Degradation and mineralization of aniline by O3/Fenton process enhanced using high-gravity technology☆
2018-08-18YuejiaoQinShuaiLuoShuoGengWeizhouJiaoYouzhiLiu
Yuejiao Qin,Shuai Luo,Shuo Geng,Weizhou Jiao,*,Youzhi Liu
1Shanxi Province Key Laboratory of Higee-Oriented Chemical Engineering,North University of China,Taiyuan,030051,China
2Department of Civil and Environmental Engineering,Virginia Polytechnic Institute and State University,Blacksburg,Virginia 24061,USA
Keywords:O3/Fenton process High-gravity technology Aniline Degradation mechanisms
ABSTRACT The degradation and mineralization of aniline(AN)using ozone combined with Fenton reagent(O3/Fenton)in a rotating packed bed(RPB)was proposed in this study,and the process(RPB-O3/Fenton)was compared with conventional O3/Fenton in a stirred tank reactor(STR-O3/Fenton)or single ozonation in an RPB(RPB-O3).Effects of highgravity factor,H2O2dosage,H2O2dosing method and initial pH on the AN mineralization efficiency were investigated in the RPB-O3/Fenton process.In addition,the behavior of Fe(II)was monitored at different H2O2dosing methods and pH values.Finally,the optimal operation conditions were determined with high gravity factor of 100,initial pH of 5,Fe(II)concentration of 0.8 mmol·L−1and H2O2dosage of 2.5 ml.Under these conditions,for aniline wastewater at the volume of 1 L and concentration of 200 mg·L−1,a fast and thorough decay of AN was conducted in 10 min,and the TOC removal efficiency reached 89%in 60 min.The main intermediates of p-benzoquinone,nitrobenzene,maleic acid and oxalic acid were identified by liquid chromatography/mass spectroscopy(LC/MS),and the degradation pathways of AN in RPB-O3/Fenton system were proposed based on experimental evidence.It could be envisioned that high-gravity technology combined with O3/Fenton processes would be promising in the rapid and efficient mineralization of wastewater.
1.Introduction
Aniline(AN,C6H7N)is an important chemical intermediate and widely applied in many fields such as petrochemicals,pharmaceuticals,explosive materials and dyes[1–3].Furthermore,AN is one of the major aromatic amines produced from the degradation of wastewater containing aromatic compounds[4,5].Thus,this is not surprising that such compounds can find their way in many types of industrial waste waters.AN is highly toxic because it can react easily in the blood to convert hemoglobin to methahemoglobin,thereby preventing oxygen uptake.Once the wastewater containing AN is discharged in to the environment,AN could be adsorbed in sediments,which may extend its persistence in the aquatic environment[6].Due to the negative impact on the human health and environment,wastes/wastewater containing AN demands a thorough removal technique before discharging into the natural water.
Nowadays,waste waters containing AN can be treated successfully through several physical processes[7](e.g.adsorption,extraction,etc.),biological treatment processes[8](e.g.activated sludge,aerobic/anaerobic bacteria,etc.)or chemical catalysis processes[6,9](e.g.photocatalytic,ozonation,etc.).Among those,ozone has been widely used in a wide array of wastewater treatment due to its high oxidation–reduction potential(2.08 V)and without secondary pollution[10,11].Concerning the AN,an aromatic compound with an unshared pair of electrons,has a high delocalization of electrons and then exhibits high reactivity towards O3[6].However,single ozonation rarely leads to total mineralization,especially for saturated compounds such as aldehydes and smallchain carboxylic acids,which are partial oxidation products of the initial organic pollutants present in the solution.In order to accomplish a fast and stronger mineralization,ozone-based advanced oxidation processes(photocatalytic ozonation,O3/H2O2,O3/Fe(II)and O3/Fenton)are becoming increasingly important as an ultimate treatment technology.Among those processes,O3/Fenton is of great impact because in addition to O3direct reactions,it combines the catalytic systems of O3/Fe(II),O3/H2O2and Fe(II)/H2O2,allowing to explore different synergies.The set of chain reactions in O3/Fenton process involved the production and decay of·OH that could react with ozone-insensitive intermediates and improve the mineralization efficiency of wastewater[12].Therefore,O3/Fenton process is promising to assist the degradation of the recalcitrant compounds in the wastewater treatment.However,the practical use of ozonation for wastewater treatment is limited by the low solubility and mass transfer efficiency of ozone[13].
