A study on simultaneous removal of NO and SO2by using sodium persulfate aqueous scrubbing☆
2018-08-18XueKangXiaoxunMaJiananYinXuchunGao
Xue Kang,Xiaoxun Ma,*,Jian'an Yin*,Xuchun Gao
1School of Chemical Engineering,Northwest University,Xi'an 710069,China
2Chemical Engineering Research Center of the Ministry of Education(MOE)for Advanced Use Technology of Shanbei Energy,Xi'an 710069,China
3Shaanxi Research Center of Engineering Technology for Clean Coal Conversion,Xi'an 710069,China
4International Scientific and Technological Cooperation Base of the Ministry of Science and Technology(MOST)for Clean Utilization of Hydrocarbon Resources,Xi'an 710069,China
5Xi'an Shaangu Power Co.,Ltd.,Xi'an 710075,China
Keywords:Simultaneous removal of NO and SO2 Sodium persulfate Ferrous sulfate Hydrogen peroxide Active radicals
ABSTRACT Nitric oxide(NO)removal and sulfur dioxide(SO2)removal by sodium persulfate(Na2S2O8)were studied in a Bubble Column Reactor.The proposed reaction pathways of NO and SO2removal are discussed.The effects of temperatures(35–90 °C),Na2S2O8(0.05–0.5 mol·L−1),FeSO4(0.5–5.0 m mol·L−1)and H2O2(0.25 mol·L−1)on NO and SO2removal were investigated.The results indicated that increased persulfate concentration led to increase in NO removal at various temperatures.SO2was almost completely removed in the temperature range of 55–85 °C.Fe2+accelerated persulfate activation and enhanced NO removal efficiency.At 0.2 mol·L−1 Na2S2O8and 0.5–1.0 mmol·L−1Fe2+,NO removal of 93.5%–99%was obtained at 75–90 °C,SO2removal was higher than 99%at all temperatures.The addition of 0.25 mol·L−1H2O2into 0.2 mol·L−1Na2S2O8solution promoted NO removal efficiency apparently until utterly decomposition of H2O2,the SO2removal was as high as 98.4%separately at 35 °C and 80 °C.
1.Introduction
The excessive emission of NOx(mainly NO and NO2)and sulfur dioxide(SO2)after fossil fuel combustion produces serious negative effects on atmosphere pollution[1]and health problems,especially for China,which has really depended on coal as the energy source during the process of industrial development.Hence,many stringent regulations and institutions have been published to limit the emission of noxious gas on the basis of the economic requirement and the national conditions.The simultaneous removal of NOxand SO2in a reactor can effectively reduce the complexity of system and the in vestment and operation cost.Of these oxides,NO2and SO2are very soluble in water and could be separatedeasily from the exhaust stream bysimplescrubbing.Whereasnitric oxide(NO),as the chief component of NOx,comprising 90%–95%of the entire emission,is quite difficult to remove by wet scrubbing because of its increasing liquid phase resistance to mass transfer[1]and very sparingly soluble,whichgreatlylimits the extensive application of simultaneous desulfurization and denitrification by wet scrubbing.In this respect,how to achieve the goal to abate the NO with high efficiency and lowcostisone of the most important and necessary is sues for the simultaneous removal of NO and SO2in the field of environmental protection.
In past few years,NO absorption in aqueous solutions by adding chemical agents such as yellow phosphorus,H2O2,NaClO2,organic hydroperoxides,KMnO4,andsodiumhypochlorite[2–7],has beenstudied extensively.However,these methods are still in laboratory exploration stage because of the formation of poisonous byproduct(ClO2)or insoluble byproduct(MnO2),secondary environmental pollutions or technical problems and the high costs.In 2010,Nymul E.Khan and Yusuf G.Adewuyi investigated for the first time the effectiveness of sodium persulfate activated by temperature in wet scrubbing of NO.The research results demonstrated that the persulfate is effective and meets many of the criteria of a wet-scrubbing agent for NO removal[8].Different from the above work of Yusuf G.Adewuyi,our experiments were without any phosphate buffer or other additive to change the pH value of persulfate solution,which should be easy for the absorption solution treatment and lower for the cost of materials[9,10].The peroxydisul fate anion(S2O82−)from sodium persulfate activation with the characteristics of more water-soluble,high efficiency,better selectivity,inexpensive price,environmentally friendly,and safe to handle,has been demonstrated to be a strong oxidizing agent and could remain active longer after delivery.Though it is being kinetically slow in original form,the sulfate free radicals(SO4•−)with E0(SO4•−)=2.6 V could be generated as an intermediate radical oxidant in the thermal-,metal,and physical-activated decomposition of persulfate anion through Eqs.(1)–(3)[1–3].Activated peroxydisulfate oxidizes via dissociation into reactive radicals[SO4•−and HO•(E0(HO•)=2.7 V)]with strong oxidation ability and the end products of SO42−and SO32−ions are produced.Tilldate,persul fate has beenusedina wetscrub ber for the treatment of toxic gases(BTEX)[11–15]in various applications[16,17].Because of its stability prior to activation,the use of peroxydisulfate is becoming increasingly popular in the decontamination of groundwater in situ chemical oxidation(ISCO)[18].Though the persulfate activated by temperature has significant synergistic effects on NO removal,the further optimization is very necessary for industrial application and environmental requirement.Therefore,some new technologies and reagents concerning simultaneous removal of NO and SO2are paid close extensive attention.

