied to hotcomponents of turbine engines to increase the operation temperature and to protect components like blades,which have a complex structure with a metallic bond coat(MCrAlY,M=Ni or Co)on a superalloy substrate for oxidation/corrosion resistance and a ceramic topcoat of yttria stabilized zirconia(YSZ)attached on the bond coating for heat protection.1,2At present,there are mainly two methods to fabricate YSZ topcoats,which are air plasma spraying(APS)and electron beam-physical vapor deposition(EB-PVD).However,these two methods also have some disadvantages.Conventional layered structured APS TBCs show high deposition rates and good thermal insulation but poor thermal shock resistance.On the contrary,columnar structured EB-PVD TBCs exhibit higher strain tolerance and better thermal shock resistance but higher thermal conductivity and lower deposition rates compared with APS TBCs.3
Recently,a new hopeful technique called plasma sprayphysical vapor deposition(PS-PVD)has been developed based on the low pressure plasma spray process(LPPS)and combines the advantages of thermal spraying(high deposition rates)and physical vapor deposition(high strain tolerance).The plasma plume of PS-PVD can expand to over 2 m long and nearly 400 mm in diameter under parameters of high power input(about 180 k W)and low work pressure(about 100 Pa).4At a high power level,the temperature of the plasma plume can exceed 6000 K.5Therefore, fine grain sized powders are enough to be evaporated and achieve an EB-PVD-like columnar coating.
High-temperature oxidation is inevitable for TBCs.A layer of thermally grown oxide(TGO)forms at service,which can inhibit the diffusion of oxygen elements into the bond coating and protect the substrate.Moreover,a thermal mismatch due to great differences of the thermal expansion coefficients between bond coating and ceramic coatings makes ceramic layer premature failure easy.Much of related literature has studied the high-temperature oxidation behaviors of TBCs,TGO growth evolution,and micro-cracks propagation within a ceramic layer.For example,Doleker and Karaoglanli6have compared the oxidation behaviors of YSZ and Gd2Zr2O7TBCs,which indicate that the Gd2Zr2O7TBCs have lower thermal conductivity,lower oxygen permeability,and higher structural stability at higher temperatures.These advantages render the Gd2Zr2O7a good alternative top coating material for TBCs.Ahmadian and Jordan7have studied the effect of rapid cycling on oxidation,microcracks,and lifetime of APS TBCs.Their results have shown that there must be very significant inelasticity presenting in crack formation and crack growth in TBCs.Chen et al.8have investigated the oxidation and crack nucleation/growth in an APS TBCs with a NiCrAlY bond coat.Their results have shown that mixed oxides form at the beginning of the thermal exposure in air,along with the formation of the Al2O3layer and cracks initiated mostly in association with the formation of (Cr,Al)2O3·Ni(Cr,Al)2O4·NiO.
Impedance spectroscopy(IS)is a cheap,sensitive,and nondestructive testing method,which has been used extensively to measure the electrical properties of ceramic materials.9–11In the past decade,impedance spectroscopy has been used to reflect the growth of TGO during oxidation and the effect of cracks propagation of the YSZ layer on YSZ electrical properties.For example,Zhang et al.12have used impedance spectroscopy to measure the relationship between the microstructure of a top coat and its electrical properties,as well as thickness and compositional changes of the TGO in environments with different oxygen partial pressures at 1050°C for TBCs produced by EB-PVD.Wang et al.13have studied the TGO growth in APS TBCs after oxidation in air at 1100°C.Ali et al.14have also adopted impedance spectroscopy to investigate the relevance between the microstructure and electrical properties of TGO in APS TBCs oxidation in air at 1150°C.
At present,all of the studies are aimed at APS TBCs and EB-PVD TBCs and the great mass of oxidation temperatures of TBCs above 950°C.The present work is the first to evaluate the oxidation behaviors of PS-PVD TBCs in air at 950°C using impedance spectroscopy.The electrochemical physical model and equivalent circuit of PS-PVD TBCs are established in the work.IS analysis of PS-PVD TBCs has shown four relaxation processes,where three of them correspond to YSZ grains,YSZ grain boundaries,the TGO,and the metal electrode effect.Furthermore,the oxidation kinetics and behaviors of pretreated and un-pretreated PS-PVD TBCs are compared.
2.Experimental
2.1.Sample preparation
Nickel-based super-alloy K 417 was cut into columnar specimens with a dimension of∅25.3 mm×6 mm as substrates,which were grit-blasted before PS-PVD.The composition of K 417 is given in Table 1.A NiCoCrAlYTa bond coating(about 100 μm)was deposited by low-pressure plasma spray(LPPS)(Guangdong Institute of New Materials,Guangzhou,China)on the surface of superalloy.The preparation of all 7YSZ coatings(about 300 μm)was carried out on a PSPVD system(Sulzer-Metco MulticoatTM,Switzerland)onto pretreated and un-pretreated bond coatings,respectively.The process of surface pretreatment for bond coating is based on the following procedures.Firstly,the surface of bond coating was polished using a polishing machine(Tegramin-25,Struers).Subsequently,the surface of bond coating was heated to 1000°C through the plasma plume of PS-PVD for 10 minutes in a vacuum chamber with addition of a certain amount of oxygen.The surface temperature of the substrate was maintained at 850 °C to 1000 °C in the process of fabricating 7YSZ coatings.The powder information and spraying parameters of PS-PVD are given in Tables 2 and 3,respectively.
2.2.High-temperature oxidation testing
All specimens were heated in air to 950°C with a rate of 10 K·min1and isothermally oxidized for 10,50,100,150,and 250 h,respectively.Three specimens were used for impedance measurements and two for microstructure analysis for each oxidation condition.
2.3.Characterization
Impedance measurements were implemented at 400°C using an Ametek Parstat 4000 electrochemical impedance spectroscopy(EIS)analyzer(Parstat4000,AMETEK Inc,USA),which was controlled by a computer.Spectra analysis was performed with the help of Zview impedance analysis software toobtain the electrical properties of TBCs.In this measurement,an alternating current(AC)amplitude of 100 mv was employed,and the AC frequency was in the range of 0.1 to 1×106Hz.The K 417 alloy substrate without TBCs was welded on stainless steel wires as an electrode.The TBC surface was coated with a platinum paint with an area of π×25 mm2,which served as the other electrode.In order to consolidate the platinum paint and enhance its adhesion to the specimen surface,the paint was sintered at 800°C for 10 min.