As a process intensification method,high-gravity technology is realized by usinga rotatingpacked bed(RPB).High centrifugal acceleration of 100–1000 times of general gravity is generated in an RPB through high rotating speed for intensifying gas–liquid mass transfer and improving chemical reaction processes[14].Liquids are spread or split into the thinner liquid film or smaller droplets by the huge shearing force under the high gravity environment,which strongly increases the gas–liquid interfacial area and decreases the transfer resistance of gas into the liquid phase[15].Jiaoet al.[16]reported that the gas–liquid mass transfer efficiency could be enhanced by 1–2 orders of magnitude in RPB.Lin et al.[17,18]has investigated the absorption of ozone from gaseous streams by Fe(II)and H2O2solution in RPB and reported that the ozone removal efficiency reached 95%and gas phase mass transfer coefficient was 1.1 s−1.Based on this,the applications of high-gravity technology for enhancing ozone mass transfer and improving ozonation efficiency of wastewater have been studied[19,20].Despite some papers published about the applications of O3/Fenton processes in RPB(RPB-O3/Fenton),the behavior of Fe(II)and H2O2was rarely concerned.On the other hand,the degradation mechanism and mineralization of wastewater(such as the removal efficiency of total organic carbon(TOC))instead of the sole indicator of the removal efficiency of target contaminants need to be extensively studied.
In this work,an RPB was employed to intensify the mass transfer of ozone and improve the mineralization efficiency of AN in O3/Fenton processes.The specific objectives were to(i)assess the synergies between the RPB and O3/Fenton process for the deep degradation of AN,(ii)gain insight in to the behavior of Fe(II)and H2O2in degradation processes,and(iii)enhance our understanding of the oxidation mechanism of AN in the aqueous systems.
2.Experimental
2.1.Chemicals and setup
Aniline,ferrous sulfate heptahydrate,hydrogen peroxide(30%),sodium hydroxide,sulfuric acid(98%),phenanthroline and other reagents were of analytical grade,which were commercially available from Tianjin Hengxing Chemical Reagent Co.,Ltd.,China,and without further purification in this study.Methanol and acetonitrile were of chromatographic grade and obtained from Kemiou Chemical Reagent Co.,Ltd.,China.All working solutions were prepared in deionized water.
The experimental setup(Fig.1)used in this study was an RPB,developed at Research Center of Shanxi Province for High Gravity Chemical Engineering and Technology(Shanxi,China).The working principle and structure of the RPB can be found in the literature[21].The details of the RPB employed in this study were shown in Table 1.
2.2.Experimental procedures
One liter of AN solution(200mg·L−1)containing the catalyst of Fe(II)was introduced into the RPB through the peristaltic pump with a flow rate of 80 L·h−1,and 2.5 ml H2O2solution was continuously fed along the reaction time into the stirred tank.Ozone was produced from pure oxygen by an ozone generator(Shandong Lubang Photoelectric Equipment Co.Ltd.,Shandong,China)and mixed with N2to adjust the required ozone concentration.The mixed gas with a flow rate of 60 L·h−1from the gas inlet flowed radially owing to a pressure gradient.Meanwhile,the liquid through a liquid distributor converged from inner edge to outer edge of the packing under the centrifugal force.In this process,ozone was absorbed into the wastewater by large contact area and under small mass transfer resistance between the gas stream and the liquid streams.Also,the ozone was catalyzed by the Fenton reagents to degrade organic compounds.The solution after the reaction flowed to liquid outlet due to gravity,and the gas stream was vented through the tail-gas absorption bottle.During the degradation of AN,the analytic samples of 2.5 ml were withdrawn off with a syringe from sampling port for analyzing at a regular time interval of 5 min.
For comparison,a similar experiment procedure was also performed in a conventional stirred tank reactor(STR).The O3-containing gas was continuously bubbled into the solution,and the catalysts of Fe(II)and H2O2were simultaneously added to start the reaction.
