Insight into the degradation mechanism of cefixime under crystallization condition☆
2018-08-18LingyuWangXiaonaLiYuminLiuDandanHanShiyuanLiuTengZhangBoYuJunboGong
Lingyu Wang,Xiaona Li,Yumin Liu,Dandan Han,Shiyuan Liu,Teng Zhang,Bo Yu,Junbo Gong*
School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
Keywords:Chemical stability Degradation kinetics Degradation Mechanism cefixime Additives
ABSTRACT The chemical stability of cefixime was determined by high-performance liquid chromatography(HPLC)under different conditions,including factors such as pH,solvents,initial concentration,temperature and additives.The degradation process follows the first-order kinetics.A pH-rate pro file exhibits the U-shape and shows the maximum stability of cefixime at pH=6.The stability in different pure solvents is ranked as acetone>ethanol>methanol>water,while the degradation rate of cefiximeexists a maximumat the ratio of0.6 in water+methanol mixtures.In addition,the degradation rate increases with the temperature increasing and the activation energy of degradation was found to be 27.078 kJ·mol−1in acetone+water mixed solvents.The addition of different additives was proven to either inhibit or accelerate the degradation.The degradation products were analyzed using HPLC,LC–MS and infrared spectroscopy,and the possible degradation pathways in acid as well as alkaline environment were proposed to help us understand the degradation behavior of cefixime.
1.Introduction
cefixime,(C16H15N5O7S2,CAS Registry No.79350-37-1,Fig.1),a broad spectrum of the third generation of oral cephalosporin,has the advantages of high effectivity,long-term,wide adaptability and so on[1,2].It is resistant to hydrolysis by most β-lactamases and can be used for the treatment of susceptible infections,including gonorrhea and otitis media.As a new generation of cephalosporins,cefixime has great potentiality in drug market because of its strong antibacterial effect and small dose[3,4].
β-Lactam antibiotics are able to kill or inhibit thegrowth of bacteria,the antibacterial activity of which mainly depends on the existence of the β-lactam ring fused to a six-membered ring.However,it is because of the instability of its structure and the complexity of the production conditions,the degradation of β-lactam antibioticss is easy to occur.Cefixime is one of widely-used cephalosporins and there are many schemes to prepare and purify the product of cefixime.However,the degradation of cefiximeis inevitable during the preparation and crystallization process because the conditions of process have to be changed successively,suchassolvents,temperature,pH,additives,concentration of reactants and so on[5-7].So it is urgentto understand the stability of cefiximein solutions with different conditions to ensure the quality and purity of the product.
When it comestothestability of cefixime,there are so mere ports on its degradation study.Jessie So fia Pamudji et al.[8]have evaluated the stability of cefixime-β-cyclodextrin inclusion complex in liquid suspension dosage form.The photo-thermal stability of cefixime was investigated by Ahmed E.M.Saeed et al.[9]in 2011.Subrata Mallick et al.[10]have studied the effect of captisol complexation and water soluble polymer on the hydrolytic degradation of cefixime in water.Satoshi Kitamura et al.[11]have represented the solid-state stability of cefixime trihydrate and so on.However,there is still a lack of knowledge about the degradation of cefixime in mixed solvents without stabilizers under stress conditions,especially under the conditions commonly used in industrial manufacture.So an in-depth systematic study of this matter is required to attain necessary information about the stability of cefixime in industrial conditions.

