Dissolution-regrowth synthesis of SiO2nanoplates and embedment into two carbon shells for enhanced lithium-ion storage☆
2018-08-18ZhijunYanXiangcunLiXiaobinJiangLeZhangYanDaiGaohongHe
Zhijun Yan,Xiangcun Li,Xiaobin Jiang,Le Zhang,Yan Dai*,Gaohong He*
State Key Laboratory of Fine Chemicals,Chemical Engineering Department,Dalian University of Technology,Dalian 116024,China
Keywords:Silica nanoplates Carbon shell Macroporous Lithium-ion battery
ABSTRACT In this work,SiO2nanoplates with opened macroporous structure on carbon layer(C-mSiO2)have been obtained by dissolving and subsequentregro wing the out ersolidSiO2layer of the aerosol-basedC-SiO2double-shellhollow spheres.Subsequently,triple-shell C-mSiO2-C hollow spheres were successfully prepared after coating the C-mSiO2templatesby the carbon layer from the carbonization of sucrose.When being applied as the anode material for lithium-ion batteries,the C-mSiO2-C triple-shell hollow spheres deliver a high capacity of 501 mA·h·g−1after 100 cycles at 500 mA·g−1(basedon the total mass of silica and the two carbon shells),which is higher than those of C-mSiO2(391 mA·h·g−1)spheres with an outer porous SiO2layer,C-SiO2-C(370 mA·h·g−1)hollow spheres with a middle solid SiO2layer,and C-SiO2(319.8 mA·h·g−1)spheres with an outer solid SiO2layer.In addition,the battery still delivers a high capacity of 403 mA·h·g−1at a current density of 1000 mA·g−1after 400 cycles.The good electro chemical per for mancecanbe attributed to the high surface area(246.7m2·g−1)and pore volume(0.441 cm3·g−1)of the anode materials,as well as the unique structure of the outer and inner carbon layer which not only enhances electrical conductivity,structural stability,but buffers volume change of the intermediate SiO2 layer during repeated charge–discharge processes.Furthermore,the SiO2nanoplates with opened macroporous structure facilitate the electrolyte transport and electrochemical reaction.
1.Introduction
Recently,lithium-ion batteries(LIBs)are very widely used in a variety of advanced technologies,like portable electronic devices,electric vehicles(EVs),energy storage systems and so on.To meet their ever growing application,it is deemed necessary to improve the energy and power density as well as the long cycling stability of LIBs[1,2].Thus,developing anode materials which possess high capacity,superior rate capability,and long cycling stability are highly desirable[3].A large number of researches have been focused on the development of alternative anode materials for applications in LIBs.Nowadays,silica is regarded as one of the most promising candidates because of its much higher theoretical specific capacity,satisfactory potentials,low cost and environmental friendliness[4–6].Not only in the Li-ion battery field,but SiO2is reported in other energy storage fields,like supercapacitor[7],Li–S Battery[8].
However,when SiO2is used as anodes in practical applications,some shortcomings arise,such as poor electrical conductivity and large volume change during charge–discharge processes[4,6,9,10].To avoid these shortcomings,nanostructured SiO2or combining SiO2with high conductivity materials,like carbon,has been proved as an effective method to improve the conductivity and structural stability of the SiO2-based active material[5,8,11–20].Guo et al.grew a layer of silica on hard carbon namednano-SiO2/carbon by a hydrothermal method,the composite displayed a reversible capacity of 600mA·h·g−1after12cycles[12].Subsequently,Li et al.had successfully fabricated sugar apple-shaped SiOx@C spheres by one-pot synthesis and annealing[16].Recently,Jiao et al.reported that hollow core–shell structure named SiO2/C@C possesses a high specific charge capacity of 669.8 mA·h·g−1at a current density of 100 mA·g−1after 100 cycles[21].However,those composites did not show good performance in the long cycling tests because of the pulverization of electrode.