Table 1 Chemical compositions of nickel-based super-alloy K 417.

Table 2 Information of spray powders.

Table 3 Coating parameters of PS-PVD.
The morphologies of 7YSZ coatings were investigated by a scanning electron microscope(FE-SEM,Nova-Nono430,FEI)equipped with an energy dispersive spectrometer(EDS).The microstructures of the YSZ coatings were characterized by a transmission electron microscope(TEM,JEM 2100F,JEOL).Before characterization of TEM,test samples were prepared by focused ion beam(FIB,450S,FEI)milling.The phase constituents were identified by X-ray diffraction(XRD,D8-Advance,Bruker)with a rate of 0.2(°)/min(Cu-Kα,incident angle 3°,and a 2θ range of 27°–33°).
3.Results and discussion
3.1.Microstructure of feedstock and as-sprayed coating
Agglomerated M 6700 YSZ powders were used as spray feedstock as shown in Fig.1.The phase constituents of feedstock and as-sprayed coating were also investigated as shown in Fig.2.Referring to the JCPDS card,M 6700 YSZ powders mainly consist of tetragonal(t)and monoclinic(m)ZrO2phases.However,no monoclinic ZrO2phase was detected in the as-sprayed coating prepared by PS-PVD based on Table 3.Furthermore,the T-ZrO2phase translated into nontransformable tetragonal ZrO2phase(t′-ZrO2).Fig.3 shows the SEM microstructure and TEM images of 7YSZ coatings prepared by PS-PVD.The surface morphology of 7YSZ coatings exhibits typical features of a ‘‘cauliflower-like” structure(as seen in Fig.3(a)).The cross-section micrograph of 7YSZ coatings shows a quasi-columnar structure with some micro/-nano solid particles adhering to these columns.Combined with the XRD analysis results in Fig.2,it can be concluded that the micro/nano solid particles may be formed by the condensation of 7YSZ powder vapor in the process of PS-PVD.15,16Besides,many secondary column grains are also observed in the coat-ing,which is mainly due to the secondary shadowing.17–19In order to observe the nano-sized secondary columnar structure more intuitively,TEM characterization was adopted.As shown in Fig.3(c),the nano-sized secondary columns are obviously observed.These secondary columns exceed 200 nm in length and nearly 100 nm in width and are separated by nano-gaps.