2.3.Experimental analyses
The indicators of the treatment efficacy of wastewater by O3/Fenton included AN removal efficiency and TOC removal efficiency.The concentration of AN was analyzed using a high performance liquid chromatography(HPLC)system(Ultimate 3000,Dionex,USA)with a C18reverse phase column(4.6 mm × 250 mm,5 μm).The UV detection wavelength was at 280 nm,and the temperature was at 20°C.The mobile phase was typically CH3OH/H2O(70/30,v/v)with a flow rate of 0.9 ml·min−1.The concentration of TOC was measured using the nonpurgeable organic carbon(NPOC)method of TOC analyzer.The removal efficiency of AN(or TOC)was calculated by the following equation:

Fig.1.Schematic diagram of the experimental setup.

Table 1 The equipment parameters of the RPB

where η(%)=the AN(or TOC)removal efficiency,C0=the initial AN(or TOC)concentration in the solution(mg·L−1),and Ct=the AN(or TOC)concentration after reaction(mg·L−1).
The concentration of ozone was measured by ozone concentration detector(UV-2200C)and the utilization ratio of ozone(E,%)was calculated by the following equation:

where Cin=the concentration of ozone in inlet of RPB(mg·L−1),and Cout=the concentration of ozone in outlet of RPB(mg·L−1).
The concentration of dissolved ferrous ion(Fe(II))in the solution was measured by phenanthroline spectrophotometric method[22].A liquid chromatography instrument coupled with a mass spectrometry detector(LC/MS,Exactive Plus,Thermo Fisher Scientific,USA)equipped with a C18reverse phase column was used for analyzing the intermediates.The mobile phase was CH3OH/H2O(80/20,v/v)with a flow rate of 0.25 ml·min−1.The column temperature was 30 °C and the injection volume was 50 μl.Mass spectrometric detection was operated with a 4.0-kV electrospray ionization(ESI)mode.The flow rate of carrier gas was 10.0 L·min−1.
3.Results and Discussion
3.1.Comparison experiments
A series of experiments were carried out in the different processes to evaluate the degradation efficiency of AN,including RPB-O3/Fenton,O3/Fenton processes in stirred tank reactor(STR-O3/Fenton),ozonation alone in RPB(RPB-O3)and AN solution in RPB bubbled with air instead of ozone(RPB-Air).As shown in Fig.2(a),loss of AN by RPB-Air was negligible and the TOC removal of AN solution was found to be<5%over 60 min in the control experiment(Fig.2(b)),so all of the disappearance during the experiment time was attributed to the reaction instead of air stripping.
Fig.2(a)showed that the AN removal efficiency was49%and 65%in 12 min by RPB-O3and STR-O3/Fenton,respectively,while a fast and thorough decay of AN(around 100%removal efficiency)was achieved in 10 min by the RPB-O3/Fenton system.The results indicated that there was possibly a synergistic effect between the RPB and O3/Fenton processes for the degradation of AN.O3is an extremely powerful oxidant and it attacks a wide range of compounds with unsaturated bonds[23].AN,an aromatic compound possessing electron donor groups,has a high electronic density in or tho-and para-positions to react actively with O3by the electro philic attack[5].However,the reaction rate constant of organic compounds with·OH induced by O3decomposition in water is in the order of 106to 109L·mol−1·s−1,which is much larger than that with ozone molecule[24].Therefore,the AN removal rate in the catalytic systems(RPB-O3/Fenton and STR-O3/Fenton)was larger than ozonation alone(RPB-O3).Compared with STR-O3/Fenton,O3/Fenton processes presented a higher activity for the degradation of AN in RPB,which was mainly due to the enhancement of ozone mass transfer efficiency under RPB.Ozone mass transfer was the rate control step for ozonation and the removal efficiency increases with the increasing of mass transfer efficiency of ozone[25].Meanwhile,the utilization ratio of ozone was calculated in the systems of RPB-O3/Fenton and STR-O3/Fenton.The results showed that the utilization ratio of ozone respectively were85%and43%inRPB-O3/Fenton and STR-O3/Fenton.The inter facial area of gas and liquid was increased and the resistance of ozone mass transfer was decreased by reducing the thickness of liquid films and/or the size of droplets in RPB.Therefore,high-gravity technology could increase the ozone mass transfer rate from the gas phase to the liquid phase in the ozonation process.