H2O2(E0=1.77 V)is widely favored as a clean oxidant by researchers,but its low oxidation ability and thermolabile nature restrict its application prospects.In recent years,some results demonstrated that the use of some complex agents such as UV/Fenton-like[19],UV/H2O2reacting systems[20–23]is effective for the simultaneous removal of NO and SO2.Subsequently,the relevant reports about NO removal by using persulfate activated by combined temperature and Fe2+or in the H2O2/S2O82−system also were published in succession[24–32].According to the existing literatures,the oxidation reactions of NO and SO2occurin persul fatesolutionthroug hdirectelectrontrans fer from the persulfate anion,free radicals(mainly SO4•−and HO•)generated following the decomposition and activation of the per sulfate and other agents,and the direct reaction of the NOxwith S(IV)and Fe2+as follows.Moreover,it is necessary to investigate the competition between SO2and other components or radicals.The effects of simultaneous removal of NO and SO2by sodium per sulfate activated by temperature or combined per sulfate and ferrous–EDTA solutions have been preliminarily investigated[8,27].However,to the best of our knowledge,the studies focused on the simultaneous removal of NO and SO2using persulfate activated by the combined temperature and Fe2+or H2O2/S2O82−system were seldom reported.
The objective of this study was to investigate the simultaneous NO and SO2removal efficiencies by sodium persulfate with Fe2+or H2O2.The effects of persulfate activation with or without Fe2+,and dual oxidant(H2O2/S2O82−)system at different temperatures on simultaneous abatement of NO and SO2were investigated.The reaction pathways of NO and SO2removal by wet scrubbing were discussed.
2.Experimental
2.1.Experimental system
As shown in Fig.1,the scrubbing system consisted of a Bubble Column Reactor made of quartz glass(43-mm i.d.×400-mm length).The concentration of the simulated flue gas was controlled by two normal mass flow meters to allow a maximum flow of 100 sccm and 1000 sccm respectively,two preservative mass flow meters to allow a maximum flow of 300 sccm(Beijing Sevenstar Electronics Co.,Ltd.)and two buffer tanks with the volume of 400 ml.The solution of the reactor was heated to desired temperature by an electric heating jacket and the solution temperature was measured by the temperature controller.The mixture gas from there actor was introduced in to the drying bottle to remove moisture and then was analyzed by the flue gas analyzer.The simulated flue gas with a total flow of 39.18 L·h−1at room temperature was introduced throug ha43-mm-diameter gas dispersion tube with poriferous silica sand fitted at the bottom of the reactor.
2.2.Experimental procedure
The chemicals used in the whole experiments included sodium per sulfate(Na2S2O8,powder,AR),ferrous sulfate hep tahydrate(FeSO4·7H2O,particle,AR),and hydrogen peroxide(H2O2,25%,AR).The concentration of N2,SO2and O2was higher than 99.9%and the NO standard mixture gas was comprised of N2and NO.The water for preparing the experiment solutions was made by the Up Water Purification System.