Herein,we report a unique structure of C-mSiO2-C triple-shell hollow spheres which have two carbon shells with SiO2nanoplates embedded.The macroporous SiO2layer with large surface area improves Li ion diffusion via the nanoscale SiO2/C shell[19].And the inner carbon shell serves as efficient backbones to SiO2nanoplates,provides the lithium-ion diffusion path.Meanwhile,the outer carbon shell can not only enhance electrical conductivity but also buffer the volume change of SiO2for lithium ion storage.As illustrated in Fig.1,the C-SiO2template is simply obtained by a one-step aerosol strategy.And by dissolution and regrowth of the SiO2layer,hydrothermal reaction,followed by a carbonization process,C-mSiO2-C triple-shell hollow spheres can be obtained.
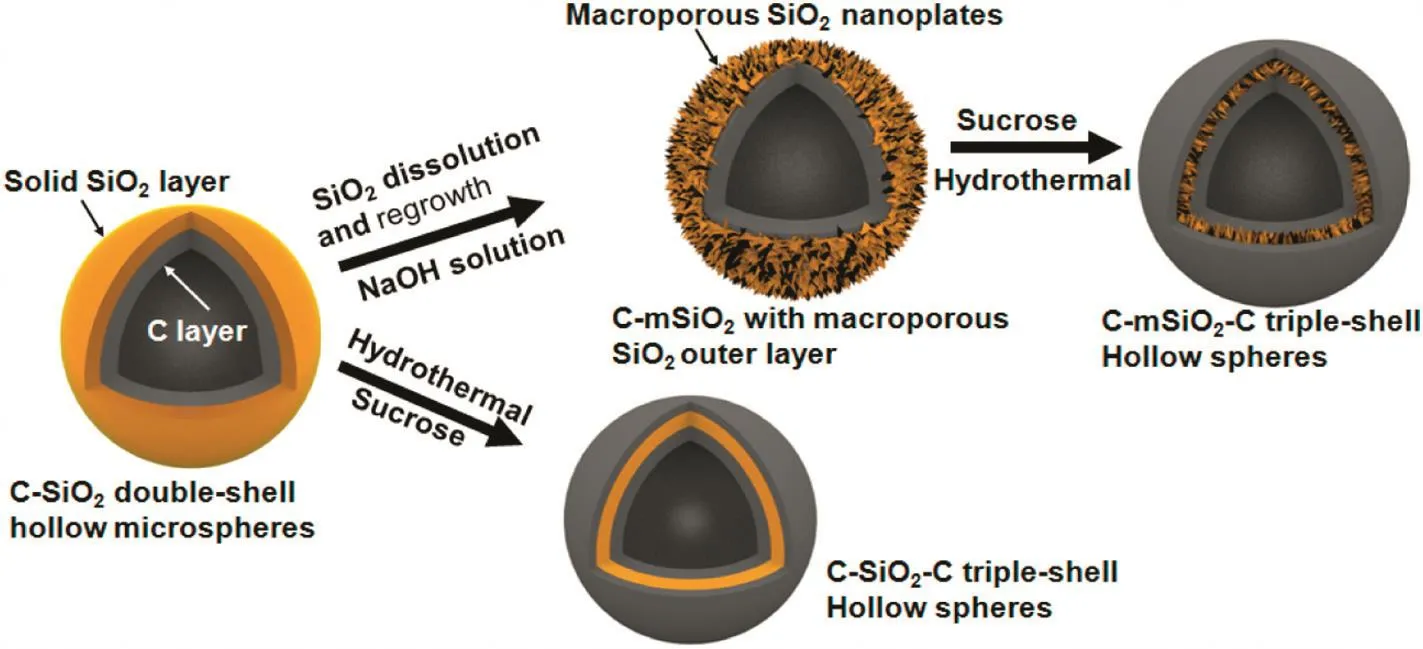
Fig.1.Schematic illustration of the formation processes for the C-mSiO2-C triple-shell hollow spheres.
