甘蓝型油菜种子萌发期耐苯磺隆种质筛选与综合评价
2018-08-10崔明圣赵愉风唐章林李加纳周清元
王 倩 崔 翠 叶 桑 崔明圣 赵愉风 林 呐 唐章林 李加纳 周清元
甘蓝型油菜种子萌发期耐苯磺隆种质筛选与综合评价
王 倩**崔 翠**叶 桑 崔明圣 赵愉风 林 呐 唐章林 李加纳 周清元*
西南大学农学与生物科技学院, 重庆 400715
本研究旨在探讨不同基因型甘蓝型油菜种子萌发期耐除草剂苯磺隆特性, 建立其筛选的评价体系, 选育萌发期耐苯磺隆的甘蓝型油菜品种(系)。采用苯磺隆耐性度量值(T值)、平均隶属函数值(ASF值)、加权耐性系数(WTC值)等指标及相关分析、频数分析、主成分分析、灰色关联度分析、聚类分析和逐步回归分析相结合的方法, 鉴定萌发期耐性。通过11份供试材料相对根长的方差分析, 确定了油菜萌发期耐苯磺隆种质资源筛选及评价研究的最佳苯磺隆浓度为25 mg kg–1。在此浓度胁迫下, 241份甘蓝型油菜的根长、芽长、率、发芽势、鲜重5个指标均存在显著性差异。根据T值的聚类分析, 将供试种质划分为5个苯磺隆耐性级别, 其中I级3份、II级30份、III级198份、IV级6份、V级4份。对苯磺隆耐性较强的甘蓝型油菜品种(系)为希望106、SWU95和WH-33, 可作为甘蓝型油菜苯磺隆耐性育种和耐性机制研究的重要资源。根长、鲜重、发芽率可作为评价油菜种质资源萌发期耐性的指标性状。
甘蓝型油菜; 耐苯磺隆; 种质资源; 综合评价; 萌发期
自1942年除草剂2,4-D问世以来, 人类使用化学除草剂防除农田杂草已有70多年的历史。西方发达国家已经在85%~100%的作物上使用除草剂[1], 我国除草剂生产和使用量也逐年增加[2]。但是, 在所施用的除草剂中, 绝大部分都残留于土壤或淋溶于水中, 真正起作用的很少[3], 极容易导致对除草剂敏感的作物死亡[4]。不科学地使用半衰期长的除草剂还会毒害下茬作物[5]。因此, 既要高效地消灭杂草, 又不使作物受到药害, 可以通过新型除草剂产品的研发和耐除草剂作物品种的培育两种途径实现, 其中后者相对比较经济, 费用一般不超过研制新除草剂的5%[6]。发掘农作物除草剂耐性基因、培育耐除草剂农作物新品种成为作物遗传育种的重要方向之一。
油菜是当今世界的主要油料作物之一, 我国油菜的种植面积、总产量均约占世界的1/3[7]。田间杂草危害可使油菜产量下降15%, 更甚者可减产50%以上[8-9]。我国油菜主产区的杂草类型大致可分为以禾本科杂草为主、以阔叶杂草为主以及禾本科与阔叶草混生3种[10]。油菜为阔叶型植物, 所以阔叶杂草就成为油菜田杂草防除的一个关键瓶颈[11]。苯磺隆作为阔叶类杂草除草剂, 在小麦田除草取得良好效果[12], 若要选育出耐苯磺隆的油菜品种, 将苯磺隆和现有油菜田单子叶除草剂混合使用, 可为油菜田杂草的有效防除提供一条新的途径[13]。因此, 培育和推广耐苯磺隆除草剂油菜品种可以有效控制阔叶草害, 减少中耕除草用工,提高油菜产量, 增加效益[14]。另外, 苯磺隆作为化学杀雄剂在油菜杂种优势利用研究中效果良好, 已经引起广泛重视[15-16], 选育具有苯磺隆除草剂耐性的甘蓝型油菜作为父本,有利于简化制种程序, 降低制种成本[16-17]。信晓阳等[14]使用苯磺隆溶液处理49个不同基因型油菜幼苗, 筛选出2个在苗期低敏感材料。曲高平等[18]用甲基磺酸乙酯(EMS)溶液对甘蓝型油菜中双9号种子诱变处理, 在M2突变体库中筛选到3株苯磺隆耐性突变体。华中农业大学报道从华双5号油菜EMS突变后代群体中鉴定出几株苯磺隆耐性突变体, 测序表明它们的突变位点均为的Pro197Ser/ Leu突变[16]。吴学莉等[17]从一个耐苯磺隆除草剂的播娘蒿天然突变体中克隆了乙酰乳酸合成酶(ALS)基因, 并通过转基因发现其表达使甘蓝型油菜对苯磺隆的耐性提高至野生型致死浓度的3倍。汪亚琴[19]以甘蓝型油菜品系甲572为受体, 转化水稻细胞色素P450基因家族成员基因, 获得了抗苯磺隆的油菜株系。有研究表明植物耐受苯磺隆除草剂主要与基因突变和非靶标酶代谢解毒能力有关[1,20-24]。同时, 田间杂草对苯磺隆耐性突变体的机制研究也相继报道, 其中黑草和看麦娘的基因在拟南芥中表达, 获得的转基因植物可表现抗多种除草剂[25-28]。在耐除草剂作物的培育中, 鉴定并获得具有天然耐药性的植物是研究的关键[29], 因此, 从现有油菜中筛选耐苯磺隆种质资源, 并挖掘内源耐苯磺隆基因源对于油菜育种具有重要的理论和应用价值。
虽然在4种磺酰脲类除草剂中, 苯磺隆的植物毒性最低[30], 且苯磺隆为短残留除草剂[31], 但是也有报道指出小麦地土壤中苯磺隆降解半衰期为5~12 d[32-33], 因此, 苯磺隆仍存在短期土壤残留现象。目前, 关于苯磺隆土壤残留对作物种子, 尤其是对油菜种子萌发的影响未见报道。本研究拟综合鉴定与评价供试品种(系)的发芽率、发芽势、鲜重、干重、根长和芽长, 筛选出萌发期耐性较强的油菜种质及相关性较强的测定指标, 研究播种期苯磺隆残留对油菜种子萌发的影响, 为苗期、蕾薹期、花期筛选耐苯磺隆油菜品种(系)提供基础, 也为进一步在遗传和育种研究中利用这些优异种质提供理论依据。
1 材料与方法
1.1 供试油菜种质资源
试验材料为国内外各农业高校、科研院所选育或收集的, 具有不同遗传背景和广泛地理来源的241份甘蓝型油菜种质(附表1), 该群体包含部分自交系和常规品种, 种子均由重庆市油菜工程技术研究中心提供。
处理药剂为10%苯磺隆除草剂(江苏瑞东农药有限公司), 有效成分含量100 g L–1, 生产用量每公顷150 g兑水600 kg (250 mg kg–1)。
1.2 方法