Fig.1.Process flow diagram(PFD).1—N2cylinder;2—O2cylinder;3—NO cylinder;4—SO2cylinder;5–8—mass flow meters;9–10—buffer tank;11—Bubble Column Reactor;12—drying bottle;13—temperature controller;14–15—three-way valve;16— flue gas analyzer.
Pure,dry nitrogen gas was bubbled through the reactor for at least 30 min to remove any dissolved oxygen before the start of the experiments.After that,the simulated flue gas was passed through the bypass line and the temperature controller was opened to heat the water in the reactor.When a stabilized reading and required temperature were obtained,appropriate quality of sodium persul fate and/or other agents were added directly in to there actor to make scrub bing solution achieve the desired concentration,and water was then added to make the total volume 500 ml.The periphery of the reactor was surrounded by silica wool for thermal preservation.For Fe2+activation experiments,the Fe2+was added into the water together with the persul fate.The outlet concentration of the flue gas(NO and SO2)from the reactor was analyzed using the flue gas analyzer(MRU GmbH.Co.,Germany).Once the persulfate was added into the reactor,the simulated flue gas was switched to the inlet of the reactor by the three-way valve and data acquisition was started.The data were collected at 8-s intervals.The reactor allo wed the gas to flow continuously in an upward direction,and the liquid phase could be operated either in batch or continuous mode.In our experiments,there actor operated under the condition of semibatch mode,that is,the gas flowed upward continuously,and the liquid was stationary.All agents are added into the aqueous solution at one time.
2.3.The removal efficiency
When the simulated flue gas was bubbled through the reactor,NO and SO2reacted with the persul fate activated by temperature and Fe2+or the dual oxidant()and then were removed.The NO or SO2removal efficiency is defined as:

where Cinand Coutare the steady-state concentration[in parts per million(ppm)]of NO or SO2as recorded from the flue gas analyzer at the inlet and outlet of the Bubble Column Reactor,respectively.
3.Results and Discussion
3.1.Effect of Na2S2O8concentration and temperature on NO and SO2removal efficiencies
In this paper,several sets of experiments at all temperatures[35,55,65,75,85,and 90 °C(±1 °C)]and persulfate concentration[0.05,0.1,0.2,0.3 and 0.5 mol·L−1(±0.5%)]were conducted to investigate the removal efficiency of NO(500 μl·L−1initial gas phase)and SO2(1000 μl·L−1initial gas phase).
The effects of temperatures and persulfate concentration on NO and SO2removal(at time≥2000 s)are illustrated in Figs.2 and 3.Fig.2 shows that at the lower temperatures of 35 °C and 55 °C,the oxidation reactions are less aggressive due to the slow generation rate of SO4•−(see Eq.(1)),which leads to a slow increase of the NO removal ef ficiency for the entire range of persulfate concentration.As Eq.(1)has shown,the rate constants of persulfate decomposition at 75°C have been reported to be 570 times greater than that obtained at 25°C.This means that the thermal activation simply enhances the generation rate of reactive radicals for similar persulfate concentration.Hence,as the persulfate concentration increases continuously,the NO removal efficiency is observed to enhance rapidly because the formation rate of SO4•−radical sfrompersul fateactivation in creases with in creasing temperature(>65 °C).Until the concentration of the persulfate reaches 0.2 mol·L−1,beyond this level,a change in the concentration of persulfate does not induce a proportional change in NO removal efficiency.The effects of temperatures on SO2removal efficiency using different persulfate concentrations are illustrated in Fig.3 with the results:i)low solubility leading to low SO2removal efficiency at the low temperatures(35°C),ii)increasing oxidation and absorption efficiency of SO2up to ~99%at the high temperatures(55–85 °C)and iii)at 90 °C,the simultaneous self-recombination and inter-combination of the radicals(SO4•−and HO•)because the excessiverelease of SO4•−results in a slightly decrease of SO2removal efficiency and exists the critical temperature.The results indicate that almost 99%SO2removal ef ficiency can be achieved with the temperature changed between 55°C and 80°C at all persulfate concentrations.Based on the mechanism presented by Bartlett and Cotman[33]for uncatalyzed decomposition of persul fate,we canassumethat the followingse to freaction pathways with their corresponding rate constants(Eqs.(4)–(15))coupled with Eq.(1)are responsible for the simultaneous removal of NO and SO2[8,29].the persulfate concentration of 0.2 mol·L−1is chosen as the optimal in future works.


