Desulfurization of liquid hydrocarbon fuels via Cu2O catalyzed photooxidation coupled with liquid–liquid extraction☆
2018-08-18XiaomingGaoJiaoFeiYanyanShangFengFu
Xiaoming Gao*,Jiao Fei,Yanyan Shang,Feng Fu
Department of Chemistry and Chemical Engineering,Shaanxi Key Laboratory of Chemical Reaction Engineering,Yan'an University,Yan'an 716000,China
Keywords:Desulfurization Photo-oxidative Gasoline Extraction Cu2O Catalyst
ABSTRACT By combining the photochemical reaction and liquid–liquid extraction(PODS),we studied desulfurization of model fuel and FCC gasoline.The effects of air flow,illumination time,extractants,volume ratios of extract ant/fuel,and catalyst amounts on the desulfurization process of PODS were analyzed in detail.Under the conditions with the air as oxidant(150 ml·min−1),the mixture of DMF–water as extract ant(the volume ratio of extract ant/oil of0.5)and photo-irradiationtime of2h,the sulfur removal rate reachedonly42.63%and39.54%for the model and FCC gasoline,respectively.Under the same conditions,the sulfur removal rate increased significantly up to 79%for gasoline in the presence of Cu2O catalyst(2 g·L−1).The results suggest that the PODS combined with a Cu2O catalyst seems to be a promising alternative for sulfur removal of gasoline.
1.Introduction
Because the sulfur-contained compounds in the fuel are converted to toxic SOx during their combustion,this not only contributes to acid rain and soil pollution,but also poisons catalysts for decontamination of car exhaust gases[1–5].Consequently,there are stringent requirements for the sulfur content in fuels in many countries[6].However,conventional hydrodesulfurization methods(HDS)can hardly produce the desired fuels that can well fit with these requirements owing to the low reactive aromatic thiophenes and their derivatives existing in the liquid fuels[7,8].Therefore,it is of great importance to develop more advanced approaches/advanced technologies to HDS to achieve deep desulfurization of fuel gasoline.
So far,various alternative techniques to HDS have been investigated to deeply desulfurize hydrocarbon fuels,such as adsorption[6,9,10],oxidative desulfurization(ODS)[11,12],and biocatalytic treatment[1].Among them,ODS is one of the most promising methods for deep desulfurization of fuels[13],because it can be carried out under very mild conditions(ambient temperature and pressure).In this process,thiophene and its derivatives are of high reactivity and thus can be easily oxidized to corresponding sulfones or sulfoxides[1].Therefore,the large polar discrepancy between these oxides and fuel molecules facilitated the removal of sulfur by the extraction or distillation[7].Hydrogen peroxide(H2O2)is the most used oxidant in the chemical oxidative desulfurization due to its high active oxygen content and environmental benign properties[13].However,introducing H2O2into fuel oil requires additional liquid–liquid phase separation process after oxidation,which will decrease the efficiency of the overall processes and causes a loss of fuel.Furthermore,the utilization and storage of large amount of H2O2are potentially dangerous.
Recently,photo-oxidative desulfurization(PODS)technique has attracted special interest in research[2,14–16],by which the oxidation and extraction are carried out at the same time.Moreover,atmospheric oxygen can be used as an oxidant in this process thus avoiding extra separation process[14].However,there is few work related to introducing a photosensitizer in a photo-oxidative desulfurization process of fuels to increase the efficiency of photochemical reaction,due to the more complicated operation required[2].
In the present work,a facile PODS method without a photosensitizer was employed to reduce the sulfur content in oil fuels such as FCC gasoline,where atmospheric oxygen and copper(I)oxide hydrothermally prepared were used as oxidant and photocatalyst,respectively.The effects of various factors including air flow,illumination time,extractant type,volume ratio of fuel/extractant,and catalyst dosage were investigated in detail to acquire the optimal desulfurization conditions.