1.2.1 萌发期最适苯磺隆处理浓度筛选 参照陈新等[34]的培养皿纸上发芽法并略有改动。以遗传背景不同的11份甘蓝型油菜品种(系)为材料(附表1), 对每个品种设置蒸馏水对照和不同浓度(250、25、2.5、0.25 mg kg–1)苯磺隆胁迫共5种处理, 各处理3次重复。在铺有2层滤纸的培养皿中加入3 mL不同浓度的苯磺隆溶液, 对照中加入相同体积蒸馏水, 将已清洗的饱满种子均匀放在滤纸上, 每皿20粒, 盖上皿盖, 于人工培养箱中培养, 设置温度为25℃, 相对湿度为85%, 光照和黑暗时间为16 h/8 h。参照汪梦竹等[35]的研究, 于第7天测定油菜主根根长, 分析并确定苯磺隆最佳胁迫浓度。
1.2.2 萌发期耐苯磺隆鉴定 用1.2.1中确定的最佳处理浓度, 以蒸馏水为对照, 依照上述方法培养241份甘蓝型油菜(附表1), 3次重复。于第3天统计种子发芽势(germination vigor, GV), 第7天统计种子发芽率(germination rate, GR), 随机选取每个重复10株幼苗分别测定其茎长(shoot length, SL)、根长(root length, RL)、鲜重(fresh weight, FW)和干重(dry weight, DW)形态指标。
1.3 数据处理与分析
利用Microsoft Excel 2013、IBM SPSS19.0[36]和DPS 2006[37]统计软件整理分析数据。
参考闫峰等[38]、Upadhyaya[39]、汪灿等[40]的方法, 以各品种6个性状测定值作为基础数据, 分析其平均数差异显著性, 按公式(1)计算对苯磺隆单项耐性系数(tribenuron-methyl tolerance coefficient, TC), 式中XCK分别表示第个指标下第个品种(系)苯磺隆胁迫和对照处理的指标测定值。针对各指标TC值, 进行简单相关分析、连续次数分布统计分析和主成分分析。
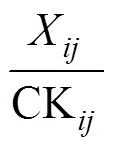
按公式(2)、(3)和(4)计算取各种质各指标的隶属函数值[(X)]和平均隶属函数值(ASF, average subordinative function value)。
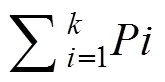
(X)(X– Xmin) /( Xmax – Xmin)= 1, 2, 3…,;1, 2, 3…,(3)

式中,P为第个综合指标贡献率, 表示第个指标在所有指标中的重要程度。X、Xmin、Xmax分别表示第个指标下第个品种的TC值及第个指标下的最小值和最大值。其中为选取的主成分个数。
根据因子权重系数(ω)和各主成分得分值[(X)], 按公式(5)计算苯磺隆耐性度量值(T, tribenuron-methyl tolerance comprehensive evaluation value)。
= 1, 2, 3, …,(5)
按公式(6)、(7)计算关联系数(ξ)及等权关联度(γ)。
ξ=,= 1, 2, 3, …,(6)
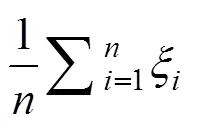
式中,ξ为关联系数,Δ为品种最优性状与第个品种(系)第个性状的绝对差值。minΔ为最小二级绝对差值, maxΔ为最大二级绝对差值,为分辨系数(取0.5)。
分别按公式(8)和(9)计算各指标权重系数[ω(γ)]和加权关联度(weight tribenuron-methyl tolerance coefficient, WTC)。


式中,γ为各指标关联度, TC为对苯磺隆单项耐性系数。
以各指标TC值为比较序列, 分别以T值和WTC值为参考序列进行灰色关联度分析, 获得各指标TC值与T值间的关联度(T)和TC值与WTC值间的关联度(WTC)。
最后基于供试甘蓝型油菜种质T值, 采用加权配对算术平均法(weighted pair group method average, WPGMA)和欧氏距离进行聚类分析, 划分苯磺隆耐性等级, 并分别以T值、ASF值和WTC值为参考序列, 对各指标TC值进行逐步回归分析, 获得回归方程。
2 结果与分析
2.1 苯磺隆胁迫浓度筛选
11份甘蓝型油菜品种在不同苯磺隆浓度处理条件下, 根长均受到不同程度的抑制(表1)。在0、0.25、2.5、25和250 mg kg–1浓度下, 根长均值分别为7.73、4.44、1.62、1.26和1.15 cm, 处理较对照分别下降36.12%、79.01%、83.64%和85.15%, 根长在2.5 mg kg–1处理时下降接近最大值, 到25 mg kg–1和250 mg kg–1处理时下降趋势变缓。11份品种在5个处理中差异均具有统计学意义, 但是25 mg kg–1胁迫处理时, 更能够区分材料本身的差异; 在25 mg kg–1具有差异的材料, 在0.25、2.5和250 mg kg–1胁迫处理均没有差异(表1)。如25 mg kg–1胁迫下, P18与所有品种(系)间差异均显著, 甲预31棚与中双12等5个品种差异显著。2.5 mg kg–1胁迫下, P18与至尊和B265品种(系)间差异不显著, 甲预31棚与6个品种(系)差异不显著。250 mg kg–1胁迫下, P18与至尊品种(系)间差异不显著, 甲预31棚与9个品种差异不显著。另外, 250 mg kg–1胁迫处理7 d后, 各品种根部萎缩生长普遍变差。因此, 25 mg kg–1浓度可作为鉴定甘蓝型油菜萌发期耐苯磺隆的适宜浓度。
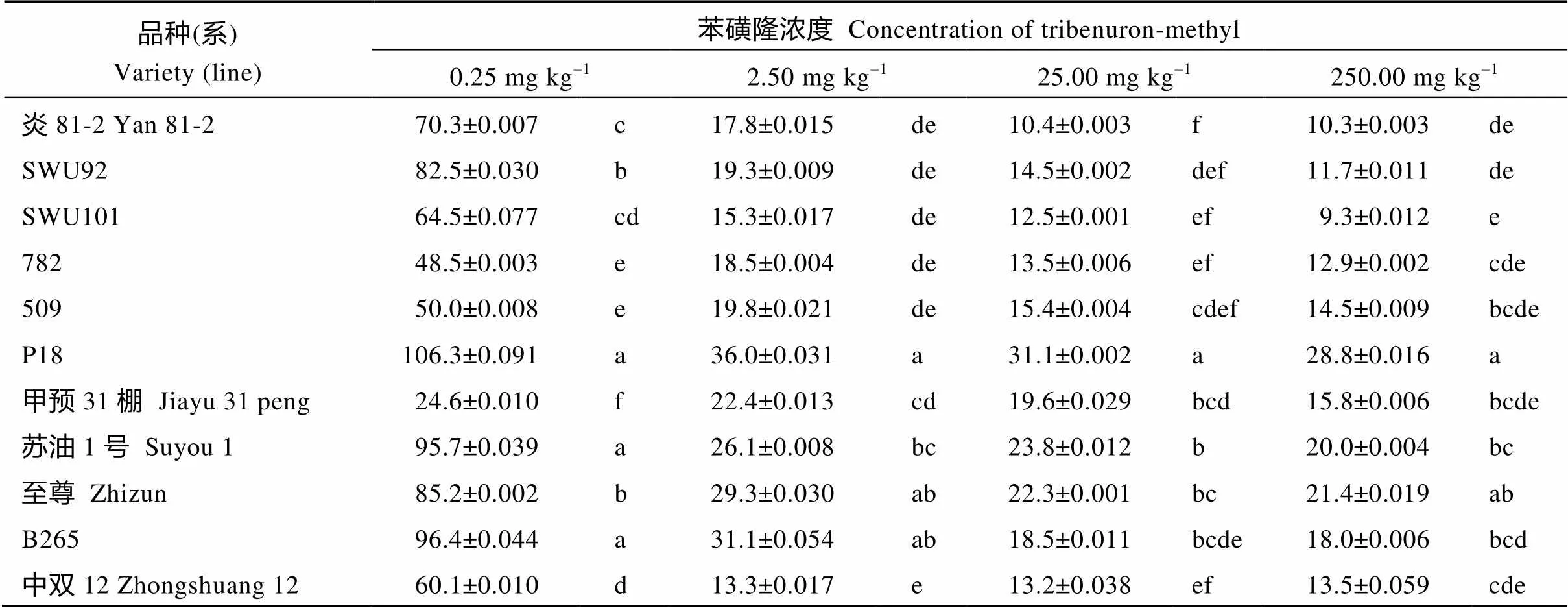
表1 不同浓度下11份品种根长相对值的显著性分析
同列标有不同小写字母者组间差异显著(<0.05)。
Values within a column followed by different letters are significant by different between groups (<0.05).