Fig.2.Dependence of NO removal on persulfate concentration at different temperatures.

Fig.3.Dependence of SO2removal on persulfate concentration at different temperatures.

Hence,reactions(16)–(19)are proposed based on the overall stoichiometries of NO and SO2oxidation by the persulfate anion[8].

The effects of persulfate concentration on NO and SO2removal at 75 and 85 °C is illustrated in Fig.4.At 0.2 mol·L−1persulfate,the NO removal efficiencies are found to be 62.91%and 73.22%at 75 and 85°C,respectively.Beyond this level,there is no obvious increase for the NO removal efficiency.The maximum removal efficiency(~99%)of SO2could be achieved at all persulfate concentration levels.Therefore,

Fig.4.Dependence of NO and SO2removal on persulfate concentration at 75 °C and 85 °C.
3.2.Effects of Fe2+concentration on NO and SO2removal efficiencies by sodium persulfate
The sodium persulfate as an oxidizer could be decomposed to produce SO4•−radicals by Fe2+at normal temperature and the Fe2+ions were oxidized to generate Fe3+ions.Moreover,the rate constants of persulfate activated by Fe2+are 3.5×105faster than that by temperature activation at70°C(seeEq.(2)).So a small quantity of Fe2+can accelerate the activation and decomposition of per sulfate to produce more SO4•−radicals,which is responsible for HO•production.However,it should be paid abundant attentions that the existence condition of Fe3+ions generated from Fe2+oxidation in aqueous solution is quite strict:the Fe3+ions easily precipitated,the precipitate reaction began at pH 2.7 and finished at pH 3.7.All of these restrict the extensive application of Fe2+ions in different fields.Fortunately,the aqueous solutions of per sulfate are in itself acidic(at pH 1.45 ± 0.1 with 0.2 mol·L−1per sulfate concentration)and the production(such as HNO3,H2SO4and small amounts of HNO2)emerged from NO and SO2oxidation and absorption process ensures that the experiments could go on smoothly in required acid condition.The produced ferric sulfate[Fe2(SO4)3]could be separated out from the reactant solution including Na2SO4,NaNO3by adding alkali liquor to form precipitate via reaction(20),which makes sure that the reaction products could be purified and utilized ulteriorly.In addition,Fe2+in the solution could react with NO to produce brown complexes[Fe(NO)SO4]according to reaction(21)and the absorption liquid could be recycled through adding C2H6OH solution as an electron donor after calefaction and desorption.