Fig.1.Chemical structure of cefixime.
To reduce the degradation in solution and optimize the process parameters for high-purity and high-yield products,we collect quantitative degradation data so as to obtain more detailed information on the degradation kinetics and mechanisms of cefixime in mixed solutions by high-performance liquid chromatography(HPLC).The study focuses on the effects of several process parameters(solvent,pH,temperature,concentration,additives)on the stability of cefixime in solutions.It must be stressed that the selection of all process parameters in this study are based on the process of preparation and crystallization of cefixime in industry.The overall degradation process was analyzed by HPLC.Then the degradation products were characterized by LC–MS and IR.According to the experimental results,the possible degradation pathways of cefixime in aqueous solution were proposed.All these studies contribute to a better insight into the chemical stability of cefixime products.
2.Experimental
2.1.Materials
Cefixime,with a mass purity of>99%,was kindly supplied by Guangzhou Baiyunshan medicine Co.Ltd.(Guangzhou,China).Methanol,ethanol and acetone(purchased from Tianjin Chemical Reagent Co(Tianjin,China))were of analytical-reagent grade.The chemicals used in HPLC(tetrabutylammonium hydroxide,phosphoric acid,acetonitrile)were purchased from Tianjin Jiangtian Reagent Co.(Tianjin,China).Distilled deionized water(conductivity=18.2 MΩ·cm)used throughout the measurement process was prepared in our laboratory.
2.2.Analytical procedures
HPLC was performed with an Agilent 1200 variable wavelength detector(VWD spectroscopy)and an Agilent extend-C18 reverse-phase column(250 mm × 4.6 mm,5 μm).The analytical method was selected according to the Chinese Pharmacopeia 2010[12].The mobile phase was made up of acetonitrile and tetra butyl ammonium hydroxide solution in a ratio of 260:90 v/v and the UV detection was made at 254 nm.The tetra butyl ammonium hydroxide solution was made by diluting 25 ml tetrabutyl ammonium hydroxide(10%water)to 1000 ml with distilled deionized water and then the pH was adjusted to 7.0 with phosphoric acid solution(1.5 mol·L−1)before mixing with the acetonitrile.
2.3.Kinetic measurements
The degradation of cefixime in different solvent systems including water,ethanol,methanol,acetone and their mixtures(1:1 v/v),was deter mined at303K with the initial concentration of5mg·ml−1in the pH of 3.01.Then the influence of pH on the degradation of cefixime in acetone-water(1:1 v/v)system was investigated at the same condition.HCl(0.1 mol·L−1)and NH3·H2O were used to adjust the pH from 1 to10,respectively.The ionic strength(μ)of all the solutions was adjusted to 0.5 mol·L−1with a solution of sodium chloride.All pH measurements were made with a pH meter(Five Easy Plus FE20,Mettler Toledo Switzerland)with an accuracy of 0.01 A.To assess the degradation kinetics of cefixime in solutions,the flasks containing the drug with known initial concentration were placed in a thermostat shaker(type 501A,Shanghai Laboratory Instrument Works Co.,Ltd.,China,with the precision of±0.1 K)to keep a certain temperature in dark in the laboratory.After definite time intervals,appropriate 5 g samples weighed by an analytical balance(type AB204-N,Mettler-Toledo,Switzerland)with a precision of±0.0001 g were withdrawn.And then they were diluted to a certain concentration to be analyzed by HPLC.
2.4.Drug degradation dynamics model
2.4.1.Determination of reaction order and activation energy
Most degradation reaction of pharmaceuticals can be regarded as zero order, first order or pseudo- first-order reaction in solutions[13,14].The relationship between the degradation rate and concentration can be expressed as Eq.(1).

where k is the reaction rate constant.It is concerned with the nature of the reactants,temperature,solvents and pH and so on.The greater the k is,the faster the degradation reaction will be.And n is the reaction order,which represents the effect of concentration on the degradation rate.
2.4.2.Activation energy
The relationship between the activation energy of degradation reaction and the reaction rate constant can be expressed as Eq.(2)[13].

where A is frequency factor which can be considered as a temperature independent constant.R is the gas constant and Tis the reaction temperature.Eais the activation energy.The reactants must acquire the minimum energy of Eato make the degradation reaction occur.Therefore,Eacan be used to compare the stability of the drugs.And by taking the natural logarithm of Eq.(2),it takes the following format as Eq.(3)

From the equation we can see that the higher the temperature is,the faster the reaction becomes.A linear relationship should be obtained when the lnk vs 1/T is plotted.Hence the activation energy Eacan be obtained from the slope of the plot.
3.Results and Discussion
3.1.Degradation kinetics of cefixime
3.1.1.Degradation of cefixime in different solvent systems
In order to select an appropriate solvent to prepare and purify the cefixime powder in industry,the temporal evolution of concentration during the degradation of cefixime in different solvent systems was analyzed by HPLC and plotted in Fig.2.It can be seen that the natural logarithm of the concentration of cefixime(lnc/c0)versus time is linear approximation in all the solvent systems and follows the apparent first-order kinetics.The degradation rate of cefixime follows the rank of acetone<ethanol<acetone/water(1:1 v/v)<ethanol/water(1:1 v/v)<methanol<water<methanol/water(1:1 v/v).Obviously,our results show that the degradation is solvent-dependent.This phenomenon is largely due to the different polarity of different solvents.And the effect of the solvents on the stability of drugs can be described as the following equation[15].