2.2 耐苯磺隆油菜种质资源筛选
2.2.1 241份甘蓝型油菜萌发期指标 对照和处理(25 mg kg–1苯磺隆胁迫)的6个性状在品种间差异均具有统计学意义, 各性状的变异系数分别为16.45%~54.56%和20.01%~54.83%, 说明试验中的241份种质在两个条件下均表现出广泛的遗传变异。在苯磺隆胁迫下, 各性状受到影响程度不同。胁迫处理下根长均值仅为0.94 cm, 较对照下降87.99%, 受抑制最严重。胁迫处理下的发芽势、发芽率、鲜重均值分别为75.77%、83.22%、0.36 g, 较对照分别下降6.81%、5.37%、5.26%。胁迫处理下芽长均值为2.37 cm, 较对照增加4.61%。在胁迫条件下, 根长、芽长、发芽势、发芽率的变异系数均增加, 其中根长的变异系数较对照增加最明显(表2)。
2.2.2 单项指标分析 供试种质在苯磺隆胁迫处理后, 与对照相比各指标均发生不同程度的变化(表3)。同一指标下不同品种对苯磺隆单项耐性系数(TC)的最大值和最小值之间差异较大, 说明不同品种间对苯磺隆的耐性不同。不同指标下TC值存在明显差异, 变异系数介于10.3%~49.0%之间, 表明各指标对苯磺隆胁迫反应的敏感性不同。此外对各单项指标进行单因素方差分析, 表明不同种质间在根长、芽长、发芽率、发芽势、鲜重5个指标下均存在具有统计学意义的差异。相关分析表明(表4), 各指标间存在一定程度的相关性。其中干重与根长呈显著负相关, 与发芽率呈显著正相关, 而与其余指标相关不显著。发芽势与发芽率呈极显著正相关, 与根长呈极显著负相关, 相关系数分别为0.687和–0.150。发芽率与根长呈显著负相关, 鲜重与芽长呈极显著正相关, 其余指标间无显著相关性。除发芽势与发芽率相关系数较高, 其余相关系数绝对值均在0.1~0.3之间。此外, 各指标TC值分布次数相差较大(图1)。根长的TC值在0.095~0.215之间的品种最多。TC>0.90的鲜重、发芽势、发芽率、芽长、干重的分布频率分别为68.0%、70.5%、80.5%、81.3%和96.3%, 各指标对苯磺隆胁迫的敏感性由强到弱依次是根长、鲜重、发芽势、发芽率、芽长、干重。因此, 如果直接采用各个指标, 由于指标间信息的叠加重合, 很难准确客观地评价各种质的耐性, 从而影响对苯磺隆耐性鉴定结果。
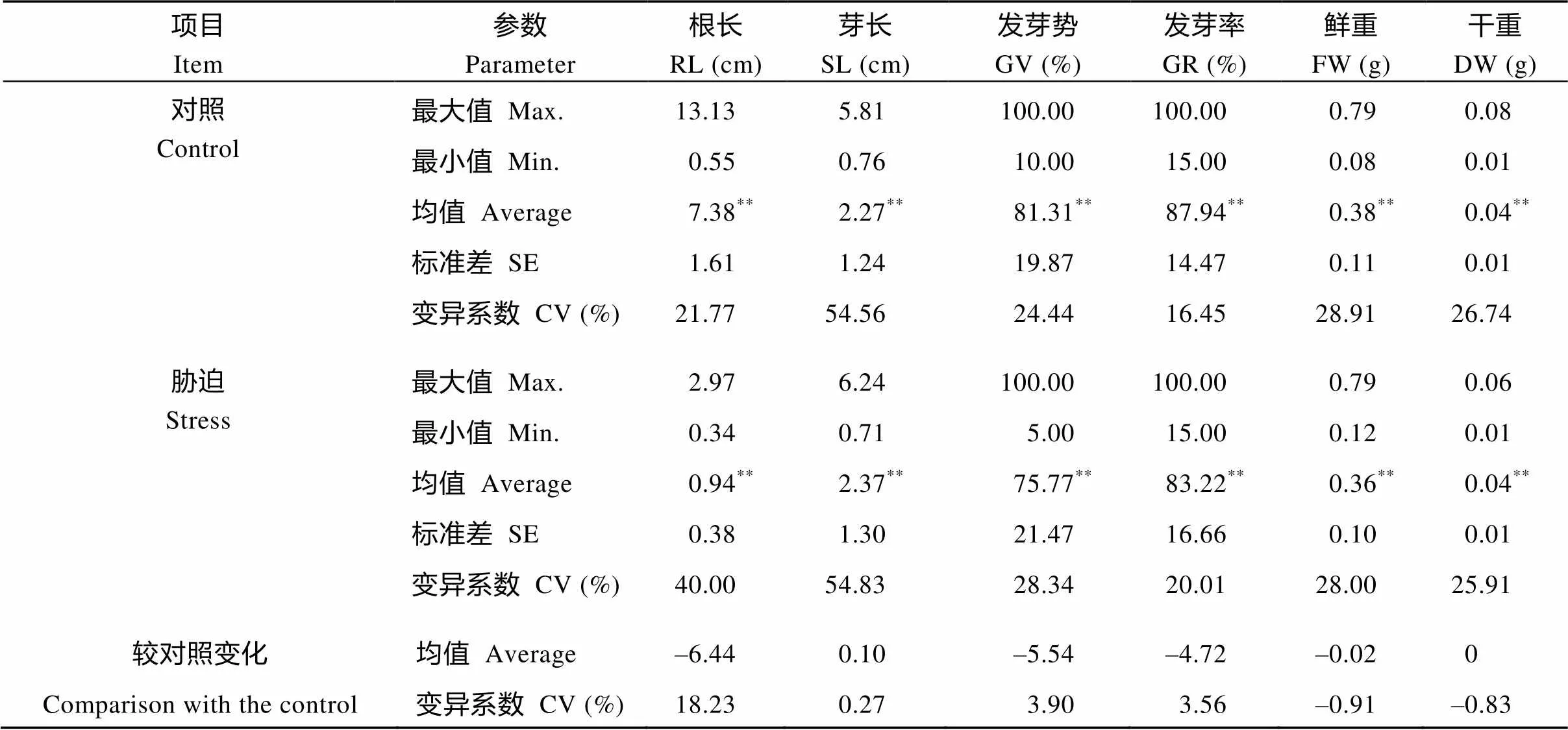
表2 苯磺隆胁迫下油菜萌发期各指标的变化
**表示在0.01水平上差异显著。
**Significant difference at the 0.01 probability level. RL: root length; SL: shoot length; GV: germination vigor; GR: germination rate; FW: fresh weight; DW: dry weight.

表3 供试油菜种质各指标的苯磺隆耐性系数
**表示在0.01水平上差异显著。
**Significant difference at the 0.01 probability level. RL: root length; SL: shoot length; GV: germination vigor; GR: germination rate; FW: fresh weight; DW: dry weight.
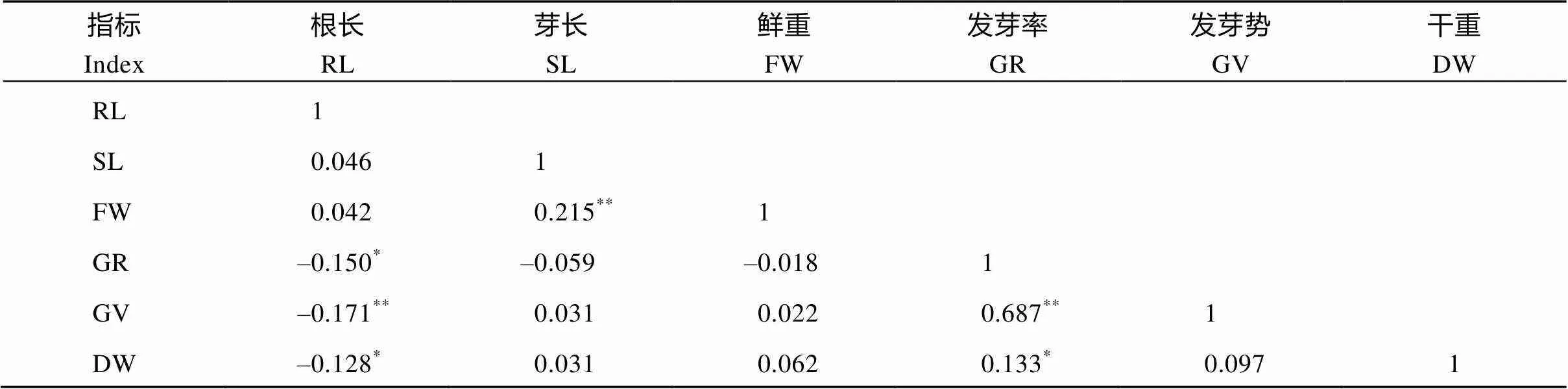
表4 供试油菜种质各指标耐性系数的相关性
*和**分别表示在0.05和0.01水平上相关显著。
*,**Significant correlations at the 0.05 and 0.01 probability levels, respectively. RL: root length; SL: shoot length; GV: germination vigor; GR: germination rate; FW: fresh weight; DW: dry weight.