The objective of this study was to experimentally investigate the Influence of various levels of Fe2+ions coupled with the thermally activated 0.2 mol·L−1persulfate on NO and SO2removal at all temperatures(35–90 °C).The experimental results are outlined as follows(Figs.5–7).As shown in Fig.4,the removal efficiency of NO is much better with Fe2+than nano-Fe2+,increased temperature leads to an increase in NO and SO2removal in the absence and presence of Fe2+.The results are divided into two disparate phenomena by the temperature of 75°C as a boundary:the NO removal efficiency shows a benign tendency,especially for a concentration of 1.0 mmol·L−1Fe2+,where the NO removal efficiency goes from 62.9%without any Fe2+to 93.5%with an Fe2+concentration of 1.0 mmol·L−1.Above 75 °C,the result tendency is changed.The Fe2+concentration of 0.5 mmol·L−1shows optimal efficiency on NO removal and the maximum increases up to 99%at 90°C.As documented in the literature,the per sulfate oxidation reaction has taken place through both direct electron transfer and free radical reactions via SO4•−radical from persulfate activation.The absorption of NO by persulfate solution is assumed to be driven mainly via reactions(5),(16)and(20)with the reactive radicals(SO4•−and HO•)generated by S2O82−activation.The persulfate activation with and Fe2+ions could generate SO4•−radicals(Eq.(2)),which might further undergo interconversion to a hydroxyl radical(HO•)depending on pH levels in accordance with Eq.(4).Meanwhile,the propagation reactions and the termination reactions involve the consumption of free radicals and the production of other radicals or transient species by Eqs.(4)–(9)and(22).NO could be oxidized to NO2through Eq.(16).The direct interactions,self-recombination and intercombination of the radicals might tend to reduce the NO oxidation efficiency represented by reactions(23)–(26),which is more prominent at the higher temperatures or higher concentration of active radicals[8,27–33].

Yang Xianliu group[34–36]presented the effects of Fe2+concentration on NO absorption rate and demonstrated that the NO absorption rates enhanced with the Fe2+concentration increased(below 5.0 mmol·L−1),but inhibited the increase with further increase(above 5.0 mmol·L−1)because of a great self-consumption of HO•radicals in a short time via reaction(21).
The results of SO2removal at 0.2 mol·L−1sodium persulfate activated by combined temperature and Fe2+are demonstrated in Fig.5.SO2could be almost completely removed when the concentration of Fe2+increases up to 1.0 mmol·L−1.At 5.0 mmol·L−1Fe2+concentration,the SO2removal efficiencies increase with the temperature increasing but still lower than others due to the reducing concentration of free radicals caused by the competition and self-recombination among Fe2+,SO4•−and HO•,as represented by Eqs.(23)–(26).Based on previous studies,the following set of reaction pathways(with their corresponding rate constants in the literature where available)are hypothesized to be plausibly responsible for the simultaneous consumption and/or oxidation of NO and SO2in temperature/Fe2+-activated aqueous persulfate as shown in Eqs.(2),(10)–(15)and(23)–(27)[8,31–33].The results also could reference previous analysis on NO removal.Fig.7 depicts the effects of temperature on NO(Fig.7a)and SO2(Fig.7b)removal using 0.2 mol·L−1persulfate at all Fe2+concentration levels.As a whole,maintaining suitable amount of Fe2+concentration to reduce the consumption for sulfate and other free radicals could guarantee advantageous NO and SO2removal efficiency.The results suggest that the concentration range of 0.5–1.0 mmol·L−1Fe2+is optimal on the NO and SO2removal process at 0.2 mol·L−1Na2S2O8solution.