where k is degradation rate constant and ε is the dielectric constant.k∞is the rate constant when ε goes to ∞.Zaand Zbare the electric charge of ion and drug respectively.When Zaand Zbcarried same type of charges,the rate constant k is in accordance with the dielectric constant of the solvents.The pharmaceutical ions of cefixime and the attack ions both have positive electric charge in pH=3.02.When the ε of solvent is relatively low,the degradation rate constant k is low accordingly.That is to say that the solvent with low ε can reduce the rate constant k.The dielectric constant of the solvents follows the rank of acetone<ethanol<methanol<water[16].So the degradation rate constant k is in the order of acetone<ethanol<methanol<water,the trend of which agrees well with our experiment.
However,the effect of solvents on the degradation of drugs is complex.It is worth noting that the degradation in methanol/water followed the order of methanol<water<methanol/water(1:1 v/v),which indicates that the degradation may be concerned with the ratio of solvent mixtures.Therefore we investigated the degradation kinetics in different volume ratios of methanol and water mixtures.The degradation rate constant k(Table1)calculated from the slope of degradation curves versus the volume ratios was plotted in Fig.3.It is obvious that the degradation rate constant k does not increase monotonically with increasing volume ratios of water.The maximum of degradation rateis reached at the volume ratio of 0.6.This phenomenon is similar to that called co-solvency[17]in dissolution behavior of many pharmaceuticals which exist extreme values at a certain ratio of solvent mixtures.So we can deduce that the effect of solvent is complex,which is related not only to dielectric constant but also the interactions between pharmaceuticals and solvents.Another reason for this phenomenon may be that,at the extreme value,the polarity of solute is similar to that of the mixed solvents.
Table 1 Reaction kinetic equations and the characteristic parameters

Fig.3.Plot of the degradation rate constant of cefixime versus the ratio of methanol–water mixtures at 303.15 K.
Generally,the acetone/water mixture is chose as the solvent for industrial crystallization.And our results also demonstrate the degradation rate in the acetone/water mixture is relatively low,which may be one of the reasons why such solvent is used in industry.In order to provide better guidance to practical production,we selected the acetone/water mixture as the solvent system to investigate the influence of pH,temperature,initial concentration and additives on the degradation of cefixime.
3.1.2.The pH-dependent degradation in the acetone/water
In the real industrial process,it is inevitable to use acid or alkali to adjust pH during the crystallization process.Therefore,the study of pH-dependent degradation of cefixime is essential.To investigate the degradation rate in acetone/water(1:1 v/v)as a function of pH(1–10),the logarithm of the concentration of cefixime(lnc/c0)versus time was plotted.The result shows that degradation process follows the first-order kinetics over the range of our studying pH.As shown in Fig.4,the degradation of cefixime in the alkaline solution is relatively faster than the acidic solution,indicating that the cefixime is more unstable in alkaline solution.While the cefixime is relatively stable in neutral solution because the degradation rate at pH=6 is slow.The effect of pH on the rate constant k can be described by the following equation[18].