2.Experimental
2.1.Synthesis of C-mSiO2-C triple-shell hollow spheres
The double-shell C-SiO2hollow sphere was prepared by one-step aerosol strategy.Firstly,1.1 g of cetyltrimethylammonium bromide(CTAB)was dissolved in 15 ml of ethanol.Then,0.95 g of FeCl3·6H2O was added into the former solution,ultrasound to dissolve.2.0 ml of 0.1 mol·L−1HCl solution containing 1 g of sucrose and 4.5 ml of tetraethyl orthosilicate(TEOS)was mixed with the above dispersion,and then the mixture was dispersed in an ultrasonication bath for 10 min.Subsequently,the solution was formed into aerosol droplets by a commercial atomizer.Meantime because of the rapid hydrolysis of TEOS,a silica shell was formed on the surface of aerosol droplets with the organic species inside[22–24],and C-SiO2hollow spheres was obtained after calcination under an N2flow at 500°C for 3 h at a heating rate of 2 °C·min−1.Then,0.2 g of obtained C-SiO2sample and 4 g of NaOH was added in 100 mldeionized water with continuous stirring for4h.In this process,the solution was heated to accelerate the dissolution of silica and then naturally cooling to regrow the dissolved silica on the carbon surface.The C-mSiO2was collected and washed several times by centrifugation with deionized water and ethanol.
Finally,0.1 g of the obtained C-mSiO2was dispersed in a solution containing 10 ml of H2O,10 ml of ethanol and 0.3 g of sucrose,and then transferred into an 80 ml polytetra fluoroethylene(PTFE)-lined stainless steel autoclave and kept at 180°C for 4 h.The production was collected and washed several times with deionized water and ethanol after the autoclave was cooled down,and then dried at 60°C overnight.The composites were annealedat800°Cfor3hunderN2flowata heating rate of 1 °C·min−1to form C-mSiO2-C[2,14].C-SiO2-C was obtained in the same way with the C-SiO2as templates.
2.2.Material characterizations
The morphologies of the obtained hollow microspheres were examined using a field emission scanning electron microscopy(FE-SEM,Nova NanoSEM 450).The microstructures and energy-dispersive X-ray spectroscopy elemental mapping images were obtained by a JEOL-2010F transmission electron microscopy(TEM).The nitrogen adsorption–desorption isotherms were measured at 77.35 K using a Micromeritics AUTOSORB-1-MP.The specific surface area was calculated by using the Barret–Joyner–Halender(BJH)method.X-ray photoelectron spectroscopy(XPS)was performed using ESCALAB 250Xi.
2.3.Electrochemical measurements
Electrochemical experiments were measured using CR2025-type coin cell sassembledinanargon- filledglovebox with Celgard 2325membrane as separator and lithium-foil as counter electrode.The electrolyte employed was 1 mol·L−1LiPF6in the mixture of ethylene carbonate(EC)and dimethyl carbonate(DMC)(1:1 in volume ratio).The working electrode was prepared by casting a mixture slurry of 80 wt%active material,10 wt%carbon black(Super-P)and 10 wt%polyvinylidenedi fluoride(PVDF)(withN-methylpyrolidoneasasolvent)on a copperfoil,the film was dried at 100°C overnight in a vacuum oven.The dried film was cut into round disks,the active material loading of the disks was1–2 mg·cm−2.Galvanostatic charge–discharge studies of the fabricated lithium-ion cells were carried out on a LAND CT2001A multichannel battery test system in the potential range of 0.005–3.0 V(vs.Li/Li+)at a current rate of 200 mA·g−1and 500 mA·g−1.