Fig.5.NO removal efficiency as a function of temperature for different concentrations of Fe2+at0.2mol·L−1.Na2S2O8,(a)0.5 mmol·L−1,(b)1.0mmol·L−1,(c)5.0mmol·L−1and(d)NO removal efficiency vs.temperatures.
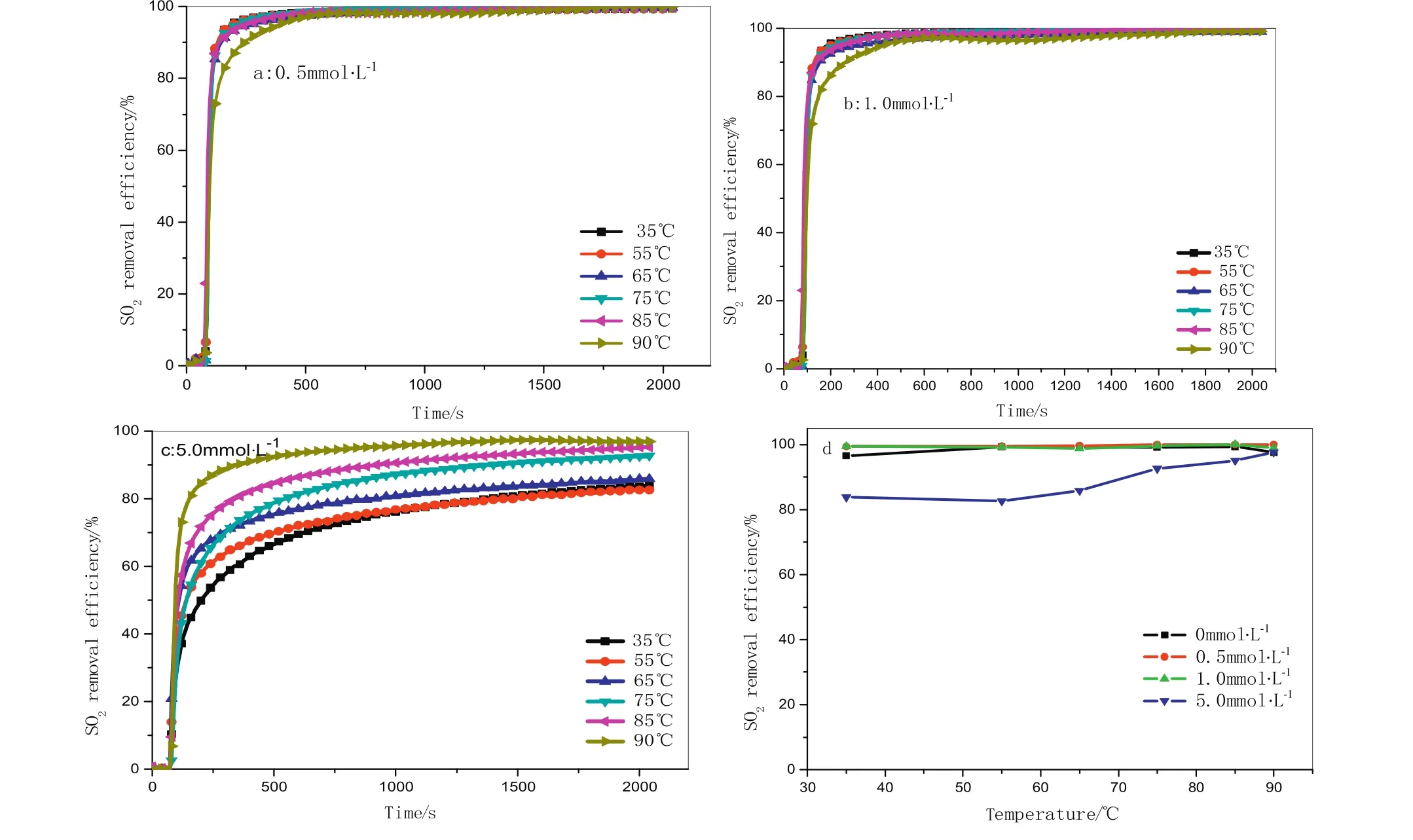
Fig.6.SO2removal efficiency as a function of temperature for different concentrations of Fe2+at0.2mol·L−1.Na2S2O8,(a)0.5mmol·L−1,(b)1.0mmol·L−1,(c)5.0mmol·L−1and(d)SO2 removal efficiency vs.temperatures.
3.3.Effect of H2O2/Na2S2O8system on NO and SO2removal efficiencies
Hydrogen peroxide,as a strong oxidant,sat is fies many of the criteria such as inexpensive,efficient,and environmentally benign,for being a candidate for the chemical oxidant used in the wet scrubbing of NO and SO2.Most of all,it could generate HO•radicals,the most powerful oxidizing species after fluorine radical[22,23].Hence,H2O2has been used for post-combustion flue gas treatment in Photochemical methods at thelowtemperature.However,the out let temperature of the fluegas from coal- fired power plants is high enough to decompose the H2O2to release heat for end productions of H2O and O2,which restricts its wide application in various industries.In view of previousresearches,the relevant experiments were conducted to investigate the effect of persulfate coupled with H2O2activated by temperature on NO and SO2removal as follows.