Fig.2.Comparison of AN degradation in the different reaction systems.(a)AN removal efficiency along with reaction time;(b)TOC removal efficiency of AN solution along with reaction time.Experimental conditions:β =100,initial pH=5,initial concentrations of AN,ozone,Fe(II)and H2O2dosage were 200 mg·L−1,36 mg·L−1,0.8 mmol·L−1and 2.5 ml,respectively.
To further investigate the efficiency of the combination of RPB with O3/Fenton processes in mineralization of AN solution,TOCs in different reaction systems were monitored and the results were illustrated in Fig.2(b).It is noted that TOC removal efficiency of AN solution all increased with the increasing reaction time in different processes.The removal efficiency of TOC was higher in STR-O3/Fenton system(63%)compared to the RPB-O3(45%),and the highest removal efficiency was observed for the RPB-O3/Fenton process(89%).Nevertheless,while AN was thoroughly removed in 10 min,the TOC removal efficiency was only 28%in RPB-O3/Fenton process.It was reported that the reaction rate constant of·OH with AN was larger than that with the intermediate products formed during degradation processes of AN[26],and thus·OH was preferred to react with AN in the initial stage when the amount of·OH was relatively insufficient.Therefore,thorough decay of AN was achieved but the degree of mineralization of wastewater was not always satisfactory,similar as the previous study[27].
3.2.Effect of operating conditions
3.2.1.Effect of high gravity factor
The high gravity factor β,a dimensionless para meter used to characterize the intensity of gravitational field,is calculated as follows[28]:

where ω is angular velocity of the rotation of rotor,s−1;r is the average of outer diameter and inner diameter of packing,m;g is gravitational acceleration(9.8 m·s−2).
As indicated in Fig.3,the TOC removal efficiency of AN wastewater by O3/Fenton processes in the RPB was enhanced from 63%to 89%by increasing the high gravity factor of the RPB from 0 to 100,and then gradually decreased when the high gravity factor was more than the optimal value.There as on was that the higher high gravity factor generated from RPB could decrease the size of droplet and the thickness of the liquid films,which increased the inter facial area.Furthermore,the increasing high gravity factor in RPB caused the turbulence of gas and liquid,making the gas/liquid interface quickly renewed and decreasing the resistance of ozone mass transfer.Therefore,the transfer of ozone from gas phase to the liquid phase,a liquid film controlled process,was enhanced by RPB,especially in higher high gravity factor.However,a much higher gravity factor exceeding the optimal value could lead to a reduced average gas/liquid contact time[29],which finally decreased the TOC removal efficiency and contributed to the deterioration of the effluent qualities.
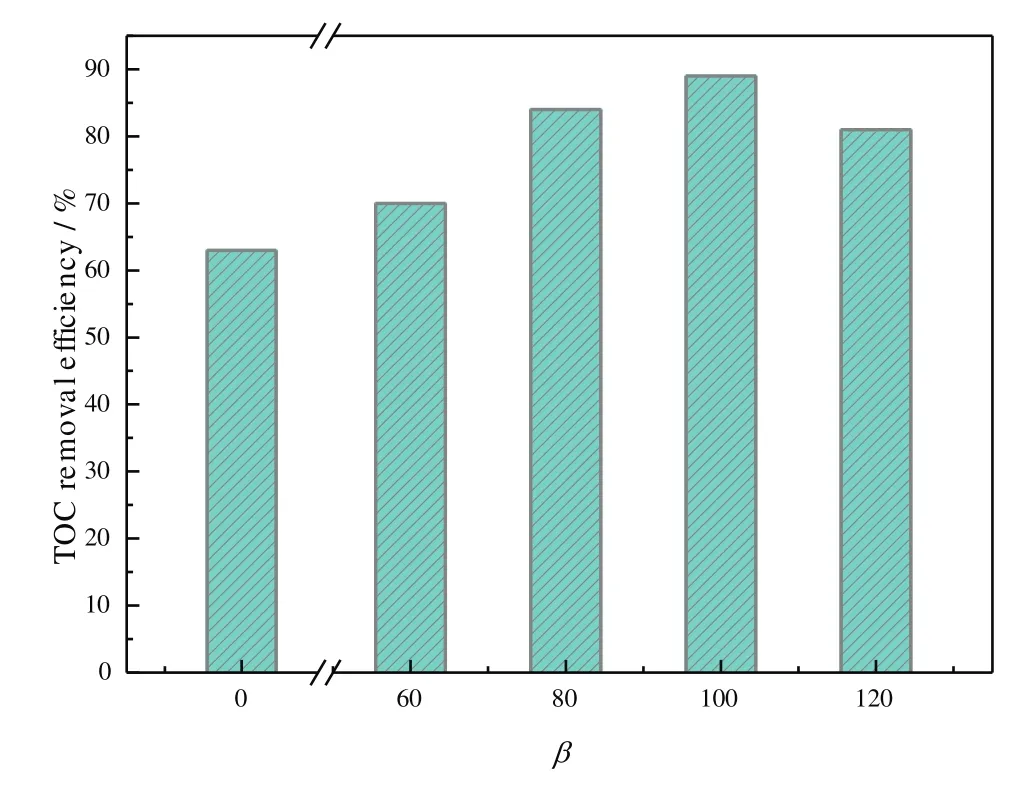
Fig.3.Effect of high gravity factor on TOC efficiency for AN wastewater.Experimental conditions:initial pH=5,t=60 min,initial concentrations of AN,ozone,Fe(II)and H2O2dosage were 200 mg·L−1,36 mg·L−1,0.8 mmol·L−1and 2.5 ml,respectively.