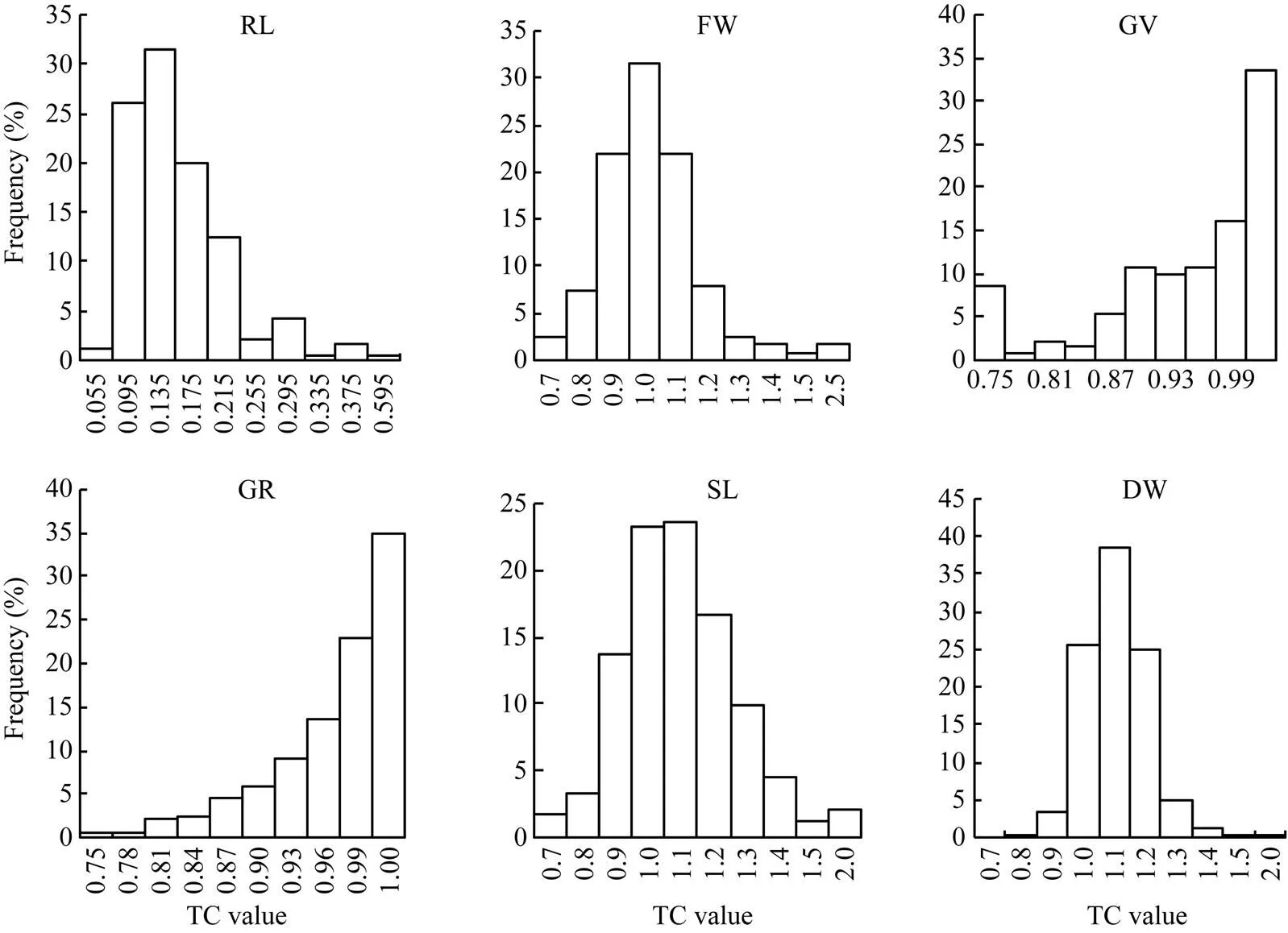
图1 241份甘蓝型油菜不同指标耐性系数(TC)的频次分布
RL: 根长; FW: 鲜重; GV: 发芽势; GR: 发芽率; SL: 芽长; DW: 干重。
RL: root length; SL: shoot length; GV: germination vigor; GR: germination rate; FW: fresh weight; DW: dry weight.
2.2.3 萌发期主成分分析 由表5可知, 第1、第2、第3和第4主成分的贡献率分别为30.17%、21.83%、18.27%和13.16%, 累计贡献率达到83.42%, 已基本代表了所测指标的信息, 可反映影响萌发期油菜耐苯磺隆性主导因素。从各个指标在综合指标的贡献率及主成分特征向量值可以看出, 第1主成分中发芽率、发芽势所占的比率较大, 第2主成分中鲜重所占的比率较大, 第3主成分中根长所占的比率较大, 第4主成分中芽长所占比率较大。
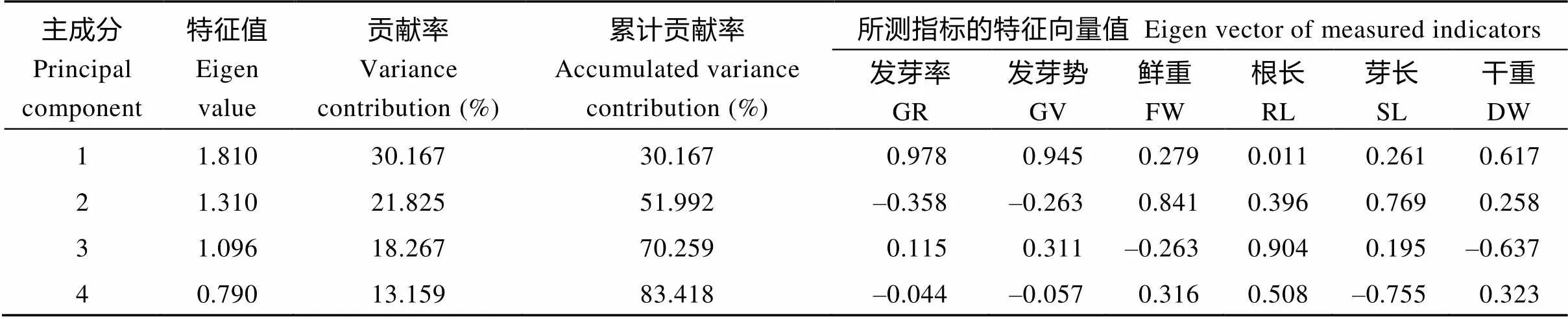
表5 各综合指标的特征值、贡献率和主成分特征向量值
GR: germination rate; GV: germination vigor; FW: fresh weight; RL: root length; SL: shoot length; DW: dry weight.
2.2.4 甘蓝型油菜品种资源耐苯磺隆性综合评价
供试种质平均隶属函数值(ASF值)介于0.155~ 0.687之间, 平均值均为0.485, 变异系数为15.3%, 根据ASF值大小对供试种质进行耐性排序, 其结果见表6。其中, 对苯磺隆耐性强的品种(系)有SWU95、SWU113、希望106和WH-33, 耐性弱的品种(系)有10-804、中双7号、中油589和10-1043, 其余种质介于两者之间。供试种质加权关联系数(WTC值)介于0.360~0.615之间, 平均值均为0.508, 变异系数为8.14%。根据WTC值大小对供试种质进行耐性排序, 对苯磺隆耐性强的品种(系)有希望106、WH-33、SWU113和SWU95, 耐性弱的品种(系)有中双7号、中油589和10-1043, 其余种质介于两者之间(表6)。供试种质苯磺隆耐性度量值(T值)介于–2.58~ +2.22之间, 根据T值大小对供试种质进行耐性排序, 对苯磺隆耐性强的品种(系)有希望106、SWU95和WH-33, 耐性弱的品种(系)有中油589和10-1043, 其余种质介于两者之间(表6)。三者之间的鉴定结果有所区别, 但供试种质耐性较强较弱的鉴定结果基本一致。为方便比较, 将ASF值、WTC值和T值的排序数值相加, 再根据累加值从小到大进行排序, 从241份种质资源的综合排序看, 其中排名前五的依次为希望106、SWU95、WH-33、SWU113和97097。从4种排序方法看, 希望106、SWU95、WH-33均表现为对苯磺隆耐性强。
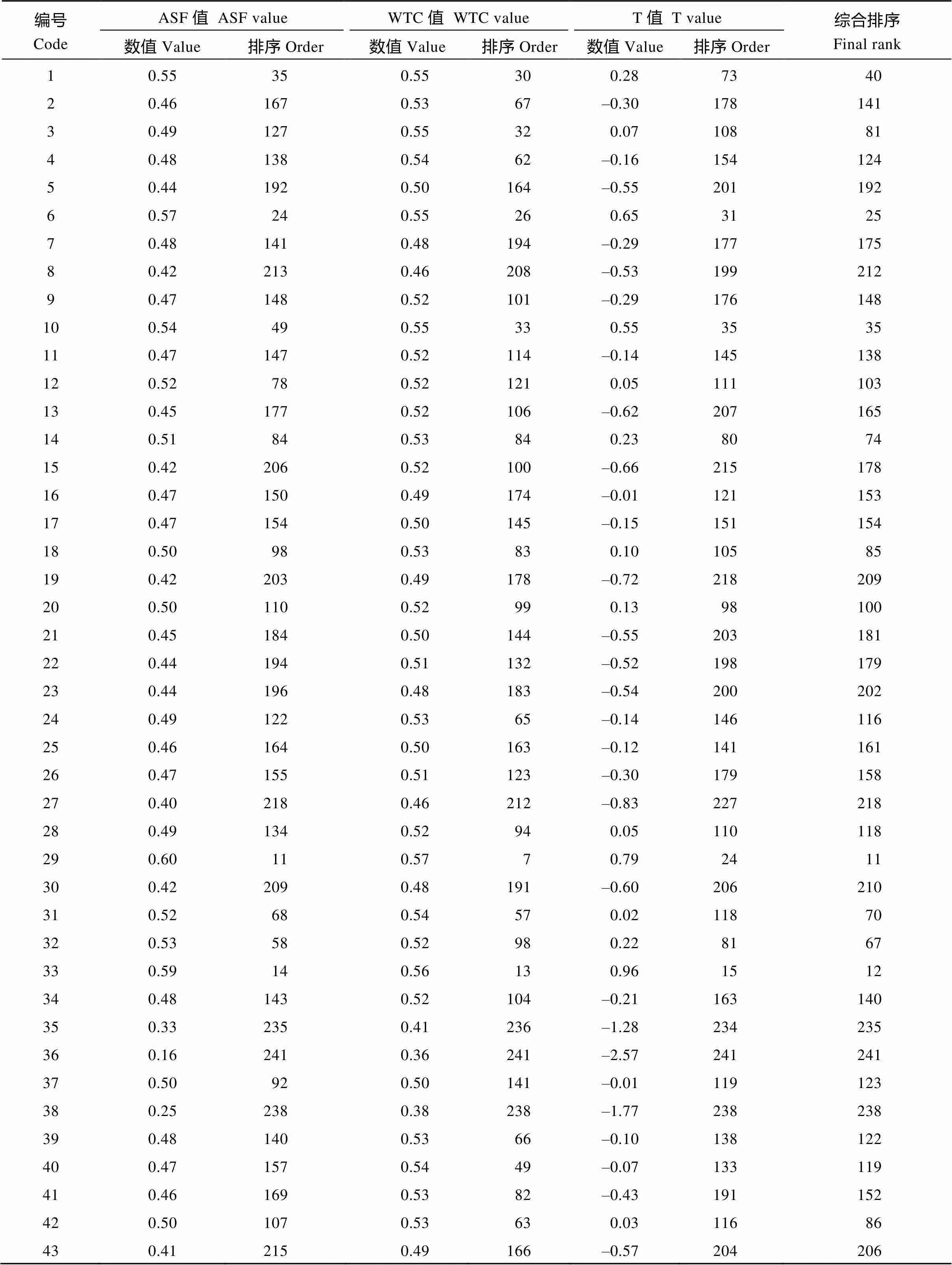
表6 苯磺隆胁迫下油菜品种资源的ASF值、WTC值、T值和综合排序
(续表6)编号CodeASF值 ASF valueWTC值 WTC valueT值 T value综合排序Final rank数值Value排序Order数值Value排序Order数值Value排序Order 440.461620.50147–0.13143156 450.441970.49175–0.39186194 460.491200.5447–0.0212287 470.392230.45223–0.74219223 480.51890.54400.356454 490.54430.53810.356352 500.53520.55250.218246 510.422040.47206–0.36185208 520.6450.5791.1365 530.501060.50156–0.07135134 540.501080.511370.366099 550.441870.48186–0.31181191 560.461630.49167–0.17157164 570.451820.47197–0.24167189 580.501030.5458–0.15152106 590.461680.48185–0.31180183 600.54500.55270.464637 610.471600.5375–0.33182144 620.51830.53860.336766 630.322360.42235–1.41236236 640.53600.54550.208658 650.54460.54520.533939 660.55340.56140.533826 670.491240.5389–0.27172131 680.501040.5373–0.11140107 690.451750.52105–0.39188162 700.52710.521020.188878 710.392220.44224–0.81225225 720.57210.54421.001220 730.292370.41237–1.47237237 740.53510.54380.139953 750.451740.5461–0.26169136 760.52690.501420.04114112 770.51850.511330.12100109 780.451730.49181–0.17155172 790.52720.521180.169284 800.402190.45222–0.77223221 810.372280.42234–0.75221229 820.54420.511240.1110182 830.422110.47198–0.64212213 840.471520.501520.02117147 850.491330.50158–0.08136150 860.451790.45215–0.10137182 870.212390.38239–1.99239239 880.56310.54590.444841 890.53630.52960.524057 900.451720.49169–0.15149166 910.432020.46209–0.22164201 920.501010.53740.326973 930.51900.511390.356189 940.52770.56220.425545 950.471560.49165–0.13144160 960.55330.56160.434931 970.52750.53700.0411577 980.53540.53760.543747 990.501020.54450.159671 1000.382260.45219–0.92231227 1010.451710.50161–0.43192180 1020.57250.53720.901734 1030.451760.51131–0.35183167 1040.53620.501430.159595 1050.422120.47205–0.68216216 1060.55360.521100.762648 1070.59130.56150.971413 1080.50930.5379–0.15150111 1090.54440.54530.435044 1100.51880.511360.2083101 1110.481370.50148–0.04127142 1120.491190.5460–0.04129102 1130.52790.501590.375991 1140.52800.53640.208464 1150.491250.52970.277592 1160.491310.51127–0.19162146 1170.52740.53850.405761 1180.471510.5292–0.16153133 1190.491210.52117–0.01120127 1200.451800.49180–0.51197193 