Fig.1 Micrograph of M 6700 powders.

Fig.2 XRD patterns of M 6700 powders and 7YSZ coatings prepared by PS-PVD.
3.2.Oxidation behaviors and kinetics of quasi-columnar structure 7YSZ TBCs
High-temperature oxidation resistance is an important criterion for evaluating the quality of TBCs.As can be seen in Fig.3(a),the quasi-columnar structure is porous since shadowing,and all the columnar structure gaps are perpendicular to the coating surface.These gaps will be a good oxygen transmission channel and shorten the diffusion path of oxygen.Thus,quasi-columnar structure 7YSZ TBCs can be easily oxidized under high-temperature environments.In order to improve the high-temperature oxidation resistance of TBCs,the surface of the bond coating is pretreated before spraying topcoats.
Fig.4(a)and(b)shows the interface micrographs of pretreated and un-pretreated as-sprayed TBCs,respectively.After surface pretreatment for the bond coating,the interface between the bond coating and top coats is flat,and a continuous TGO layer of 1 μm thick(dTGOis the thickness of the TGO layer)can be observed in Fig.4(a).Furthermore,there are no voids in the interface of pretreated as-sprayed TBCs.However,for un-pretreated TBCs,a discontinuous thin TGO layer with no oxidation resistance can be observed in the interface(as shown in Fig.4(b)),which may be produced in the process of preparation of bond coats or in the pro-heating process before spraying topcoats.Considering that the preparation of quasi-columnar 7YSZ coatings by PS-PVD is mainly vapor deposition,the ragged interface would increase the shadowing effect,and thus voids are observed in the interface as well as the ceramic layer near the interface.
After oxidation for 250 h,the interface micrographs of pretreated and un-pretreated TBCs are presented in Fig.4(c)and(d),respectively.For pretreated TBCs,a continuous TGO layer with flatting is detected in the interface,and the thickness of the TGO layer is increased to 2 μm(as shown in Fig.4(c).Meanwhile,no voids and cracks are observed in the interface after oxidation for 250 h.However,for un-pretreated TBCs,a continuous but uneven TGO layer is observed in Fig.4(d)and the average thickness of the TGO layer is nearly 3.5 μm.Besides,some microcracks are found in the interface and ceramic layer near the interface.Because voids and porous are formed inside the ceramic layer near the interface and the interface itself,the internal bonding strength of the ceramic layer and the interfacial bonding strength will be reduced.In addition,the ragged surface of the bond coating will result in a stress concentration more easily at the interface in the process of oxidation.When the stress of TGO growth exceeds the interfacial bonding strength,microcracks will initiate at the interface and extend through the pores inside the ceramic layer to the interior of the ceramic coatings.
In order to quantify the degree of improvement of the pretreatment process on the oxidation resistance of quasicolumnar 7YSZ coatings,the oxidation kinetics curves of pretreated and un-pretreated TBCs are fitted using the power function equation(shown in Eq.(1)).The fitting curves are shown in Fig.5.F1 and F2 represent the oxidation kinetics curves of un-pretreated and pretreated TBCs,respectively.

where δ (μm)is the thickness of the TGO layer,and t(h)is the oxidation time.The constants a,b and index n are determined by the experimental data.The fitting equations are as follows:
Equation of curve F1:

Equation of curve F2:

Eq.(2)show that b2=4.56×10-3μm=4.56 nm,indicating that the average TGO thickness of the un-pretreated asspray TBC is 4.56 nm.This discontinuous and thin TOP layer in Fig.4(b)can be considered to have little or no oxidation resistance,so the thickness of the TGO layer can be ignored,i.e.,b ≈ 0.However,Eq.(3)show that b3=0.96 μm ≈ 1 μm,indicating that the average TGO thickness of the pretreated as-sprayed TBC is 0.96 μm,which coincides with the measured TGO thickness in Fig.4(a).At the same time,it can be seen from the trend of the fitting curves of F1 and F2 that the un-pretreated and pretreated TBCs both have undergone rapid oxidation and a slow oxidation process.Soboyejo et al.20have studied the high-temperature isothermal oxidation behavior of thermal barrier coatings prepared by APS,indicating that the TGO growth kinetics is parabolic in the temperature range between 900 and 1100°C.In this work,combined with the index n2=n3=0.47≈0.5 in Eqs.(2)and(3),it can be considered that the quasi-columnar structure TBC prepared by PS-PVD is accordance with the Wagner parabolic function law.In order to obtain the oxidation rate constants of the un-pretreated and pretreated TBCs,Eqs.(2)and(3)are converted according to the Wagner law(Eq.(4)),as shown in Eqs.(5)and(6),respectively.

Fig.3 SEM micrographs and TEM image of the YSZ top coat prepared by PS-PVD.

Fig.4 Interface micrographs of TBCs as-sprayed and after oxidation for 250 h.

Fig.5 Oxidation kinetics fit curves of 7YSZ coatings prepared by PS-PVD.

where δ (μm)is the thickness of the TGO layer,t(s)is the oxidation time,and kc(cm2·s-1)is the reaction rate constant.

As can be known from Eqs.(5)and(6),the oxidation rate constants for un-pretreated and pretreated TBCs are kc5=0.101 × 10-12cm2·s-1and kc6=0.115 × 10-13cm2·s-1,respectively.kc5/kc6=8.78≈9,indicating that the oxidation resistance of the TBC after pretreatment is increased nearly 9 times than that of the un-pretreated TBC.
Fig.6 shows the surface and fracture cross-section SEM micrographs of the 7YSZ top coat after oxidation for 10 h and 250 h at 950°C in comparison with their original morphology before oxidation.Shrinkage cracks on the 7YSZ coating surface after oxidation 10 h are clearly observed in Fig.6(c),which have been formed mainly due to sintering.Meanwhile,some secondary columnar grains are also melting together since the grains sintering(as shown in Fig.6(d)).After oxidation for 250 h,the widths of the original shrinkage cracks are slightly increased,and some small shrinkage cracks near the original shrinkage ones are also observed in Fig.6(e).Moreover,almost all secondary columnar grains are melting together,and the contour of the grains can hardly be observed in Fig.6(f).All in all,the quasi-columnar structure 7YSZ coating is sintering during the oxidation process,resulting in the formation of shrinkage cracks on the coating surface and the melting together of the secondary columnar grains.

Fig.6 Surface and cross-section micrographs of the pretreated PS-PVD-7YSZ top coating.
3.3.IS analysis of quasi-columnar structure TBCs
3.3.1.Impedance spectra and equivalent circuit of quasicolumnar structure TBCs
Fig.7(a)shows a Nyquist plot(high frequency is amplified),and Fig.7(b)shows a Bode plot from impedance measurements for pretreated PS-PVD TBCs,subjected to oxidation at 950°C for up to 250 h.The two figures show a lot of electrical responses,especially four semicircles in Fig.7(a)and distinct θ peaks in Fig.7(b)are observed,which are due to four relaxation processes.Inferred from many references,21–23the four relaxation processes can be attributed to YSZ grain(G)(approx.105–106Hz),YSZ grain(including secondary columnar grains)boundary(GB)(approx.104–105Hz),a thermal grown oxide(TGO)layer(approx.101–104Hz),and an electrode response(E)(approx.10-1–101Hz),respectively.It should be noted,specifically in the Bode plot,that with an increase of oxidation time,the TGO response is more remarkable.
In order to obtain the electrical properties of the 7YSZ coating and TGO layer,it is inevitable to establish an equivalent circuit for fitting the measured impedance spectra,and an electrochemical physical model of PS-PVD TBCs is also established in Fig.8.It should be pointed out that the YSZ grain boundaries(YSZ-GB)consist of voids or cracks in the ceramic layer and the secondary columnar grain boundary.A constantphase element(CPE)is adopted instead of a capacitance in the equivalent circuit,being more suitable to describe the behavior of a non-ideal,i.e.,not pure,capacitor due to dispersion in the electrode and chemical inhomogeneity.A simulation result and a measured impedance spectrum are shown in Fig.9.The simulation results are in good agreement with the measured results.The electrical properties(resistance and capacitance)from the simulation are therefore taken as the measured results.