2.Experimental
2.1.Preparation of photocatalyst
All chemicals used in the experiment were of analytical grade and used as received.In a typical hydrothermal synthesis,a 0.5 mol·L−1NaOH solution was added dropwise to 10 ml of aqueous solution of CuCO3·Cu(OH)2·xH2O(1.0 g)and glucose(1.34 g)with stirring.Then,the above solution was stirred for 30 min after the pH of solution reached to 12.The suspended liquid prepared was hydrothermally treated in a microautoclave of 25 ml internal volume at 90°C for 10 h,and cooled in the oven overnight.Finally,the obtained red Cu2O powder was filtrated,washed with distilled water and dried at 100°C for 24 h.
2.2.Characterization
The phase and composition of the samples were identified by X-ray diffraction(XRD)using monochromatized Cu Kαradiation under 40 kV and 100 mA and with the 2θ ranging from 20°to 80°(Shimadzu XRD-7000).The morphologies and microstructures of the samples were analyzed using the scanning electron microscope(SEM,JEOL JSM-6700F).The FT-IR diffraction pattern of the samples was identified by an FTIR Spectrometer(IR)using KBr and with the range from 200 cm−1to 4000 cm−1(Shimadzu IR Prestige-21).
2.3.Photo-oxidative desulfurization
For comparison,a model gasoline(MG)with the sulfur concentration of 860 μg·g−1was prepared by adding thiophene of 600 μl into n-octane(500ml).Furthermore,the real FCC gasoline(FG)used here was obtained from a local oil re finery with the sulfur content of 740 μg·g−1.
2.3.1.PODS without catalyst
PODS experiments were carried out in a photochemical reactor(XPAII,Nanjing Xujiang Machine Plant)equipped with a magnetic stirrer,quartz cap,UV light and re flux condenser.A typical procedure was as follows:10 ml of MG or FG solution was placed in the quartz tube combined with an extractant(acetonitrile,methanol,dimethylformamide(DMF)or DMF/water solution)at various volume ratios of oil to extractant.The resulting mixture was stirred vigorously and photo-irradiated by a high pressure mercury lamp(400 W)in the presence of air bubbling at a given flow rate at the controlled temperature of 25°C.The reaction mixtures were sampled at certain intervals and analyzed to determine the total sulfur content in the oil by a WK-2D total sulfur analyzer.
2.3.2.PODS with Cu2O
The PODS with Cu2O was also carried out according to the procedures described above.Prior to illumination,the oil,extractant and catalyst were mixed well with stirring in the reactor under the dark for 1 h to ensure adsorption/desorption equilibrium was reached.The catalyst was centrifuged off after photo-irradiation,and the sulfur concentration in the oil phase was analyzed by a WK-2D total sulfur analyzer.
3.Results and Discussion
3.1.Analysis of structure characterization
The XRD patternsof theas-prepared samples(Fig.1)show thesamples prepared in different times are well crystallized.The diffraction peaks at 2θ of 29.68°,36.41°,42.36°,52.41°,61.48°,73.65°,and 77.41°can be indexed to Cu2O(JCPDS No.78-2076),indexed to(110),(111),(200),(211),(220),(311),and(222)planes,respectively[17].Moreover,with the increase in the hydrothermal time,the peak intensity of the(111)plane is enhanced,which indicates that the crystallized phase is increased gradually.Furthermore,as the hydrothermal time is 12 h,the diffraction peaks of other impurities are not observed,indicating that the Cu2O has high purity.
The morphologies of the Cu2O sample were characterized by SEM.Fig.2 shows a SEM image of Cu2O synthesized at 12 h,which consists of the uniform cube with the length of 1.0–2.0 μm.
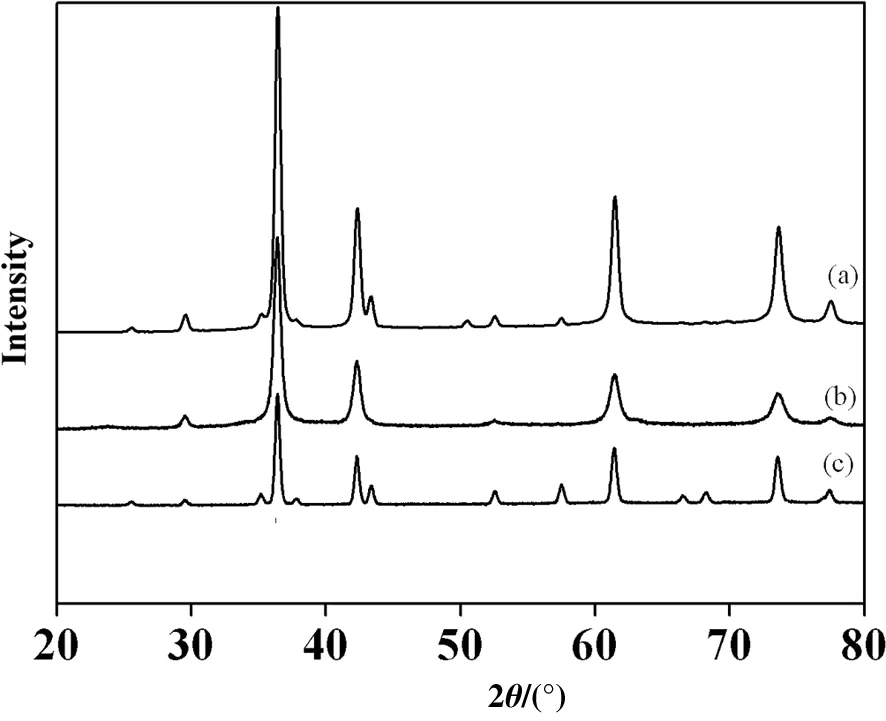
Fig.1.The X-ray diffraction patterns of Cu2O synthesized at different times:(a)6 h,(b)8 h,(c)10 h.