3.Results and Discussion
The morphologies of the obtained C-SiO2,C-mSiO2,C-SiO2-C and C-mSiO2-C hollow spheres are shown in Fig.2.Firstly,C-SiO2double-shell hollow microspheres were prepared by a rapid and facile aerosol based process,the formation mechanism have been given elsewhere[23–26].From Fig.2a,the hollow spheres possess a relatively smooth surface with diameters ranging from 0.5 to 2 μm,characteristics of the aerosol particles.From the broken hollow spheres(inset in Fig.2a)and the TEM image(Fig.S1a),the inner carbon layer and outer solid SiO2layer can be clearly identified.The energy-dispersive X-ray spectroscopy(EDS)elemental mapping and line-scanning further con firm the shell is composed of Cand SiO2(Fig.S1b,c).We suppose that such hollow particles without any porous structure in the shells have a low surface area and pore volume,and limited diffusion process of electrolyte solution and ions.Our hypothesis is that the outer silica layer can be rearranged by exploiting the dissolution–regrowth process while maisntaining the integrityofthechemicallystableinnercarbonlayer.Interestingly,therearrangement result in formation of double-shelled C-mSiO2hollow spheres with a porous silica layer,a large void space between the two layers,which are favorable for transportation of electrolyte solution and buffering volume change of the silica layer.Fig.2b and c show that hollow microspheres with obvious double-shell structure(~50 nm between the two layers)were obtained compared with the C-SiO2aerosol particles in Fig.2a and Fig.S1a.The dissolution of the silica layer and its regrowth on the carbon layer has been studied systematically by monitoring the structure evolution of the particle outer surface(Fig.S2).The HRSEM image(inset in Fig.2b)shows that C-mSiO2spheres are composed of an inner carbon layer and outer macroporous SiO2nanoplates,proving happens of dissolution and regrowth of SiO2.
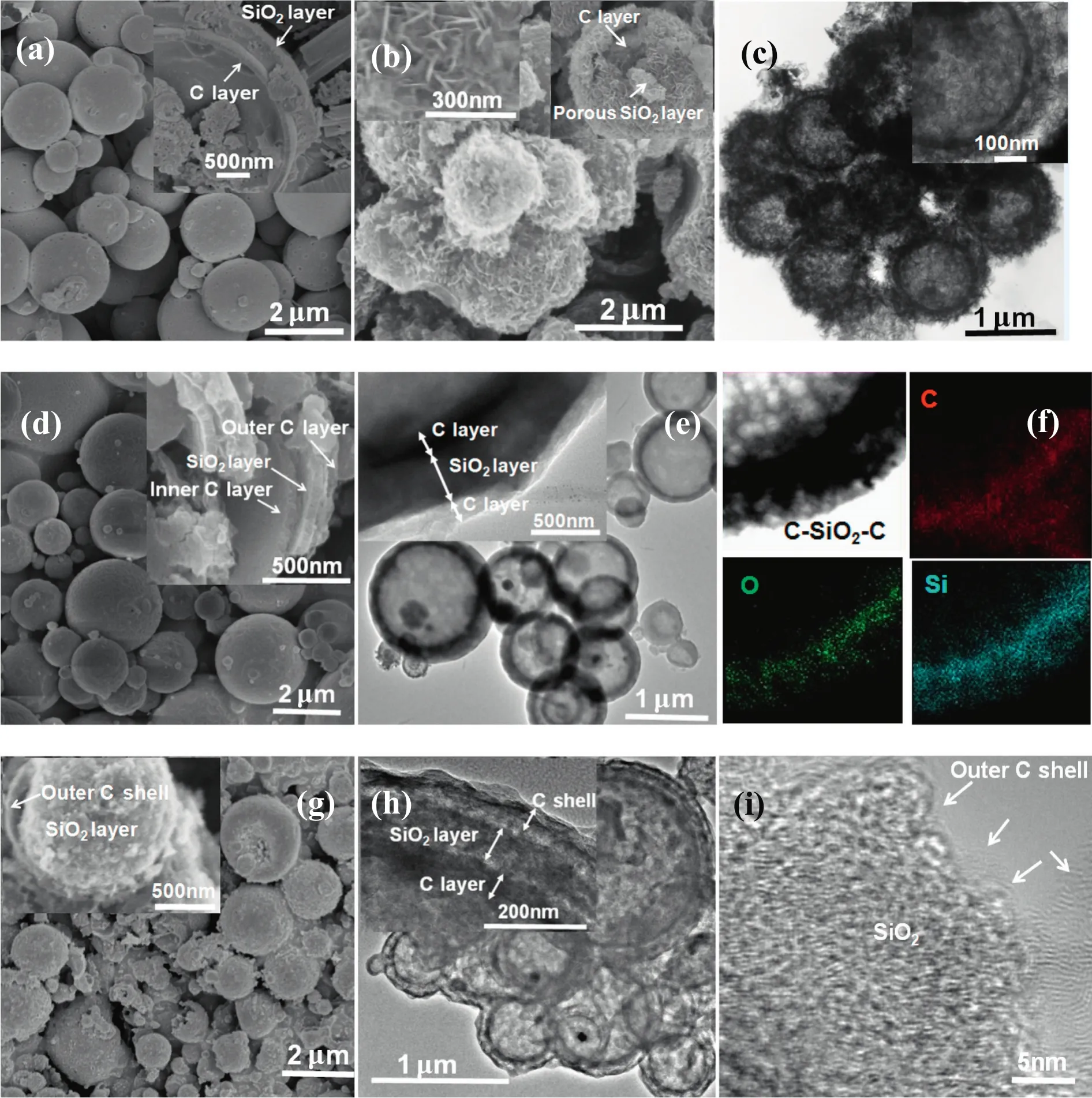
Fig.2.(a)SEMimageofC-SiO2hollowaerosolparticles,(b)(c)SEMandTEMimagesofC-mSiO2,(d)(e)(f)SEM,TEM and EDS images of C-SiO2-C,(g)(h)(i)SEM,TEMandHRTEMimagesof C-mSiO2-C.