Fig.7.Dependence of NO and SO2removal efficiency on FeSO4concentration in the presence of 0.2 mol·L−1Na2S2O8at different temperatures(35–90 °C).

Fig.8.Dependence of NO removal on H2O2concentration without any persulfate at 50°C.
Fig.8 illustrates the NO removal efficiency as a function of time at some H2O2concentration and determined the optimum concentration of H2O2of 0.25 mol·L−1at 50 °C for subsequent experiments.The effects of the H2O2/Na2S2O8system at different temperatures on NO removal efficiency are shown in Fig.9a.At 35°C,the presence of H2O2generates an inconspicuous role in promoting NO removal efficiency due to low concentration of active radicals including SO4•−radicals generated from Na2S2O8activation and HO•radicals generated from Na2S2O8or H2O2decomposition.However,with the addition of 0.25mol·L−1H2O2into0.2mol·L−1Na2S2O8,the NO removal efficiency increases by20%–30%for a set time as temperature increases from55°C to 80 °C than that in the single oxidant(S2O82−)system.On the one hand,this is apparently due to the extra addition of H2O2,which generates more radicals including SO4•−,HO•and hydroperoxyl radicals(HO2•,E0=1.60 eV,Eqs.(29)and(33))from Na2S2O8activation and H2O2decomposition,and finally enhances the removal efficiency of NO.On the other hand,high temperature also accelerates the decomposition of H2O2to produce H2O and O2through Eqs.(28)–(31)[9,10,24,25,39]and finishes the assignment role as an oxidant or a production agent of free radicals within a short time.The starting time,in which,the removal efficiency of NO is improved obviously,moves forward gradually and the working time shortens continually along with the rise in system temperature.This maybe could be explained as follows:the heat from part of H2O2decomposition promotes persulfate activation to produce more radicals for the formation of a reaction system with stronger oxidizability,another part of unreactive H2O2takes part in the con summation and the regeneration of H2O2,HO•,HO2•andO2simultaneously by Eqs.(28)–(31).Meanwhile,the O2may also participate in NO oxidation.Corresponding with that,the decomposition of H2O2would increase the concentration of the O2with the similar tendency via reactions(30)–(31)and(34)as shown in Fig.9b.


where the collision partner,X,is water.
However,it should be noted that the dual-oxidant system does not cause a doubled overall performance.It is highly possible that the interference among different radicals would occur during the reaction process.It was known that 2 mol of SO4•−radicals could be generated by direct heat of 1 mol of S2O82−ion(Eq.(1))and 2 mol of OH•radicals could be formed by direct heat of 1 mol of H2O2(Eq.(28))in the single oxidant solution(S2O82−or H2O2),respectively.In the combined H2O2/S2O82−reaction system,increased temperature from 55 °C to 80 °C led to generate more radicals and increase the whole concentration of the free radicals in aqueous solution.However,more radicals would also be consumed to form much weaker oxidants(O2,HO2•and H+ions)as represented in Eqs.(33)–(36)[37]and the reaction system is in a strong acid condition,which was not beneficial to NO removal[2].It is concluded that the observation of decreasing NO removal efficiency slightly after complete decomposition of H2O2compared with that of single oxidant(Na2S2O8)is due to increasing H+ion concentration in the reaction system.


Fig.9.Dependence of NO removal on 0.25mol·L−1H2O2at 0.2 mol·L−1persulfate,(a)NO removal efficiency pro file at all temperatures;(b)O2concentration pro file at all temperatures.