1210.50990.52950.0810796 1220.422070.47201–0.65213214 1220.422070.47201–0.65213214 1230.382250.43227–0.62208219 1240.471590.50155–0.40189171 1250.441900.46207–0.19161195 1260.53650.511400.425475 1270.441890.48190–0.63209207 1280.51870.52109–0.05131114 1290.392210.44225–0.77224224 1300.422080.51135–0.44193185 1310.422100.49176–0.17156188 1320.402170.46210–0.76222217 1330.6170.56210.971310 1340.54470.54510.514242 1350.432000.48195–0.36184203 1360.412140.45220–0.90230222 1370.422050.48192–0.71217211 1380.501110.53880.1010297 1390.50940.5456–0.1113988 1400.51820.55340.179159 1410.491260.521030.1397113 1420.58190.55240.722717 1430.491230.51128–0.04128129 1440.501090.5387–0.04125110 1450.471460.52112–0.17159145 1460.55390.57100.802218 1470.441980.49182–0.55202204 1480.55370.56170.524130 1490.392200.44226–0.81226226 1500.491290.52116–0.25168143 1510.50960.501530.08106125 1520.491320.50162–0.07134151 1530.501130.521200.07109120 1540.491300.50154–0.24166155 1550.432010.45217–0.65214215 1560.491280.521190.1790117 1570.59160.5760.663015 1580.57200.56230.603322 1590.56260.56200.405832 1600.50950.54390.1010368 1610.451830.48184–0.45194198 1620.6360.56111.2556 1630.441950.50149–0.46195187 1640.56280.55290.891821 1650.491170.462110.7128126 1660.352320.42230–1.29235233 1670.461700.51130–0.49196170 1680.491350.5369–0.04126115 1690.59120.5780.83209 1700.53560.54500.307049 1710.501140.501500.2379121 1720.471580.5377–0.26170137 1730.52730.491730.3565104 1740.53640.54430.307150 1750.51810.511290.435179 1760.58180.5750.543616 1770.53550.55360.159451 1780.491150.52930.10104105 1790.441910.52108–0.64211173 1800.6810.5842.1622 1810.52660.521070.415665 1820.57230.55310.792323 1830.52700.511250.326880 1840.441930.47200–0.12142184 1850.6820.6021.1274 1860.53530.511260.931655 1870.481420.49171–0.26171163 1880.54450.491790.861972 1890.481390.48187–0.05130157 1900.372290.45221–1.19233230 1910.172400.37240–2.14240240 1920.53610.521150.464563 1930.382240.43229–0.63210220 1940.412160.45218–0.14148205 1950.481450.53780.287493 1960.441860.47202–0.28173199 1970.51860.481880.5143108 1980.491180.501570.04112132 1990.6730.6212.2111 2000.461650.46213–0.28175190 2010.50970.52910.188983 2020.451780.48193–0.39187196 2030.6090.56181.03108 2040.6080.53681.34424 2050.461610.49177–0.28174176 2060.362300.42233–0.89229232 2070.59150.56121.0497 2080.56300.55370.822127 2090.451850.45216–0.23165200 2100.491160.50146–0.14147139 2110.59170.56191.011114 2120.471490.47203–0.17158174 2130.471530.46214–0.02123168 2140.56290.55280.563428 2150.6740.6031.8533 2160.431990.47199–0.19160197 2170.362310.43228–0.75220228 2180.52670.52900.208569 2190.501050.511220.297294 2200.451810.491680.2676149 2210.54480.54440.435243 2220.501120.491720.2478128 2230.57220.54460.792529 2240.56270.54480.672933 2250.50910.511380.356690 2260.481360.501510.04113135 2270.55400.521110.356260 2280.481440.48189–0.03124159 2290.60100.54541.07819 2300.52760.511340.169398 2310.461660.48196–0.06132169 2320.441880.50160–0.41190186 2330.501000.472040.2577130 2340.53570.491700.633276 2350.372270.42231–0.87228231 2360.55380.55350.484436 2370.55320.521130.425356 2380.54410.54410.444738 2390.53590.53710.198762 2400.332340.5380–0.57205177 2410.342330.42232–1.18232234
2.2.5 灰色关联度分析 各指标TC值与T值间的关联度大小依次为根长、鲜重、发芽率、发芽势、干重和芽长, 反映了各指标TC值与T值的密切程度, 这与各指标对苯磺隆胁迫反应的敏感性基本吻合。各指标TC值与WTC值间的关联度大小依次为根长、鲜重、芽长、发芽势、发芽率和干重(表7)。

表7 供试油菜种质各指标TC值与T值和WTC值的关联度及各指标权重
GR: germination rate; GV: germination vigor; FW: fresh weight; DW: dry weight; RL: root length; SL: shoot length.