With C-SiO2and C-mSiO2hollow spheres as templates,triple-shell hollow spheres of C-SiO2-C and C-mSiO2-C were prepared with sucrose as the carbon source.As evident by TEM and SEM image in Fig.2d and e,theC-SiO2-Chas a hollow structure and triple-layered shell.The sucrose coating and the following carbonization processes do not destroy morphology of the C-SiO2hollow spheres.The EDS elemental mapping further con firms the formation of C,SiO2and C triple-layered structure(Fig.2f).For the C-mSiO2-C triple-shell hollow spheres,however,a distinct space between the SiO2and outer C shell can be easily identified(inset in Fig.2g).The TEM images in Fig.2h further con firm the existence of larges pace bet ween the in termediatesilicalayer and the out erorinner carbon layer.In addition,besides formation of the outer carbon layer,the hydrothermal process also forms an ultrathin(1–2nm)conformal carbon coating on the SiO2nanoplates(Fig.2i),which can facilitate the fast electron transfer and protect the silica layer during the lithium insertion and desertion process.
The porous structure andsurface area of the C-mSiO2-C and C-SiO2-C(Fig.S3)electrode materials are investigated through the nitrogen adsorption–desorption measurement.The BET specific surface area and pore volume of C-mSiO2-C was measured to be 246.7 m2·g−1and 0.441 cm3·g−1respectively,which are much higher than those of CSiO2-C sample(48.7 m2·g−1and 0.069 cm3·g−1),on account of SiO2nanoplates with opened macroporous structure enhancing the adsorption capacity.Accordingly,the dissolution and regrowth of SiO2not only induce formation of porous SiO2nanoplates and large space between the protect carbon layer,but largely increase the specific surface area and pore volume of the C-SiO2-C electrode materials.The corresponding pore size distribution calculated by BJH method(Fig.S3b)reveals that both of the samples have a relatively narrow pore size around3.9nm,while an additional broad peak represents the mesopores range from 5 to 10 nm.The unique porous structure may be favorable to achieve high capacity and long cycling life for lithium storage,due to the void space buffering the volume changes during repeated charge–discharge processes.In order to elucidate the chemical characteristics and binding con figuration of the SiO2in porous carbon,X-ray photoelectron spectroscopy(XPS)analysis was conducted(Fig.S4).It presents the high-resolution XPS spectra of C1sand Si2pfor C-mSiO2-C and C-SiO2-C triple-shell hollow spheres.The binding energy scales of the spectra were calibrated by assigning the most intense C1speak a binding energy of 284.6 eV[27],then the only intense Si2ppeak at 102.8 eV(for C-SiO2-C)and 102.7 eV(for C-mSiO2-C)related to Si4+,suggesting the single amorphous phase of SiO2,which demonstrates that SiO2was not reduced to other forms of Si–O composites.