Fig.10.Dependence of SO2and NO removal on 0.25 mol·L−1H2O2at 0.2 mol·L−1persulfate,(a)SO2removal efficiency pro file at all temperatures;(b)NO/SO2removal efficiency vs.temperatures.
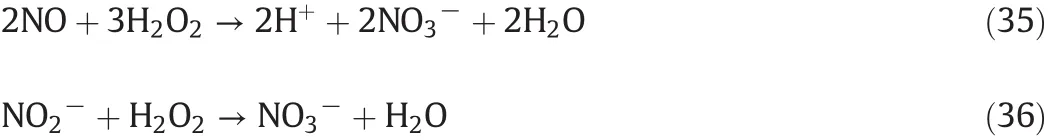
Fig.10a and b showed the effects of 0.2 mol·L−1sodium persulfate coupled with 0.25 mol·L−1H2O2on SO2removal at all temperatures.The results display that the best removal efficiency of SO2appeared at 35 °C,75 °C and 80 °C(>98%).At the low temperature(35 °C),the decomposition rate of H2O2is slower and the dual-oxidant promoted SO2to beremoved effectively.With the temperature increasing,though the accelerated degradation of H2O2decreases the concentration of H2O2,higher temperature would also accelerate the dual-oxidant system to produce more active radicals,which is beneficial for SO2removal.So with the temperature increasing,the SO2removal efficiency is improved.Except the reaction pathways listed in previous work,as Eqs.(37)–(39)have shown,the substantial quantity of H+ion also contributes for SO2removal in the S2O82−/H2O2system[37–40].

Then it follows that the H2O2/S2O82−system showed a significant effect on simultaneous desulfurization and denitrification from the flue gas.
4.Conclusions
The simultaneous removal of NO and SO2by using persulfate aqueous scrubbing has been studied in a Bubble Column Reactor under different activation conditions(heat or heat/Fe2+)and dual-oxidant system(H2O2/S2O82−),and the reaction pathways were discussed.The research results were shown as follows.
NO removal efficiency increased as the persulfate concentration increased up to 0.2 mol·L−1,beyond this level,the efficiency increased hardly for higher persulfate concentration.SO2could be removed by 99%in the temperature range of55–85°Cat all persulfate concentration levels.
Small addition of Fe2+ionsinto 0.2 mol·L−1persulfatecould enable persulfate solution to produce more active radicals and increased the NO removal efficiency,up to 93.5%with 1.0 mmol·L−1Fe2+at 75 °C and 99.0%with 0.5 mmol·L−1Fe2+at 90 °C.At the range of 0.5–1.0 mmol·L−1Fe2+,SO2was almost completely removed.However,beyond 1.0 mmol·L−1Fe2+,the removal efficiencies of NO and SO2decreased rapidly due to the higher competition between SO4•−radicals and excess Fe2+ions.
For the dual H2O2/S2O82−system,the persulfate solution could be activated to form more radicals(SO4•−,HO•and HO2•)and other oxidant(O2).Meanwhile,the H2O2decomposition was also sped up.NO removal efficiency was higher than 91%at 80 °C,0.2 mol·L−1Na2S2O8and 0.25 mol·L−1H2O2,SO2removal efficiency was larger than 98%at all temperatures except for 55°C.
The research results from the present work would provide some theoretical guidance for further studies,optimization and industrial applications of NO and SO2removal technology.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Understanding the Influence of microwave on the relative volatility used in the pyrolysis of Indonesia oil sands☆
- Degradation and mineralization of aniline by O3/Fenton process enhanced using high-gravity technology☆
- Three-liquid-phase extraction and separation of V(V)and Cr(VI)from acidic leach solutions of high-chromium vanadium–titanium magnetite☆
- Insight into the degradation mechanism of cefixime under crystallization condition☆
- Experimental and simulation of the reactive dividing wall column based on ethyl acetate synthesis☆