2.2.6 聚类分析及耐性级别的划分 基于T值, 在欧氏距离为0.75处将241份供试种质分为5类(附图1)。其中第I类为高度耐苯磺隆的品种(系), 有SWU95、希望106和WH-33共3份; 第II类为耐性品种(系), 共30份, 占总数的12.4%; 第III类为耐性一般品种(系), 共198份, 占总数的82.2%; 第IV类为不耐苯磺隆的品种(系)共6份; 第V类为高度不耐苯磺隆的品种(系), 有10-1043、10-804、中双7号和中油589共4份。根据供试材料对苯磺隆的耐性聚类分析及耐性级别划分结果, 对其耐性评价指标分级统计表明, 除芽长外, 其余单项指标的隶属函数值、ASF值、WTC值和T值均随对苯磺隆耐性级别的升高而增大(表8)。
2.2.7 回归方程 分别以ASF值、WTC值和T值为参考序列, 对供试种质各指标TC值进行逐步回归分析, 得到的3个回归方程的相关系数≈ 1,检验均达极显著水平(表9), 说明回归方程最优, 模型拟合度好, 用这3个方程进行甘蓝型油菜种质资源萌发期对苯磺隆耐性评价的效果较好。根据T值与各指标TC值的回归方程可知, 在甘蓝型油菜种质资源萌发期耐性鉴定中, 有选择性地测定与T值密切相关的指标, 如根长、鲜重、发芽率可有效鉴定油菜种质资源对苯磺隆的耐性。

表8 供试油菜种质苯磺隆耐性评价指标的分级
I、II、III、IV、V表示不同耐性级别。
I, II, III, IV, and V represent different tribenuron-methyl tolerance levels. GR: germination rate; GV: germination vigor; FW: fresh weight; DW: dry weight; RL: root length; SL: shoot length.

表9 供试油菜种质耐性模型预测
1、2、3、4、5、6分别为发芽率、发芽势、鲜重、干重、根长、芽长的TC值。
1,2,3,4,5, and6are TC values of germination rate, germination vigor, fresh weigh, dry weight, root length, and shoot length respectively.
3 讨论
种子萌发是植物生长发育的前提, 是对环境因素最为敏感的阶段。苯磺隆作为一种化学除草剂, 在杀死杂草的同时, 对作物也是一种非生物逆境胁迫。随着化学除草剂在农业生产中的广泛应用, 杂草对化学除草剂的耐性增强, 化学除草剂的使用量大幅度增加, 非合理使用导致化学除草剂在土壤中的残留越来越多, 对种子的萌发影响会越来越大。苯磺隆在现阶段油菜生产中使用量较少, 但播种前结合其他化学除草剂防除田间杂草时, 苯磺隆残留就可能影响油菜种子萌发, 目前未见关于油菜种子萌发过程中受苯磺隆影响的公开报道。本研究认为萌发期最佳苯磺隆胁迫浓度为25 mg kg–1, 为生产上除草剂浓度的1/10, 这一结果与前人[14]筛选浓度不同, 可能与研究对象、处理方式以及测定指标的不同有关。
不同作物或同一作物的不同品种对除草剂敏感性不同[14,24,41-46], 植物存在天然耐性基因, 通过除草剂的选择, 可筛选出耐除草剂作物[47]。本研究中25 mg kg–1苯磺隆胁迫条件下, 所有供试油菜种质指标均受到不同程度的影响, 且其影响差异均具统计学意义。牛志峰等[42]研究表明不同小麦品种种芽对苯磺隆的耐药性明显较种根强, 娄国强等[43]研究发现苯磺隆对不同小麦品种安全性存在较大差异, 且对根长的抑制作用明显大于株高。本研究单项耐性系数表明, 根长受抑制最严重, 这与前人研究结果基本一致[29,42,48]。另外, 频次分析表明, 发芽势、发芽率指标在0.90 近年来, 随着作物耐逆性研究的发展, 采用多种综合评价, 已在燕麦[34]、花生[39]、薏苡[40]等作物耐逆性鉴定中得到初步应用, 可以避免单一指标的片面性和不稳定性, 而目前作物耐除草剂研究中评价方法较单一。本研究采用ASF值、T值和WTC值等综合评价其对苯磺隆耐性, 确定T值为主要综合评价指标, 以ASF值和WTC值作为辅助综合评价指标, 进行较确切的聚类分析和耐药型划分, 并结合灰色关联分析、逐步回归分析筛选关键性状指标, 建立了拟合度较好的回归方程。采用ASF值、T值和WTC值对供试油菜种质综合排序, 可消除因各指标单位不同带来的影响。据此综合评价, 选出耐性强的品种(系) SWU95、希望106和WH-33, 耐性弱的品种(系) 10-1043、10-804、中双7号和中油589。种质资源的鉴定结果为油菜其他生育时期耐苯磺隆种质资源筛选和鉴定奠定了基础, 也为油菜耐苯磺隆遗传和育种研究提供了理论依据。 采用苯磺隆耐性度量值(T值)、平均隶属函数值(ASF值)和加权耐性系数(WTC值)为综合评价指标, 结合相关分析、频数分析、主成分分析、灰色关联度分析、聚类分析和逐步回归分析等方法, 对241份甘蓝型油菜品种的萌发期耐性鉴定发现, 根长、鲜重、发芽率可作为油菜品种的萌发期耐苯磺隆能力和品种选育时优先考虑指标。根据T值进行聚类分析, 可将供试苯磺隆种质划分为5个耐性级别, 其中I级3份、II级30份、III级198份、IV级6份、V级4份。希望106、SWU95和WH-33为苯磺隆耐性较强的种质, 可作为甘蓝型油菜的苯磺隆耐性育种和耐性机理研究的重要资源。 附图和附表 请见网络版: 1) 本刊网站http://zwxb. chinacrops.org/; 2) 中国知网http://www.cnki.net/; 3) 万方数据http://c.wanfangdata.com.cn/Periodical-zuowxb. aspx。 [1] 张朝贤, 胡祥恩, 钱益新. 国外除草剂应用趋势及我国杂草科学研究现状和发展方向. 植物保护学报, 1997, 24: 278–282 Zhang C X, Hu X E, Qian Y X. Trend of herbicides use in developed countries and current research and future directions in weed science research in China., 1997, 24: 278–282 (in Chinese with English abstract) [2] 张朝贤, 倪汉文, 魏守辉, 黄红娟, 刘延, 崔海兰, 隋标峰, 张猛, 郭峰. 杂草抗药性研究进展. 中国农业科学, 2009, 42: 1274–1289 Zhang C X, Ni H W, Wei S H, Huang H J, Liu Y, Cui H L, Sui B F, Zhang M, Guo F. Current advances in research on herbicide resistance., 2009, 42: 1274–1289 (in Chinese with English abstract) [3] 吴春华, 陈欣. 农药对农区生物多样性的影响. 应用生态学报, 2004, 15: 341–344 Wu C H, Chen X. Impact of pesticides on biodiversity in agricultural areas., 2004, 15: 341–344 (in Chinese with English abstract) [4] 单正军, 陈祖义. 除草剂对非靶植物(农作物)的危害影响及控制技术. 农药科学与管理, 2007, 28(9): 50–54 Shan Z J, Chen Z Y. Harm and control technology of herbicides to non target plants (crops)., 2007, 28(9): 50–54 (in Chinese with English abstract) [5] Brighenti A M, Moraes V J, Oliveira Jr R S, Gazziero D L P, Voll E, Gomes J A. Persistência e fitotoxicidade do herbicida atrazine aplicado na cultura do milho sobre a cultura do girassol em sucessão., 2002, 20: 291–297 [6] 林长福. 玉米田化学除草现状及发展趋势. 农药, 1999, (9): 3–4 Lin C F. Present situation and development trend of chemical weed control in maize field., 1999, (9): 3–4 (in Chinese with English abstract) [7] 王汉中. 我国油菜产需形势分析及产业发展对策. 中国油料作物学报, 2007, 29: 101–105Wang H Z. Strategy for rapeseed industry development based on the analysis of rapeseed production and demand in China., 2007, 29: 101–105 (in Chinese with English abstract) [8] Zhou W J, Yoneyama K, Takeuchi Y, Iso S, Rungmekarat S, Chae S H, Sato D, Joel D M. In vitro infection of host roots by differentiated calli of the parasitic plant orobanche., 2004, 55: 899–907 [9] Song W J, Zhou W J, Jin Z L, Gao D D, Joel D M, Takeuchi Y, Yoneyama K. Germination response of Orobanche seeds subjected to conditioning temperature, water potential and growth regulator treatments., 2005, 45: 467–476 [10] 俞琦英, 周伟军. 油菜田的杂草发生特点及其防治研究概况. 浙江农业科学, 2010, (1): 123–127 Yu Q Y, Zhou W J. Study on weed occurrence characteristics and control in rape field., 2010, (1): 123–127 (in Chinese) [11] 张宏军, 贾富勤, 张佳, 李晓晶. 杂草对灭生性除草剂百草枯的抗性问题. 农药科学管理, 2003, 24(12): 26–29 Zhang H J, Jia F L, Zhang J, Li X J. The resistant weeds of no selective herbicide-paraquat., 2003, 24(12): 26–29 (in Chinese with English abstract) [12] 张宏军, 刘学, 张佳, 崔海兰, 张朝贤, 朱文达. 我国油菜田除草剂登记和使用情况. 科技创新导报, 2008, (15): 252–253 Zhang H J, Liu X, Zhang J, Cui H L, Zhang C X, Zhu W D. Herbicide registration and usage in rape fields in China., 2008, (15): 252–253 (in Chinese) [13] 孙妍妍, 曲高平, 黄谦心, 吕金洋, 郭媛, 胡胜武. 甘蓝型油菜耐苯磺隆突变体基因分析与SNP标记. 中国油料作物学报, 2015, 37: 589–595 Sun Y Y, Qu G P, Huang Q X, Lyu J Y, Guo Y, Hu S W. SNP markers for acetolactate synthase genes from tribenuron-methyl resistant mutants inL., 2015, 37: 589–595 (in Chinese with English abstract) [14] 信晓阳, 曲高平, 张荣, 庞红喜, 吴强, 王发禄, 胡胜武. 不同品种油菜对苯磺隆耐药性差异的鉴定. 