Fig.4.Influence of the pH on the degradation of cefixime at 303.15 K.
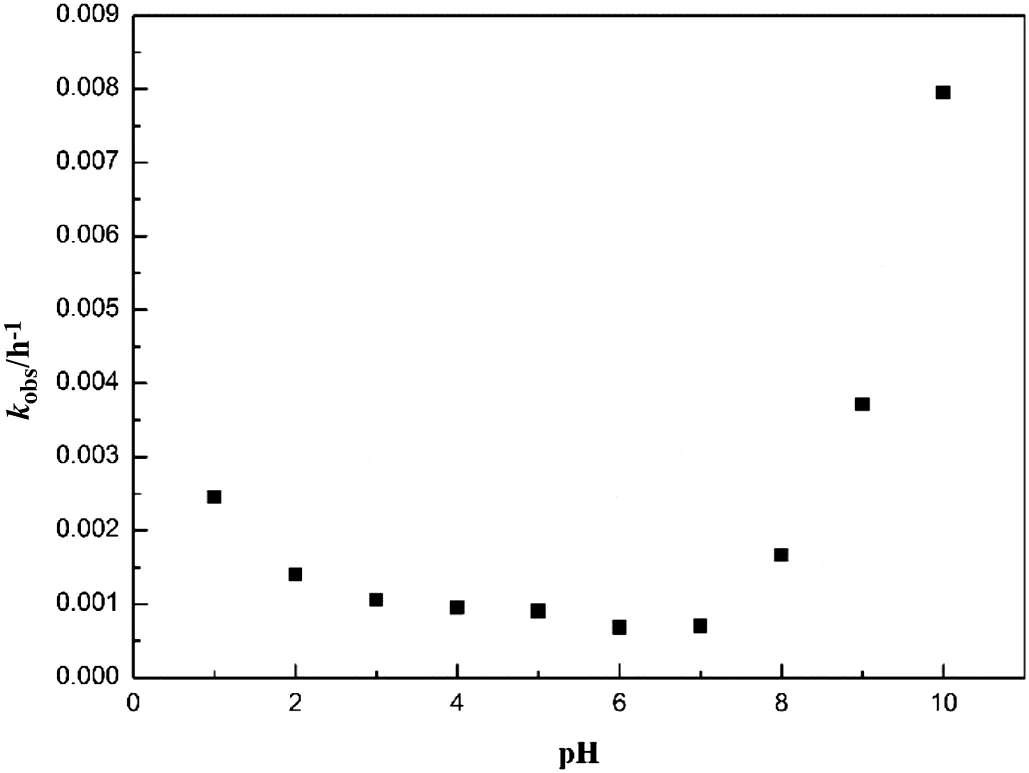
Fig.5.Plot of the degradation rate constant of cefixime versus pH.

Fig.6.Influence of the temperature on the degradation of cefixime in acetone+water.

Fig.7.Plot of the logarithm degradation rate constant of cefixime versus 1/T.

Fig.8.Influence of the initial concentration on the degradation of cefixime.

Fig.9.Influence of different additives on the degradation of cefixime:(a)inhibition(b)acceleration.

Fig.10.Chemical structure of cephalosporins.

where k is the sum of specific rate constants.kO,kH+,and kOH−are the rate constants catalyzed by H2O,H+,and OH−,respectively.Meanwhile the[H+]and[OH−]are the activity of H+andOH−,respectively.There action is mainly acid-catalyzed in the acidic environment,while it is mainly alkali-catalyzed in the alkaline environment.The equation can be simplified as the Eqs.(6)and(7)in the acidic and alkaline environment,respectively.

It can be concluded that the slope of the plot of acid-catalyzed degradation is negative,while the slope of alkali-catalyzed degradation is positive.The curves of k-pH have different shapes,such as U-shape,S-shape,V-shape and so on[19-22].The k-pH pro file,obtained from first-order kinetic plots of cefixime,was constructed as shown in Fig.5.The k-pH pro file is U-shaped and it shows maximum stability of cefiximeata bout pH=6.Considering the degradation result,it is better to control the pH at 6–8 where the degradation of cefixime can be reduced and high yields can be obtained.The degradation in acidic environment is relatively low and the isoelectric point of cefiximeis pH=2.1,so the pH=2.1 could be chosen as the terminal point of crystallization.
3.1.3.Temperature effect on the degradation of cefixime in the acetone/water