3.2.2.Effect of the dosage and the dosing method of H2O2
The effect of H2O2dosages on TOC removal efficiencies of RPB-O3/Fenton system was evaluated by conducting experiments using different H2O2dosages(0–4.5 ml).As shown in Fig.4(a),adding H2O2was an effective method of AN mineralization,and the TOC removal ef ficiency significantly improved compared with that without the addition of H2O2.It was observed that the TOC removal efficiency increased with the increasing of H2O2volume until an optimum dosage was reached.However,further increase of H2O2amount might decrease the TOC removal efficiency and the optimum H2O2volume of 2.5 ml gave a better TOC removal efficiency in this study.That was due to there as on that the generated ·OH by the decomposition of H2O2shown in Eqs.(4)–(6),which promoted the removal efficiency and improved the effluent qualities[30].However,ifH2O2dosage was greater than the optimum value,excessive H2O2would react with ·OH in the Eqs.(7)–(9)to reduce the amount of·OH and have negative effect on the organic degradation efficiency.Similarly,the effect of H2O2dosage was observed in lower high gravity factor(β=40).
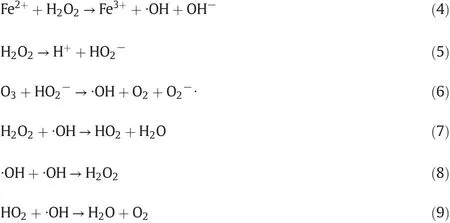
In addition to the H2O2dosage,dosing method of H2O2also affected the treatment efficiency due to the instability of H2O2and strong activity of·OH[31].Commonly,2.5mlofH2O2was added at the beginning of the process,named as batch experiment,promoting a high·OH and thus a rapid oxidation degradation.Nevertheless,high·OH concentration production during the initial stages caused competitive scavenging reactions between Fe(II),H2O2,O3and ·OH(such as the Eqs.(7)–(9)),which reduced the amount of·OH available for the degradation of contaminants and caused an inefficient consumption of H2O2.The alternative of H2O2dosing method was the continuous feeding upon the reaction time(2.5 ml of H2O2was continuously fed along the reaction time of 60 min),named as continuous run.In order to explore the H2O2dosing method,different H2O2dosing methods(continuous run and batch experiment)were chosen to conduct experiments.Meanwhile,the concentration of Fe(II)was monitored along the experiment,shown in Fig.4(b).