Fig.7 Impedance spectroscopy of pretreated PS-PVD TBCs after oxidation at different times.

Fig.8 Electrochemical physical model and equivalent circuit for the PS-PVD TBC system.
3.3.2.Relation between impedance spectra and TGO growth
The TGO layer of the pretreated PS-PVD TBCs as-sprayed and after oxidation for 50 h,100 h,and 250 h have been analyzed by EDS as in Fig.10.The EDS results show that the TGO layer mainly consists of alumina,and that the oxygen atom content after oxidation for 100 h obviously decreases.As the TGO layer fully consists of alumina,it is possible to calculate the TGO thickness according to the relationship between resistance and thickness as follows:

where R is the resistance,d is the thickness of the electrode,namely the thickness of TGO,A is the area of the electrode,and ρ is the resistivity(specific resistance)of the electrode material;for alumina,its value of resistivity is constant at the preset temperature.

Fig.9 Simulation spectra based on the equivalent circuit and the measured spectra of the as-spray TBCs.

Fig.10 EDS analysis of the TGO layer after oxidation.
As shown in Fig.11,the resistance of TGO is fitted according to the equivalent circuit plotted as a function of the square root of the oxidation time.Clearly,there is a linear relation between the TGO resistance and the square root of the oxidation time,indicating that the oxidation of 250 h in air at 950°C follows a parabolic law and that the method of characterizing the TGO thickness by its resistance measured by fitting the equivalent circuit is feasible.
The relationships between the resistance and capacitance of the TGO layer and the TGO thickness are presented in Fig.12.Clearly,the resistance of the TGO increases and the capacitance decreases with an increase of the TGO thickness.However,the above relationships are not absolutely linear,and both of the slopes increase slightly during oxidation from 50 to 250 h.According to Eq.(7)and the following Eq.(8)analysis,it indicates that the resistivity(ρ)increases and the relative dielectric constant(ε)decreases during oxidation.


Fig.11 Resistance of the TGO as a function of the square root of the oxidation time.
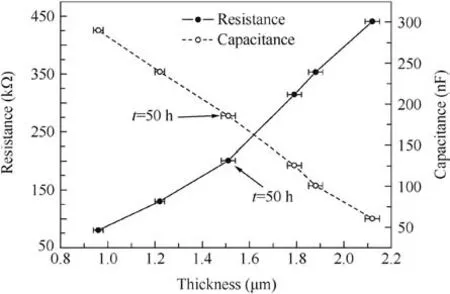
Fig.12 Resistance and capacitance of the TGO as a function of the TGO thickness.
where C is the capacitance,ε is the relative dielectric constant,ε0is the space dielectric constant,and A is the area of the electrode.An increase of the resistivity may be due to TGO densification with oxidation.As is well known,Al2O3is a p-type semiconductor.Its resistivity increases with decreasing oxygen partial pressure that usually decreases with decreasing porosity within a material.24Usually,the oxygen partial pressure is proportional to the oxygen atom content of TGO layers.Therefore,combined with the result in Fig.10,it has been demonstrated that a denser alumina has a smaller oxygen partial pressure within the TGO layer,which will lead to a higher resistivity.However,the reason for the relative dielectric constant’s decreasing is ill-defined and needs to be researched continually.
3.3.3.Relation between impedance spectra and 7YSZ coating prepared by PS-PVD