Fig.2.The SEM photograph of the Cu2O sample synthesized at 10 h.
Fig.3 shows the FT-IR spectra of the sample synthesized at 10 h.The absorption peaks appear in the vicinity of the wave number of 625 cm−1owing to the Cu--O--Cu stretch and bending,which indicates that the sample synthesized consists of Cu2O[18].

Fig.3.The FT-IR diffraction patterns of sample synthesized at 10 h.
3.2.The PODS of model gasoline(MG)
3.2.1.The effect of air flow
We examined the performance of PODS on MG,and a series of optimization tests have been carried out.Fig.4 shows the desulfurization efficiency of PODS on MG as a function of air flow.

Fig.4.The effect of air flow rate on desulfurization efficiency of PODS for MG.Reaction conditions:temperature of 298 K;photo-irradiation time of 2 h;DMF as the extract ant and the volume ratio of DMF/oil at 0.5;the initial sulfur content of MG at 860 μg·g−1.
Without illumination,the sulfur removal reached to only 9.65%by bubbling air,indicating just a few sulfur compounds transfer from nonpolar oil to polar DMF phase by simply mixing the two phases.However,the sulfur content in treated MG decreased observably in the presence of photoirradiation with air.This suggests the sulfur compounds like thiophene can be converted to highly polarized compounds by the photooxidation process[19,20],which are difficult to distribute into the nonpolar oil phase.Moreover,the sulfur removal initially increased as the air flow increased from 0 to 150 ml·min−1,but declined slightly with further addition of the flow rate.It is self-evident that the higher the rate of air flow,the more dissolved is the oxygen in the reaction system before it reaches the dissolution equilibrium.This would enhance the formation of excited oxygen under photoirradiation,which is beneficial to oxidation of the thiophene to polar sulfide.Thus,it is reasonable to observe that further increasing the air flow cannot enhance the oxygen content in the mixed liquid when the rate of air flow reaches to 150 ml·min−1.Furthermore,continuous air flow would favor the volatilization of oil,leading to decline of the desulfurization efficiency.So,the following study was performed at the air flow of 150 ml·min−1in considering the treatment efficiency.
3.2.2.The effect of extractant
To make the PODS process more efficient,the extractant must be carefully selected to satisfy a number of requirements.In view of polarity of oxidizing sulfur species,the extractant must be of high polarity to avoid being dissolved in the oil.Here,we evaluated the performance of several polar solvents as extractant in the PODS process,such as water,methanol,acetonitrile and DMF.

Fig.5.Effect of different polar extracting solvents on desulfurization efficiency of PODS for MG.Reaction conditions:298 K;photo-irradiation time of 2 h;air flow of 150 ml·min−1;the volume ratio of extract ant/oil at 0.5;the initial sulfur content of MG at 860 μg·g−1.
As shown in Fig.5,the highest sulfur removal rate of 47.5%can be achieved when we use DMF as extract ant during the PODS process.However,the corresponding oil recovery rate was only92.2%after treatment,decreasing economic efficiency greatly.To improve the total efficiency,water was added into DMF to adjust its properties related to its solubility of sulfur compounds and oil rejection.Subsequently,we investigated the effect of water content in DMF to desulfurization efficiency.Fig.6 shows that the sulfur removal declined with the increase of concentration of water in DMF,while the corresponding oil recovery was enhanced.This indicates that the solubility of both organosulfur compounds and oil in the mixed extract ant is reduced as the water content in DMF increases.In conclusion,the increase of water content in extract ant plays a dual role in affecting the efficiency of PODS.Thus,considering the desulfurization and economic efficiency,the water con tent cannot be fixed at too high a value in extract ant.The foll owed study on the PODS process was performed at the water content of 10 wt%in the mixed extract ant.

Fig.6.The effect of water content in DMF on desulfurization efficiency of PODS for MG.Reaction conditions:at 298 K;irradiation time(2 h);air flow(150 ml·min−1);the volume ratio of extractant/oil at 0.5;the initial sulfur content of MG at 860 μg·g−1.
Table 1 shows the variations in sulfur removal and oil recovery with respect to the volume ratio of extract ant/oil.Obviously,the sulfur removal increased substantially from 8.1%to 64.86%as the volume ratio of extractant/oil increased from 0 to 1,which then decreased slowly with a further increase of the volume ratio.On the other hand,as expected,the oil recovery reduced gradually from 99.81%to 77.09%with the increase of the volume ratio of extractant/oil.Although the sulfur removal reached the maximum of 64.86%at the volume ratio of extractant/oil of 1,the relative oil recovery was still low(91.53%).Thus,the compromised volume ratio of extractant/oil of 0.5 was used for further experiments.