Fig.3.Charge–discharge pro files of C-mSiO2-C(a),C-mSiO2(b),C-SiO2-C(c),and C-SiO2(d).
The charge–discharge pro files at typical cycles of C-mSiO2-C(Fig.3a),C-mSiO2(Fig.3b),C-SiO2-C(Fig.3c),and C-SiO2(Fig.3d)electrodes were tested at a current density of200mA·g−1for the first three cycles and the following 100 cycles between the voltage limits of 0.05–3.0 V.For example,as shown in Fig.2a,there is a relatively long plateau at around 0.7 V.And it can only be found on the first discharge curve,which can be explained by the formation of SEI film[28–30].The initial dis charge capacity of the C-mSiO2-C is 648 mA·h·g−1,and the charge capacity is 412.7 mA·h·g−1,with an initial coulombic efficiency of 64%.The discharge capacity after the 2nd cycle is stabled at about 523.8 mA·h·g−1.By dissolution and regrowth of SiO2layer,the obtained SiO2nanoplates with opened macroporous structure may make the reaction more easily.And after 3 cycles,the electrode delivers a high coulombic efficiency,which has been maintained over 98%in the following cycles,as a result of the stable SEI films on the electrode and SiO2nanoplates[28,31–33].However,without opened macroporous structure or outer carbon layer,the in itial capacity of the C-SiO2onlyreaches355.4mA·h·g−1,with a coulombic efficiency of 48.24%.By contrast,C-mSiO2and C-SiO2-C have little higher coulombic efficiency of 55.23%and 53.75%and specific capacities.These results further con firm the unique porous SiO2nanoplates and the sandwich-like structure with two carbon layer play a dominant role to improve the Li-ion storage performance of C-mSiO2-C electrode material.
Fig.4a demonstrates the cycling performance of C-mSiO2-C,C-mSiO2,C-SiO2-C and C-SiO2composites at a current density of 200 mA·g−1with avoltagerange of0.005–3.0V(vs.Li+/Li).It is found that the discharge capacity of C-SiO2double-shell hollow microspheres decreases rapidly with cycling and remains only 304.1 mA·h·g−1at the 100th cycle.The capacity of the 100th cycle merely retains 68.2%of the fifth cycle,which can be attributed to the C-SiO2composites pulverization caused by the large volume change.The C-mSiO2with macroporous SiO2outer layer delivers a capacity of 337.4 mA·h·g−1.The high cycling capacity may be due to that macroporous SiO2nanoplates have the capability of buffering the volume changes of SiO2during the repeated charge–discharge processes.Meanwhile,C-SiO2-Ctriple-shell hollow spheres deliver a higher reversible capacity of 377.5mA·h·g−1thanks to the uniform outer carbon coating layer.After coated by the carbon layer from the carbonization of sucrose,the cycling performance of the obtained triple-shell structured C-mSiO2-C composite has been significantly improved.It delivers the highest reversible capacity of 501.5 mA·h·g−1and excellent cycle stability with the capacity retention of about 97%as against the tenth cycle after 100 cycles.Moreover,the coulombic efficiency of the C-mSiO2-C composite rapidly increases from 63%of the first cycle to 92%of the second cycle and maintains nearly 100%in the subsequent cycles.The inner and outer carbon layer can enhance the electrical conductivity of electrode as well as accommodate the volume changes of the SiO2component during the cycling.