Fig.11.The degradation mechanism of cephalosporin antibiotics.
The effect of temperature on the stability of cefixime was studied at pH=3.04 with an initial concentration of 5 mg·ml−1at 283.15 K,293.15 K,303.15 K and 323.15 K,respectively.It can be found that all curves presented in Fig.6 are for the apparent first order reaction.The temperature has a remarkable effect on the reaction and a higher temperature results in a significant increase in the degradation rate.It can be quantitatively described by the Arrhenius equation as described in the Section 2.4.2.Then a curve of lnk versus 1/T was plotted(Fig.7)to evaluate the apparent activation energy Ea.The fitting equation is lnk=3.812–3256.8901/T and the Eaobtained from the slope was 27.078kJ·mol−1.According to the results,the temperature for the crystallization was chosen to be293.15K in order to reduce the degradation in solution.
3.1.4.Initial concentration on the degradation of cefixime in the acetone/water
To carry out a comparative study of cefixime degradation in aqueous solutions with different initial concentrations, the logarithm of residual cefixime concentration was plotted versus time for three different initial concentrations (Fig. 8). It can be seen that the plots are a set of parallel lines,which indicates that the overall degradation rate constants almost remain unchanged and were independent of initial drug concentration
3.1.5.Additives on the degradation of cefixime in water
It is well-known that the additives can Influence the solubility,habit,and purity of the crystallization products.In addition,they may lead to the stabilization of the drug or the acceleration of its breakdown in solution,which largely depends on the nature of degradation reaction and the structure of additive.Many studies have investigated the impact of additives including excipients on the degradation of drugs in order to optimize the degradation process.The stabilizing effect of α-,β-and γ-cyclodextrins on prostacyclin have been explained by Hirayama et al.[23].Suetal.have studied the effect of different polymers on the solution stability of furacilin[24].The effects of various excipients on the stability of perindopril tert-butylamine have been described by Zhang et al.[25].
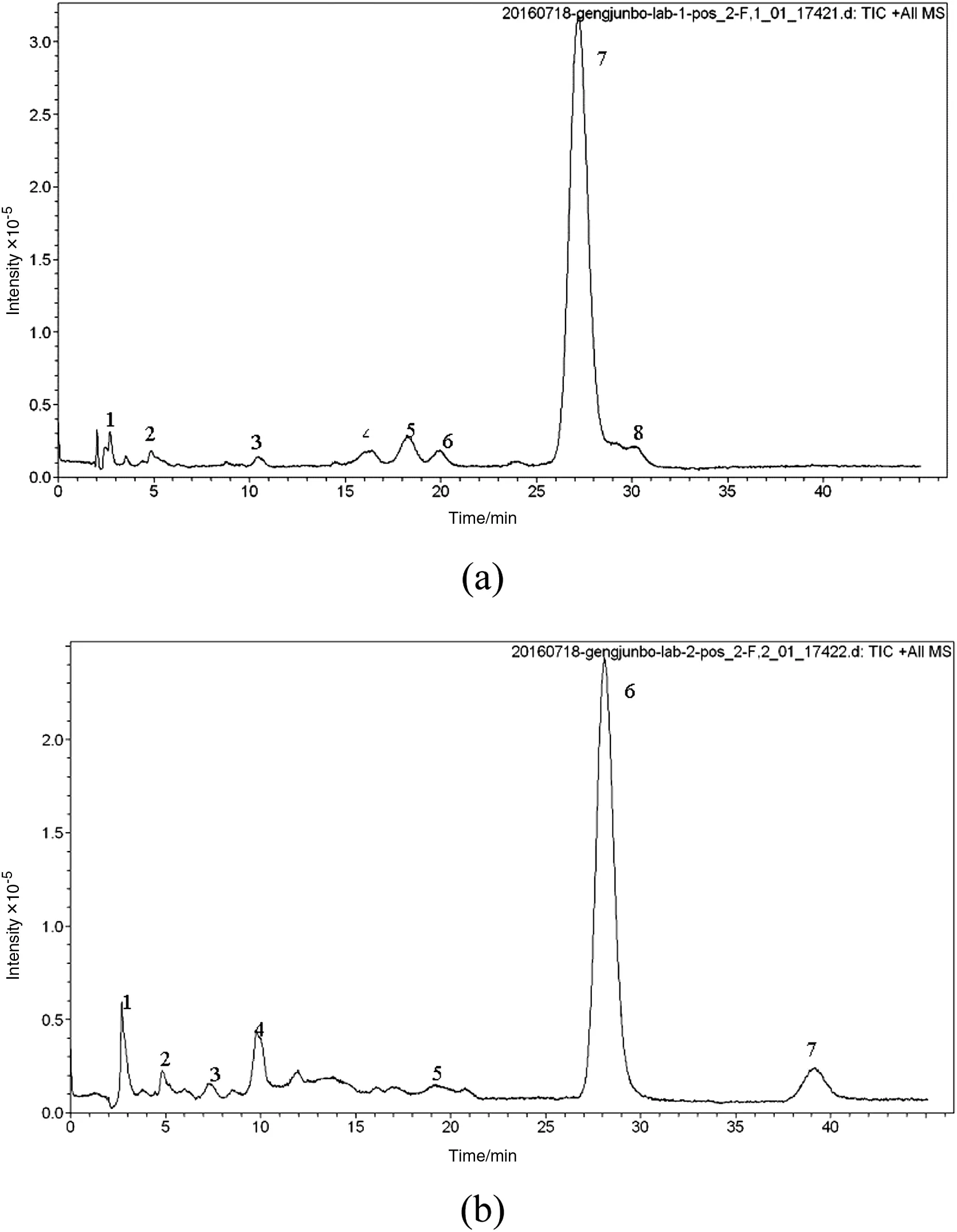
Fig.12.LC of degradative cefixime in acid solution(a)alkaline solution(b).