西北农业学报, 2014, 23(7): 68–74 Xin X Y, Qu G P, Zhang R, Pang H X, Wu Q, Wang F L, Hu S W. Identification of the tribenuron-methyl tolerance in different rapeseed genotypes., 2014, 23(7): 68–74 (in Chinese with English abstract) [15] Yu C, Hu S, He P, Sun G, Zhang C, Yu Y. Inducing male sterility inL. by a sulphonylurea herbicide, tribenuron-methyl., 2006, 125: 61–64 [16] Li H, Li J, Zhao B, Wang J, Yi L, Liu C, Wu J, King G J, Liu K. Generation and characterization of tribenuron-methyl herbicide-resistant rapeseed () for hybrid seed production using chemically induced male sterility., 2015, 128: 107–118 [17] 吴学莉, 易丽聪, 侯凡, 吴江生, 姚璇, 刘克德. 表达播娘蒿突变基因DsALS–108的抗苯磺隆甘蓝型油菜植株构建. 农业生物技术学报, 2016, 24: 469–477 Wu X L, Yi L C, Hou F, Wu J S, Yao X, Liu K D. Generation of tribenuron-methyl herbicide resistant rapeseed () plants expressing mutated gene DsALS-108 of flixweed ()., 2016, 24: 469–477 (in Chinese with English abstract) [18] 曲高平, 孙妍妍, 庞红喜, 吴强, 王发禄, 胡胜武. 甘蓝型油菜EMS突变体库构建及抗除草剂突变体筛选. 中国油料作物学报, 2014, 36: 25–31 Qu G P, Sun Y Y, Pang H X, Wu Q, Wang F L, Hu S W. Ems mutagenesis and als-inhibitor herbicide-resistant mutants ofL., 2014, 36: 25–31 (in Chinese with English abstract) [19] 汪亚琴. 水稻抗除草剂基因CYP81A6转化油菜的研究. 华中农业大学硕士学位论文, 湖北武汉, 2013 Wang Y Q. The Expression of Rice Herbicide Resistance Gene CYP81A6 inMS Thesis of Huazhong Agricultural University, Wuhan, Hubei, China, 2013 (in Chinese with English abstract) [20] 邱芳心, 杜桂萍, 刘开林, 毛爱星, 罗坤. 杂草抗药性及其治理策略研究进展. 杂草科学, 2015, (2): 1–6 Qiu F X, Du G P, Liu K L, Mao A X, Luo K. Research progress on weed resistance to herbicides and control methods., 2015, (2): 1–6 (in Chinese with English abstract) [21] 信晓阳. 油菜苯磺隆抗性研究与转基因抗除草剂油菜的选育. 西北农林科技大学硕士学位论文, 陕西西安, 2013 Xin X Y. Charaterization of Resistance to Tribenuron-Methyl in Rapeseed (L.) and Development of Transgenic Rapeseed with Herbericide–Resistance. MS Thesis of Northwest A&F University, Xi’an, Shaanxi, China, 2013 (in Chinese with English abstract) [22] Koger C H, Poston D H, Hayes R M, Montgomery R F. Glyphosate-resistant horseweed () in Mississippi., 2004, 189: 820–825 [23] Kuk Y I, Kim K H, Kwon O D, Lee D J, Burgos N R, Jung S, Guh J O. Cross-resistance pattern and alternative herbicides for Cyperus difformis resistant to sulfonylurea herbicides in Korea., 2004, 60: 85–94 [24] 刘伟, 王金信, 杨广玲, 毕建杰, 隋标峰. 不同小麦品种对苯磺隆耐药性差异及其机理. 植物保护学报, 2005, 32: 300–304 Liu W, Wang J X, Yang G L, Bi J J, Sui B F. Different of tolerance and mechanism of various wheat varieties to tribenuron-methyl., 2005, 32: 300–304 (in Chinese with English abstract) [25] Heap I M. International survey of herbicide-resistant weeds., 1990, 4(1): 220 [26] Deng W, Yang Q, Jiao H T, Zhang Y Z, Li X F, Zhang M Q. Cross-resistance pattern to four AHAS-inhibiting herbicides of tribenuron-methyl-resistant flixweed () conferred by Asp-376-Glu mutation in AHAS., 2016, 15: 2563–2570 [27] Sun J, Wang J X, Zhang H J, Liu J L, Bian S N. Study on mutations in ALS for resistance to tribenuron-methyl inL., 2011, 10: 86–91 [28] Cumminsa I, Wortleyb David J, Sabbadin F, Heb Z, Coxona C R, Strakera H E, Sellarsa J D, Knighta K, Edwardsc L, Hughesd D, Kaundund S S, Hutchingsd S J, Steela P G, Edwardsb R. Key role for a glutathione transferase in multiple–herbicide resistance in grass weeds., 2013, 110: 5812–5817 [29] 商璐. 抗草甘膦大豆种质挖掘及抗性机制研究. 东北农业大学硕士学位论文, 黑龙江哈尔滨, 2016 Shang L. Study on Screening and Resistant Mechanism of Germplasm Resources to Glyphosate–Resistance in Soybean. MS Thesis of Northeast Agricultural University, Harbin, Heilongjiang, China, 2016 (in Chinese with English abstract) [30] Kotoula-Syka E, Eleftherohorinos I G, Gagianas A A, Sficas A G. Phytotoxicity and persistence of chlorsulfuron, metsulfuron- methyl, triasulfuron and tribenuron-methyl in three soils., 2010, 33: 355–367 [31] 邹月利, 陶波. 磺酰脲类除草剂的降解机制及代谢产物的研究进展. 农药科学与管理, 2011, 32(10): 24–31Zou Y L, Tao B. Research advance on the degradation mechanism and degradation products of sulfonylurea herbicides., 2011, 32(10): 24–31 (in Chinese with English abstract) [32] 王正贵. 除草剂对小麦产量和品质的影响及其残留特性. 扬州大学博士学位论文, 江苏扬州, 2011 Wang Z G. Effects of Herbicides on Grain Yield and Quality in Wheat and Relevant Residual Behavior. PhD Dissertation of Yangzhou University, Yangzhou, Jiangsu, China, 2011 (in Chinese with English abstract) [33] 江改青. 小麦和土壤中苯磺隆与氯氟吡氧乙酸残留分析方法及消解动态研究. 安徽农业大学硕士学位论文, 安徽合肥, 2009 Jiang G Q. Study on Analytical Mechods and Dynamics of Tribenuron-methyI and Fluroxypyr Residues in Wheat and Soil. MS Thesis of Anhui Agricultural University, Hefei, Hanhui, China, 2009 (in Chinese with English abstract) [34] 陈新, 张宗文, 吴斌. 裸燕麦萌发期耐盐性综合评价与耐盐种质筛选. 中国农业科学, 2014, 47: 2038–2046 Chen X, Zhang Z W, Wu B. Comprehensive evaluation of salt tolerance and screening for salt tolerant accessions of naked oat (L.) at germination stage., 2014, 47: 2038–2046 (in Chinese with English abstract) [35] 汪梦竹, 慕小倩, 李玉菲, 崔宏安, 郭媛, 胡胜武. 油菜和小麦种苗根系对乙草胺的耐性差异分析. 植物保护学报, 2017, 44: 337–342 Wang M Z, Mu X Q, Li Y F, Cui H A, Guo Y, Hu S W. Analysis of acetochlor tolerance in root ofL. andL., 2017, 44: 337–342 (in Chinese with English abstract) [36] 刘安芳, 伍莲. 生物统计学. 重庆: 西南师范大学出版社, 2013. pp 279–285 Liu A F, Wu L. Biostatistics. Chongqing: Southwest China Normal University Press, 2013. pp 279–285 (in Chinese) [37] 唐启义, 冯明光. DPS数据处理系统: 实验设计统计分析及数据挖掘. 北京: 科学出版社, 2007. pp 636–644, 682–690, 1027–1036 Tang Q Y, Feng M G. DPS Data Processing System: Statistical Analysis and Data Mining of Experimental Design. Beijing: Science Press, 2007. pp 636–644, 682–690, 1027–1036 (in Chinese) [38] 闫锋, 崔秀辉, 李清泉, 王成, 曾玲玲, 刘峰, 马波, 袁明. 绿豆品种的灰色关联度分析及综合评价. 中国种业, 2011, (增刊2): 31–33 Yan F, Cui X H, Li Q Q, Wang C, Zeng L L, Liu F, Ma B, Yuan M. Grey relational grade analysis and comprehensive evaluation of mungbean (L.) germplasm resources., 2011, (suppl-2): 31–33 (in Chinese with English abstract) [39] Upadhyaya Hari D. Variability for drought resistance related traits in the mini core collection of peanut., 2005, 45: 1432–1440 [40] 汪灿, 周棱波, 张国兵, 张立异, 徐燕, 高旭, 姜讷, 邵明波. 