Table 1 The effect of different volume ratios of extractant/oil on desulfurization efficiency of PODS for MG①
3.3.The PODS of FCC gasoline(FG)
The PODS of FG(740 μg·g−1)was performed with a volume ratio of extractant/oil of 0.5 at 298 K as a function of photo-irradiation time,where the mixture of water–DMF(water content of 10 wt%)was used as the extractant.It can be seen in Fig.7 that the total sulfur content in treated FG decreased with the photo-reaction proceeding.However,this trend becomes faint as the photo-irradiation time exceeds 2 h.The sulfur removal only increased from 39.54%to 43.23%with the illumination time prolonged from 2 to 5 h.On the other hand,the experimental data were also analyzed using a pseudo- first-order model.If the photooxidation of organosulfur compounds keeps pseudo- first order kinetics in the PODS process,the plots of−ln(St/S0)vst would result in a linear relationship.Meanwhile,S0and Stare the sulfur content of treated FGattime zero and timet(h),respectively.Fig.8 showed that the value of the linear regression correlation coefficient(R2=0.995),is close to unity.This indicates strongly that the photooxidation of organosulfur compounds for FG by the PODS process follows the pseudo- first-order kinetics.

Fig.7.Variation of the total sulfur content and removal rate with irradiation time in treated FG after the PODS process.Reaction conditions:298 K;air flow(150 ml·min−1);the mixture of water-DMF(water content of 10 wt%)as extract ant;the initial sulfur content of FG at 740 μg·g−1.
In the experiments above,a satisfactory sulfur removal for FCC gasoline can never be obtained during the PODS process regardless of operating parameters,although it has been con firmed that organosulfur compounds can be removed from oil.The highest sulfur removal for FG reached is only 39.54%even at the relative optimal reaction conditions(temperature:298 K;extract ant:the mixture of water–DMF with water content of 10 wt.%;photo-irradiation time:2 h).As a result,copper(I)oxide prepared in our work was used as photocatalyst in the reaction system to further improve the efficiency of PODS.Fig.9showed that the sulfur removal rate is significantly enhanced in the presence of Cu2O,reaching its maximum value of 78.62%for Cu2O dosage being 2 g·L−1.The desulfurization efficiency is much higher than the value obtained without catalyst under the same conditions.Although the photo-oxidation is promoted by the Cu2O catalyst during the PODS process,the photoscattering and exclusion of light inevitably occur due to the presence of fine particles in the liquid.These negative effects would suppress the efficiency of photo-catalysis.Thus,the sulfur removal rate initially increased with the enhancement of the catalyst dosage,and then decreased slightly when too much Cu2O was added in the PODS system.

Fig.8.Pseudo- first-order kinetics of photooxidation of organosulfur compounds in the PODS process.

Fig.9.Effect of Cu2O dosage on desulfurization efficiency of PODS for FG.Reaction conditions:298 K;irradiation time(2 h);air flow(150 ml·min−1);the mixture of water–DMF(water content of 10 wt%)as extract ant;the volume ratio of extractant/oil at 0.5;the initial sulfur content of FG at 740 μg·g−1.
4.Conclusions
The results obtained in this study have demonstrated that the organosulfur compounds can be removed efficiently from oil fuel through the PODS process under mild reaction conditions.The sulfur compounds in FCC gasoline can be oxidized by air through the photooxidative process in the presence of Cu2O,the polarity of solvent plays an important role in determining the desulfurization efficiency and oil recovery,and the photooxidation of organosulfur compounds agreed with a pseudo- first-order reaction kinetics.Under the optimal reaction conditions,the sulfur level in FCC gasoline can be reduced markedly from 740 to about 420 μg·g−1,whereas,with the catalyst,the sulfur removal rate for FCC gasoline can be enhanced to 79%.Thus,the use of PODS with the Cu2O catalyst shows a good promise for desulfurization of FCC gasoline under mild conditions.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Understanding the Influence of microwave on the relative volatility used in the pyrolysis of Indonesia oil sands☆
- Degradation and mineralization of aniline by O3/Fenton process enhanced using high-gravity technology☆
- Three-liquid-phase extraction and separation of V(V)and Cr(VI)from acidic leach solutions of high-chromium vanadium–titanium magnetite☆
- Insight into the degradation mechanism of cefixime under crystallization condition☆
- Experimental and simulation of the reactive dividing wall column based on ethyl acetate synthesis☆