Fig.13.The possible degradation products of cefixime.
Referring to the literatures and the preparations of cefixime tablets,we studied about the effects of 19 different additives including the excipients and polymer son the degradation of cefixime in water.The concentration of the cefixime was prepared to be 10 mg·ml−1and the concentration of additives was 0.5wt%.The results are shown in Fig.9.The additives inhibiting the degradation of drugs are shown in Fig.9(a)and the acceleration of its breakdown is shown in Fig.9(b).The results show that the amino acids(glycine,DL-methionine),cellulose ethers(HPMC,MC,CMC)and magnesium stearate could obviously decrease the degradation rate.And the SDS,AEO,PEG2000 and mannitol have slight inhibition on the degradation of cefixime(Fig.9(a)).While the degradation process was accelerated by the PVP,Vitamin C,citric acid,sucrose,lactose,PEG8000 as well as Span 80.The effect of β-cyclodextrin and vitamin C are unconspicuous according to our experiment.There is no doubt that the experimental results provide a good guidance for selecting an appropriate additives to improve the stability of cefixime in solution.

Table 2 Related substances of cefixime in acid solution detected by LC-EAIMS/MS

Table 3 Related substances of cefixime in alkaline solution detected by LC-EAIMS/MS
3.2.Degradation mechanism and pathway of cefixime in solutions
The degradation mechanism of cephalosporin antibiotics has been a hot research topic for decades.As we can see(Fig.10),the molecular structure of cephalosporin antibiotics is quite similar to penicillin antibiotics,which may be prone to degradation.Generally,the degradation prefers to occur in the β-lactam ring,the group of carboxyl in the C4-position,the side chain amino fraction in the C7-position and the side chain of C3-positon.When the R2 is vinyl,the degradation in the side chain in the C3-positon is likely to intramolecular cyclize with the carboxyl in C4 position,as a result a five-membered ring lactone is formed[26-28].The degradation mechanism of cephalosporin in the side chain inthe3-positonisshownin Fig.11a.Inaddition,theβ-lactamring of ten opens as the nucleophiles attack the carbonyl carbon,which is described in Fig.11b[24,29,30].Then there will be decarboxylation or cyclization between the carboxyl carbon and the side chain amino fraction in the C7-position[23].This is easy to happen in most cephalosporin antibiotics,resulting in the drug inactivation.Besides,there often exists a conversion between the Δ2-double bond and the Δ3-double bond[24,31](Fig.11c).Remarkably,the degradation process of cefdinir is quite similar to that described in Fig.11c.
As a widely used cephalosporin,the degradation of cefixime show some similarities with other cephalosporins.In order to identify the degradation products to get a better understanding of the degradation of cefixime,the cefixime stored in alkaline solution at pH=9 and in acidic solution at pH=1.02 for 24 h at 303.15 K.Then the degradation products were determined by LC–MS,infrared spectroscopy.From the LC spectra of the degradation products of cefixime(Fig.12),we can see that the degradations in alkaline solution and acidic solution produce similar degradation products expect for a small molecule which may be due to the cleavage of the cefixime in alkaline solution(Table 3).There are approximately eight substances(Fig.13)detected by the LC–MS in our study,including some isomers.Different substances have similar structure and different fragment ions,which can help figure out the probablede gradation products.Referring to the six the dition of European Pharmacopoeia about the related impurities of cefixime[32-34],we speculate the reasonable degradation pro ducts according to frag mentions.The eight possible degradation products are presented in Fig.13,Table 2 and Table 3.