薏苡种质资源萌发期抗旱性鉴定及抗旱指标筛选. 植物遗传资源学报, 2017, 18: 846–859 Wang C, Zhou L B, Zhang G B, Zhang L Y, Xu Y, Gao X, Jiang N, Shao M B. Identification and indices screening of drought resistance in Job’s tears germplasm resources at germination stage., 2017, 18: 846–859 (in Chinese with English abstract) [41] 于泉林, 武宝悦. 不同作物对苯磺隆残留敏感性室内模拟研究. 河北职业技术师范学院学报, 2003, 17(1): 16–19 Yu Q L, Wu B Y. Different crops on the sensitivity of the indoor simulation of tribenuron-methyl residues., 2003, 17(1): 16–19 (in Chinese) [42] 牛志锋, 杜慧玲. 不同小麦品种对苯磺隆除草剂的耐药性研究. 山西农业科学, 2008, 36(2): 28–29 Niu Z F, Du H L. Study on tolerance of different wheat variety to tribenuron-methyl., 2008, 36(2): 28–29 (in Chinese with English abstract) [43] 娄国强, 吕文彦, 职明星. 苯磺隆、苄嘧磺隆对不同小麦品种安全性及叶绿素含量的影响. 中国农学通报, 2005, 21(10): 317–320 Lou G Q, Lyu W Y, Zhi M X. Studies on safety tribenuron- methyl and bensulfuron-methyl and their impact to the content of chlorophyll., 2005, 21(10): 317–320 (in Chinese with English abstract) [44] 范志金, 钱传范, 于维强, 陈俊鹏, 李正名, 王玲秀. 氯磺隆和苯磺隆对玉米乙酰乳酸合成酶抑制作用的研究. 中国农业科学, 2003, 36: 173–178 Fan Z J, Qian C F, Yu W Q, Chen J P, Li Z M, Wang L X. Study on enzymatic inhibition of acetolactate synthase from maize (L.) by chlorsulfuron and tribenuron-methyl., 2003, 36: 173–178 (in Chinese with English abstract) [45] 李脉泉, 化宿南, 郭兵福, 刘明, 宋健, 陈建港, 周福来, 于莉莉, 陶波, 邱丽娟. 大豆微核心种质对草甘膦的耐受性鉴定. 植物遗传资源学报, 2015, 16: 940–946 Li M Q, Hua S N, Guo B F, Liu M, Song J, Chen J G, Zhou F L, Yu L L, Tao B, Qiu L J. Identification of glyphosate-tolerance in soybean mini-core collection., 2015, 16: 940–946 (in Chinese with English abstract) [46] 王园园. 棉花草甘膦自然抗性评价及抗性基因源挖掘研究. 中国农业科学院博士学位论文, 北京, 2015 Wang Y Y. Identification of Natural Resistance to Glyphosate in Gossypium and the Excavation of Glyphosate-resistant Gene Resources inRaces. PhD Dissertation of Chinese Academy of Agricultural Sciences, Beijing, China, 2015 (in Chinese with English abstract) [47] 苏少泉. 抗咪唑啉酮类除草剂作物的发展与未来. 现代农药, 2006, 5(1): 1–4 Su S Q. The development and future of imidazolinone herbicide-resistant crops., 2006, 5(1): 1–4 (in Chinese) [48] 王米道, 程凤侠, 司友斌. 铜与草甘膦复合污染对小麦种子发芽与根伸长的抑制作用. 生态毒理学报, 2009, 4: 591–596 Wang M D, Cheng F X, Si Y B. The inhibition of the combined pollution of copper and glyphosate to the seed germination and root elongation of wheat., 2009, 4: 591–596 (in Chinese with English abstract) [49] 杜小娟, 梁婷婷, 慕小倩. 8种常用除草剂对黄芩种子萌发及幼苗生长的影响. 西北农业学报, 2012, 21: 202–206 Du X J, Liang T T, Mu X Q. Effects of eight herbicides on seed germination and seedling growth ofGeorg., 2012, 21: 202–206 (in Chinese with English abstract) Screening and Comprehensive Evaluation of Germplasm Resources with Tribenuron-methyl Tolerance at Germination Stage in Rapeseed (L.) WANG Qian**, CUI Cui**, YE Sang, CUI Ming-Sheng, ZHAO Yu-Feng, LIN Na, TANG Zhang-Lin, LI Jia-Na, and ZHOU Qing-Yuan* College of Agronomy and Biotechnology, Southwest University, Chongqing 400715, China The purpose of this study is to explore the characteristics of tribenuron-methyl tolerance in different genotypes of, and establish the evaluation system for screening and breeding new cultivars with tribenuron-methyl tolerance. The germination vigor was measured on the third day after treatment and the germination rate, shoot length, root length, dry weight, as well as fresh weight were measured on the seventh day. The identification of tolerance at germination stage was performed by tribenuron-methyl tolerance comprehensive evaluation value (T), average subordinative function value (ASF) and weight tribenuron-methyl tolerance coefficient (WTC) in correlation analysis, frequency analysis, principal component analysis, grey analysis, cluster analysis and stepwise regression analysis. Based on the variance analysis of relative root length index in 11 tested materials, we determined the optimum treatment concentration (25 mg kg–1) for selecting and evaluating the tolerant germplasm resources to tribenuron-methyl in napeseed. Under the optimum concentration, the single factor variance analysis showed significant differences in root length, shoot length, germination rate, germination vigor and fresh weight among 241 accessions of. The clustering analysis according to T value exhibited that the tested cultivars were roughly divided into five tribenuron-methyl tolerance grades, including three in grade I, 30 in grade II, 198 in grade III, six in grade IV, and four in grade V. We conclude that the varieties (lines) with strong tribenuron-methyl tolerance at germination stage are Xiwang 106, SWU95, and WH-33, which could be used as the germplasm materials for the study on tolerance breeding and mechanism. Furthermore, the root length, fresh weight and germination rate could be used as index traits to evaluate the tolerance of rapeseed germplasm resources during germination stage. L; Tribenuron-methyl tolerance; germplasm resources; comprehensive evaluation; germination stage 2018-03-26; 2018-04-18. 10.3724/SP.J.1006.2018.01169 周清元, E-mail: zhouqy2005@163.com **同等贡献(Contributed equally to this work) 王倩, E-mail: 734747691@qq.com; 崔翠, E-mail: cuigreeny@163.com 2017-11-20; 本研究由国家现代农业产业技术体系建设专项(CARS-12), 国家科技支撑计划项目(2013BAD01B03-12), 重庆市社会事业与民生保障科技创新项目(cstc2016shmszx0756)和西南大学博士启动基金项目(swu113064)资助。 This study was supported by the China Agriculture Research System (CARS-12), the National Key Technology Support Program of China (2013BAD01B03-12), the Science and Technology Committee of Chongqing (cstc2016shmszx0756), and the Doctoral Start-up Fund of Southwestern University (swu113064). URL:http://kns.cnki.net/kcms/detail/11.1809.S.20180418.0952.002.html4 结论