Fig.13 Resistance and capacitance of 7YSZ coating prepared by PS-PVD as a function of the oxidation time.
The relationships between the resistance and capacitance of the YSZ grains and the oxidation time are presented in Fig.13(a).Both the resistance and capacitance of the YSZ grains show no apparent change during oxidation,which indicates that oxidation at 950°C has little effect on the electrical properties of YSZ grains,namely,the YSZ grains have no significant microstructure or chemical compositional change during oxidation.At the same time,the relationships between the resistance and capacitance of the YSZ grain boundary and the oxidation time are given in Fig.13(b).Clearly,the resistance of the YSZ grain boundary increases and the capacitance decreases with prolonging oxidation time.Nevertheless,the tendencies of the resistance’s increase and the capacitance’s decrease become slow.Zhang et al.12have studied the relationships between micro-cracks in the YSZ coating prepared by EB-PVD and the resistance of the YSZ grain boundary,indicating that the resistance of the YSZ grain boundary increases with increasing micro-cracks and that the resistance decrease of the YSZ is mainly due to the sintering of YSZ coating during the oxidation process.In addition,Karaoglanli et al.25and Lv et al.26have studied the high-temperature oxidation behavior and the sintering effect on the mechanical properties of thermal barrier coatings prepared by APS.Results show porosity decreasing and crack healing of ceramic coatings resulting from the sintering of ceramic coating during the oxidation process.In this work,combined with the analytical result from Fig.6,it can be inferred that a rapid increase of the resistance for the YSZ grain boundary during initial oxidation is due to the formation of shrinkage micro-cracks,although the sintering of portion secondary columnar grains is present in the 7YSZ coating.However,with an increase of the oxidation time,the resistance value of the YSZ grain boundary increases slowly,which is mainly caused by the serious sintering of secondary columnar grains in the YSZ layer.Besides,the slight increase of shrinkage micro-cracks width during oxidation can also be one of the reasons.On the other hand,the above reasons are also suitable for explaining the capacitance variation of the YSZ grain boundary and will not be described in detail.
4.Conclusions
High-temperature oxidation of TBCs prepared by PS-PVD is accomplished at 950°C and investigated by impedance spectroscopy assisted with SEM.The TGO mainly consists of alumina,and the oxidation resistance of the TBC after pretreatment is improved nearly 9 times than that of the unpretreated TBC.The relation among TGO growth,microstructure of YSZ coating,and their corresponding electrical properties can be summarized as follows:
(1)The resistance and resistivity of the TGO layer are related to the thickness and porosity of the TGO layer,respectively.The resistance of the TGO layer increases with prolonging oxidation time resulting in an increase of the TGO thickness following a parabolic law.The resistivity of the TGO layer increases after oxidation for 50 h due to a decrease of its porosity,indicating that the protective capability of the TGO is strengthened with increasing oxidation time.
(2)A rapid increase of the resistance for the YSZ grain boundary during initial oxidation is due to the formation of shrinkage micro-cracks on the surface of YSZ coating,although the sintering of portion secondary columnar grains is present in the 7YSZ coating.However,the main reason why the resistance value of the YSZ grain boundary increases slowly with an increase of the oxidation time is that the sintering of secondary columnar grains in the YSZ layer is more serious,a slight increase of shrinkage micro-cracks width during oxidation being another possible reason.
Acknowledgements
We would like to acknowledge financial supports from National Key Research Program(No.2017YFB0306100),Guangdong Academy of Sciences(No.2017GDASCX-0843),Guang-dong Technical Research Program (No’s.201707010385, 2014B070706026, and 2013B061800053),Guangdong Natural Science Foundation (No.2016A030312015),National Natural Science Foundation of China(No.51501044),and Guangzhou Technical Research Program(No.201707010385).
杂志排行
CHINESE JOURNAL OF AERONAUTICS的其它文章
- Ion engine grids:Function,main parameters,issues,configurations,geometries,materials and fabrication methods
- Aeroelastic stability analysis of heated flexible panel subjected to an oblique shock
- Damage localization eあects of the regenerativelycooled thrust chamber wall in LOX/methane rocket engines
- Receptivity and structural sensitivity study of the wide vaneless diあuser flow with adjoint method
- Large-eddy simulation and linear acoustic modeling of entropy mode oscillations in a model combustor with coolant injection
- Development of secondary flow field under rotating condition in a straight channel with square cross-section