It could be seen in Fig.4(b)that the higher TOC removal rate occurred at the initial stage in the batch experiment,due to the rapid and intensive·OH generation and thus the rapid oxidation degradation of organic compounds.However,the TOC removal efficiency of continuous experiments at 89%came from behind and became greater than that at 82%of batch experiment after 60 min.These phenomena could be explained as follows:the overall amount of H2O2was continuously added,realizing a better availability of H2O2throughout the entire processes time and consistently promoting the TOC removal efficiency,which con firmed the aforementioned discussion.Moreover,the evolution of Fe(II)concentration was drastically decreased in both the batch and continuous experiments(Fig.4(b)),whereas reduction amount of Fe(II)concentration after experiment in continuous run(around 43.7%)was less than that in batch experiment(around 70%).In the RPB-O3/Fenton system,O3and/or H2O2are decomposed catalytically byFe(II)to·OH with oxidation product of Fe(III),suchasEqs.(10)and(4).On the other hand,this catalytic reaction is propagated from Fe(II)regeneration,which takes place by the reduction of Fe(III)with H2O2(Eq.(11))and/or with organic radical intermediates·R(Eq.(12))[32].Thus,the concentration of Fe(II)or Fe(II)/H2O2ratio was also higher in continuous experiment,achieving the decomposition of H2O2into·OH instead of O2.It was worth noting from the Fig.4(b)that the concentration of Fe(II)with a reproducible dip occurred at 20min in bat chrun,which indicated that the H2O2was depleted during 20 min and thus the increase of TOC removal efficiency along the reaction time was limited.Nevertheless,the regeneration of Fe(II)by intermediates·R resulted in a slight recovery of Fe(II)concentration for the batch experiment(from 20 min to 60 min,Fig.4(b)),with the similar results as previous study[33].

Fig.4.Effect of H2O2dosage on TOC efficiency for AN wastewater at different high gravity factor(a);variation of Fe(II)concentration and TOC removal efficiency with reaction time at different dosing method of H2O2(b).Experimental conditions:initial pH=5,t=60 min,initial concentrations of AN,ozone and Fe(II)were 200 mg·L−1,36 mg·L−1and 0.8 mmol·L−1,respectively.

3.2.3.Effect of initial pH
The initial pH of wastewater plays an important role on the oxidation efficiency in catalytic system by influencing the behavior of H2O2,and the iron(II,III)recycling processes.It is known that OH−can accelerate the generation of·OH through the decomposition of O3molecule,thushigh pH value is helpful for the in direct reaction of O3in somecase.However,in strong alkaline solution,·OH is rapidly converted to its conjugate base·O−that has a lower oxidation potential compared with·OH[34],causing an unsatisfactory mineralization.On the other hand,Fenton processes has high catalytic activity under acidic condition(pH=2–4),which is attributed to the stability of Fe(II)during this pH range[24].The effect of initial pH on TOC removal efficiencies of RPB-O3/Fenton system was investigated during the pH range of 2–7 and the results were illustrated in Fig.5.In addition,in order to gain an understanding into the behavior of Fe(II)during the experiment,the concentration of Fe(II)at 30 minwas measured in different pH values,shown in the histogram of Fig.5.
As shown in Fig.5,the concentration of Fe(II)was decreased with the increase of the pH value.However,Fe(II)concentration in the RPB-O3/Fenton was always more than that in RPB-O3/Fe(II)at different pH values.This was because that the presence of H2O2in the system accelerated the regeneration of Fe(II),which efficiently compensated for the decrease of Fe(II)to some extent in the degradation processes.Nevertheless,when pH=6,the Fe(II)concentration decreased from 0.8 mmol·L−1in the beginning to 0.35 mmol·L−1at 30 min in the RPB-O3/Fenton system,which meant that Fe(II)concentration was disastrous and the catalytic action of Fe(II)was insufficient when pH>6.
It was also found from Fig.5 that the TOC removal efficiency(71%of pH=2,75%ofpH=3)was relatively low under the strong acid at RPBO3/Fenton processes.It has been reported that H2O2partially dissociates in alkaline to generate HO2−species that consequently reacts favorably with ozone molecules to generate ·OH(Eqs.(5)–(6)).In addition,the reaction rate constant between the HO2−and O3was greater than that between the H2O2molecule and O3[35].Thus,under relatively higher pH,the generation of·OH would be relatively stronger due to HO2−species,and it was found that the poor treatment efficiency occurred under acid condition(pH<5).On the other hand,with the continuous increase of pH value(pH>5),the Fe(II)gradually became instable and the consumption of catalyst was useless for the mineralization of wastewater.Thus,the initial pH=5 was considered as appropriate pH value for RPB-O3/Fenton processes because of the sufficiently catalytic effect.