Fig.14.Comparison of IR spectra for cefixime and the degradation products of cefixime.
IR spectra were acquired for the degradation products and are shown in a overlay with spectra for cefixime in Fig.14.The peak within the range of 1850–1600 cm−1present in the cefixime IR spectra but not in the spectra for its degradation product,which is attributable to the disappearance of C=O stretching in β-lactam ring of cefixime due to the open of thering.The change of C--Ostretching vibration in carboxyl would cause the decrease of the peak at 1300–1200 cm−1.
According to the results of MS and IR,a possible degradation mechanism was proposed and it is shown in Fig.15.There will be the opening of β-lactam ring in both acid and alkaline solution when the ion attacks on theβ-lactam ring,following the rearrangement of the olefinic bonds.Then the ring-opening product is likely to decarboxylate or intramolecularly cyclize with the carboxyl in C4 position to form a five-membered ring lactone.There may be an esterification at the carboxyl in the methoxyimino of the side line.In the alkaline environment,there will be an additional rearrangement followed by hydrolytic N--O and C--C bond fission at C-6 position and decarboxylation of the intermediate amide to form a small molecular substance.All these suppositions are based on the characterization results in our experiment.
4.Conclusions
Our studies demonstrate that the degradation kinetics of cefixime matches well with the first-order reaction. The pH-rate profile is U-shaped and shows minimum degradation rate at pH=6. The composition of solvent system leads to the difference of stability of cefixime. The Acetone/Water mixtures is commonly used in the industrial crystallization and the degradation in such solvent is relatively low. Additives can not only inhibit degradation, but also play a role in accelerating degradation, which depends mainly on the type of additives. The temperature has a remarkable effect on the degradation reaction and a higher temperature results in a significant increase in the degradation rate. In addition, the possible degradation mechanism in acid and alkaline environment were presented respectively.All these studies are helpful for the optimization of the production of cefixime,which not only minimize the degradation but also improve the yield and quality of the cefixime products.

Fig.15.Degradation pathway of cefixime in aqueous solution(a)pH=1,(b)pH=9.
Nomenclature
c initial concentration,mg·ml−1
c0residual concentration,mg·ml−1
Eathe activation energy,kJ·mol−1
[H+] the activity of hydronium,mol·L−1
k degradation rate constant,mg·ml−1·h−1
kH+ hydronium ion-catalyzed rate constant,mg·ml−1·h−1
kOH2O catalyzed rate constant,mg·ml−1·h−1
kOH− hydroxide ion-catalyzed rate constant,mg·ml−1·h−1
k∞the rate constant when ε goes to ∞,mg·ml−1·h−1
[OH−] the activity of hydroxide ion,mol·L−1
R gas constant,J·mol−1·K−1
T the reaction temperature,K
Zathe electric charge of drug,C
Zbthe electric charge of ion,C
ε the dielectric constant,F·m−1
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Selective aerobic oxidation of p-cresol with co-catalysts between metalloporphyrins and metal salts☆
- Degradation and mineralization of aniline by O3/Fenton process enhanced using high-gravity technology☆
- Three-liquid-phase extraction and separation of V(V)and Cr(VI)from acidic leach solutions of high-chromium vanadium–titanium magnetite☆
- Experimental and simulation of the reactive dividing wall column based on ethyl acetate synthesis☆
- Modeling investigation of geometric size effect on pervaporation dehydration through scaled-up hollow fiber NaA zeolite membranes☆