In addition,Fig.4b reveals the cycling performance of all four composites at a current density of 500 mA·g−1.Composites besides C-mSiO2-C shows a low initial charge capacity,with the enhancement of the Li+diffusion kinetics and the further formation of Si,the reversible capacity increases in the following cycles[15].On the contrary,C-mSiO2-C delivers a good capacity because of its unique structure.At the 100th cycle,C-mSiO2-C composites still deliver a high capacity of 501 mA·h·g−1,which is much higher than that of other electrode materials(C-mSiO2:391 mA·h·g−1,C-SiO2-C:370 mA·h·g−1,C-SiO2:320 mA·h·g−1).The cells of C-mSiO2-C and C-SiO2-C after 100 cycles were disassembled and the electrodes were characterized by SEM,as shown in Fig.S5.In the case of SiO2,the C-SiO2-C spherical morphology becomes fragmented while C-mSiO2-Cstill can keep the original appearance.To investigate the stability of this unique structure and the effect of carbon layer,longterm cycling test of the C-mSiO2-Cwas carried out at a current density of 1000 mA·g−1(Fig.4e).Fortunately,the C-mSiO2-C still delivers 403 mA·h·g−1after 400th cycle.Consequently,the C-mSiO2-C triple shell hollow spheres exhibits excellent stability and specific capacity.

Fig.4.The cycling performance of the C-mSiO2-C,C-mSiO2,C-SiO2-CandC-SiO2composites at a current density of 200 mA·g−1(a)and 500 mA·g−1(b),and(c)rate capability tests for C-mSiO2-C,C-mSiO2,C-SiO2-C and C-SiO2electrodes,(d)EIS spectra of the four samples,(e)Long-term cycling performance of the C-mSiO2-C at a current density of 1000 mA·g−1.
The rate performance of the composites at different current densities ranging from 0.1 A·g−1to 2 A·g−1is shown in Fig.4c.It is found that CSiO2and C-SiO2-C anodes suffer a fast capacity attenuation.For example,C-SiO2anodes reversible capacity decreases from 518 mA·h·g−1(at0.1A·g−1)to76.5mA·h·g−1(at2A·g−1).Conversely,the discharge capacities of C-mSiO2-C anodes were stabilized at around 512,466,387,296,239 and 196 mA·h·g−1when cycled at 0.1,0.2,0.5,1,1.5 and 2 A·g−1,respectively.Importantly,when the original current density was recovered to 0.1 A·g−1,a high specific capacity of 517 mA·h·g−1could still be obtained,and it is even a little higher than that at the first10cycles.The good electrochemical properties are related to the unique structure of the C-mSiO2-C triple-shell hollow spheres.
Impedance experiments are shown in Fig.4d.The semicircle at high frequency can be ascribed to the charge transfer resistance,which is linked to the electrochemical reaction between the particles or between the electrode and the electrolyte.The sloping line is related to lithiumion diffusion in the active material.It can be seen that the impedance of C-mSiO2-C triple-shell hollow spheres is lower than that of others,which means the highest electrical conductivity and ion diffusion rate of the C-mSiO2-C electrode material.
4.Conclusions
In summary,we have successfully synthesized C-mSiO2-C tripleshell hollow spheres which have outer and inner carbon layers and intermediate macroporous SiO2nanoplates.This structure buffers the volume change of silica during repeated charge–discharge processes and provides higher electric conductivity for electron transport.Furthermore,the SiO2nanoplates with opened macroporous structure facilitate the electrolyte transport and electrochemical reaction.Consequently,C-mSiO2-C triple-shell hollow spheres still deliver a high capacity of 501 mA·h·g−1after 100 cycles at a current density of 500 mA·g−1and long good cycling performance,which is much higher than that of other electrode materials.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2018.01.016.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Understanding the Influence of microwave on the relative volatility used in the pyrolysis of Indonesia oil sands☆
- Degradation and mineralization of aniline by O3/Fenton process enhanced using high-gravity technology☆
- Three-liquid-phase extraction and separation of V(V)and Cr(VI)from acidic leach solutions of high-chromium vanadium–titanium magnetite☆
- Insight into the degradation mechanism of cefixime under crystallization condition☆
- Experimental and simulation of the reactive dividing wall column based on ethyl acetate synthesis☆