Fig.5.Effect of initial pH on TOC efficiency for AN wastewater in RPB-O3/Fe(II)(dotted line)orRPB-O3/Fenton system(solid line)and the Fe(II)concentration at 30 min in RPB-O3/Fe(II)(red column,left)or RPB-O3/Fenton system(green column,right).Experimental conditions:β=100,t=60 min,initial concentrations of AN,ozone,Fe(II)and H2O2dosage were 200 mg·L−1,36 mg·L−1,0.8 mmol·L−1and 2.5 ml,respectively.

Fig.6.The possible pathways of AN mineralization by RPB-O3/Fenton system.
3.3.Decomposition mechanism
The intermediates of AN in the RPB-O3/Fenton were identified by LC/MS,and p-benzoquinone,nitrobenzene,maleic acid and oxalic acid were the main intermediates.Based on the products analysis,possible oxidation pathways for the AN degradation were proposed and illustrated in Fig.6.
Previous studies[27,36]havere ported that the·OH plays avital role on the degradation of organic compounds during advanced oxidation process,with predominant high redox potential(E0=2.8 V)and electronic affinity of 569.3 kJ[37].The degradation mechanisms of·OH include elect rophilic addition,hydrogen abstraction reaction and electron transfer,which could degrade the organics to small molecules by destruction of carbon chain structure.For AN,the amine group was electron donating for the electrophilic aromatic substituents,which increased the electron density at the ortho-and para-positions,favoring the electrophilic addition of·OH.AN was easily attracted by ·OH through an electrophilic addition to the aromatic ring,with the formation of a p-nitrophenol.Therefore,·OH could attack the p-nitrophenol molecule,resulting in the occurrence of deamination,and the formation of hydroquinone.It is well-known that hydroquinone is very easily oxidized to p-benzoquinone[38],thus it is probably formed by reaction of·OH.Furthermore,reactions of the primary intermediates mentioned above(such as p-benzoquinone)with·OH led to the destruction of benzene ring and formation of aliphatic compounds,which could be further degraded to carboxylic acids,such as maleic acid and oxalic acid identified in this study.Finally,maleic acid was directly mineralized to CO2and H2O via the oxalic acid,being accelerated by reaction of such diacids and its intermediates with·OH[27].On the other hand,AN could also be transformed into aminophenyl radical through the electron transfer of·OH.Accordingly,the addition of·OH to aminophenyl radical resulted in the formations of quinoneimine,hydroxyaniline,and even further the p-benzoquinone.
4.Conclusions
The experiments for degradation and mineralization of AN wastewater by O3/Fenton system were processed,and the high gravity technology was applied to enhance the ozone mass transfer from the gas phase to the liquid phase.The results of comparison experiments between RPB-O3,STR-O3/Fenton and RPB-O3/Fenton systems indicated a synergistic effect between the RPB and Fenton process for the degradation of AN.Effects of high gravity factor,H2O2dosage,H2O2dosing method and initial pH on the AN mineralization efficiency were studied in RPB-O3/Fenton process.The TOC removal efficiency of AN wastewater was enhanced by increasing the high gravity factor.Increasing the H2O2dosage accelerated the degradation rate,but excessive H2O2addition contributed to the deterioration of the effluent qualities.Moreover,H2O2dosing method of continuous experiment compared with batch experiment gained a higher reactivity of Fe(II)and more satisfactory mineralization efficiency of wastewater.The optimum operation conditions were obtained at high gravity factor of 100,initial pH of 5,with 0.8 mmol·L−1of Fe(II)and 2.5 ml of H2O2for RPB-O3/Fenton system.Under the optimized conditions,the removal efficiency of AN was 100%in 10 min and the TOC removal efficiency reached 89%in 60 min.Finally,the degradation pathways of AN in RPB-O3/Fenton system were proposed based on the investigation of various intermediates.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Selective aerobic oxidation of p-cresol with co-catalysts between metalloporphyrins and metal salts☆
- Three-liquid-phase extraction and separation of V(V)and Cr(VI)from acidic leach solutions of high-chromium vanadium–titanium magnetite☆
- Insight into the degradation mechanism of cefixime under crystallization condition☆
- Experimental and simulation of the reactive dividing wall column based on ethyl acetate synthesis☆
- Modeling investigation of geometric size effect on pervaporation dehydration through scaled-up hollow fiber NaA zeolite membranes☆
