Synthesis,CrystalStructure,and Catalytic Activities of[Zn(Pybox)Br2]
2018-08-02ZHIXueTingZHUMengZengJIAWeiGuo
ZHI Xue-Ting, ZHU Meng-Zeng, JIA Wei-Guo
(College of Chemistry and Materials Science,Anhui Normal University,Wuhu 241000,China)
Abstract:A zinc complex(Dm-Pybox)ZnBr2(1)(Dm-pybox=2,6-bis[4’,4’-dimethyloxazolin-2’-yl]pyridine)has been synthesized and characterized by multinuclear NMR spectroscopy,elemental analysis and infrared spectroscopy.And the solid-state of complex 1 has been confirmed by X-ray crystallography(CCDC no.1435705).Crystal Data for C15H19N3O2ZnBr2(M=498.52g/mol):monoclinic,space group P21/c,a=10.2422(19)Å,b=16.141(3)Å,c=11.513(2)Å,β=99.128(2),V=1879.2(6)Å3,Z=4,F(000)=984,μ=5.570mm-1,ρc=1.762g/cm3,The final R1 was 0.0584(I>2σ(I))and wR2 was 0.1773(all data).And 1 is efficient catalyst for the Knoevenagel condensation reaction between ferrocenecarboxaldehyde and activated methylene compound at room temperature in water.
Key words:zinc;complex;crystal structure;catalyst
INTRODUCTION
Zinc complexes are the most important transition metal complexes catalysts because of their abundant,cheap,and non-toxic properties.The zinc complexes have been widely used in organic synthesis by chemists during the last decades[1,2].The zinc-catalyzed or zinc-mediated bond formation,particularly C-X(X=C,N,O)bonds formation have been well studied in recent years[3-10].The Knoevenagel condensation reaction using aldehydes as starting materials is one of the useful methods for C-C bond formation[11,12].The Knoevenagel condensation reaction between ferrocenecarboxaldehyde and methylene compound catalyzed by Zn(II)complexes with pincer ligand has been reported.And the zinc complexes with pincer ligands were efficient catalysts for this transformation[13].
In order to understand the chemistry of zinc complex well and the coordination anion effect on Knoevenagel condensation reaction,herein,we report the synthesis of(Dm-Pybox)ZnBr2(1)(Dm-pybox=2,6-bis[4’,4’-dimethyloxazolin-2’-yl]pyridine)and its application for Knoevenagel condensation reaction.The results indicated that the complex(Dm-Pybox)ZnBr2was more efficient than the complex(Dm-Pybox)ZnCl2[13].The difference in catalytic activity is most likely due to the bromide anion being a better leaving group compared to chloride anion.
1 EXPERIMENTAL
1.1 Materials and instruments
The manipulations were carried out under inert gas using standard Schlenk techniques.The starting materials,2,6-bis[4’,4’-dimethyloxazolin-2’-yl]pyridine(Dm-pybox)was obtained using literature method[14].Other chemicals were commercial and used as received.Multinuclear NMR spectroscopy(1H and13C NMR)were recorded on a 500 MHz NMR spectrometer at room temperature.Element analyses were tested on an Elementar III vario EI Analyzer.IR spectra were determined on a Niclolet AVATAR-360 spectrometer.
1.2 Synthesis of complex 1.
To Dm-pybox(1a)(136 mg,0.5 mmol)in dry 20 mL methanol and 15mL CH2Cl2was added ZnBr2(113mg,0.5mmol)in a 100mL round-bottomed flask at room temperature.The mixture was stirred for 24h and then the solvent was removed under vacuo.The resulting solid was washed with methanol,and then dried.The product was recrystallized from CH2Cl2/hexane to give white crystal.Yield:(217mg,87%).1H NMR(500MHz CDCl3):1.68(s,4CH3,12H),4.49(s,2CH2,4H),8.05(d,pyridine,2H),8.29(t,pyridine,1H)ppm;13C NMR(125MHz CDCl3):160.47,143.23,142.80,125.07,84.59,68.28,28.72.Anal.Calcd(%)for C15H19Br2N3O2Zn:C 36.37;N 8.49;H 3.87.Found(%):C 36.41;N 8.46;H 3.86.IR(KBr cm-1):2970(m),1660(m),1648(m),1596(s),1570(m),1450(m),1386(m),1366(m),1290(m),1223(s),1145(m),1069(m),1032(m),966(m),921(m),843(m),764(m),688(m).
1.3 General procedure for Knoevenagel condensation reaction
(Dm-Pybox)ZnBr2(1)(1.2mg,0.0025mmol,0.01 equiv)was added in H2O(1mL),then ferrocenecarboxaldehyde(53.4mg,0.25mmol,1.0 equiv)and activated methylene compound(0.5 mmol,2.0 equiv)were added.The mixture was stirred at room temperature for a few minutes,then the product was obtained after column chromatography.
2-Ferrocenylidenemalononitrile(2a)[13].(61mg,94% Yield).1H NMR(300MHz,CDCl3)δ 7.72(s,1H),4.98(s,2H),4.85(s,2H),4.33(s,5H).
3 5-Ferrocenylidene-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione(2b)[13].(80mg,91% Yield).1H NMR(300MHz,CDCl3)δ 8.45(s,1H),5.32(s,2H),4.91(s,2H),4.28(s,5H),3.39(s,3H),3.36(s,3H).
5-Ferrocenylidene-2,2-dimethyl-1,3-dioxane-4,6-dione(2c)[13].(78mg,96% Yield).1H NMR(300MHz,CDCl3)δ 8.38(s,1H),5.24(s,2H),4.91(s,2H),4.28(s,5H),1.75(s,6H).
Ethyl 2-cyano-3-ferrocenylacrylate(2d)[13].(67mg,89% Yield).1H NMR(300MHz,CDCl3)δ 8.16(s,1H),5.00(s,2H),4.70(s,2H),4.29(q,J=7.08Hz,2H),4.24(s,5H),1.35(t,J=7.05Hz,3H).
1.4 Structure determination
Diffraction data ofcomplex 1 was got on Bruker AXS SMART APEX diffractometer,using Mo Kα radiation(λ=0.71073Å)with a CCD area detector.All the data were collected at 298K and the structure was solved by direct methods,then subsequently refined on F2by using full-matrix least-squares techniques(SHELXL)[15],SADABS[16]absorption corrections were applied to the data.The calculation was performed using the Brucker Smart program.The details of the crystallographic data are shown in Table 1,and selected bond distances and angles are listed in Table 2.
The crystal data of complex 1 has been deposited with the Cambridge Crystallographic Data Centre(CCDC no.1435705).
2 RESULTS AND DISCUSSION
2.1 Synthesis of complex 1
(Dm-Pybox)ZnBr2(1)(87%)was obtained by reaction of ZnBr2with Dm-Pybox compound in CH2Cl2/MeOH at room temperature.Complex 1 was fully characterized by NMR spectroscopy,elemental analysis and infrared spectroscopy.The1H NMR spectra of 1 show singlet resonances due to methyl fragment at δ 1.65ppm,the pyridyl group appear two resonances at δ 8.04 and 8.26ppm,and the signal of the methylene in the oxazoline rings appear at δ 4.23ppm.And infrared spectrum of complex 1 in the solid state exhibits intense C=N stretching of oxazoline at about 1648cm-1.
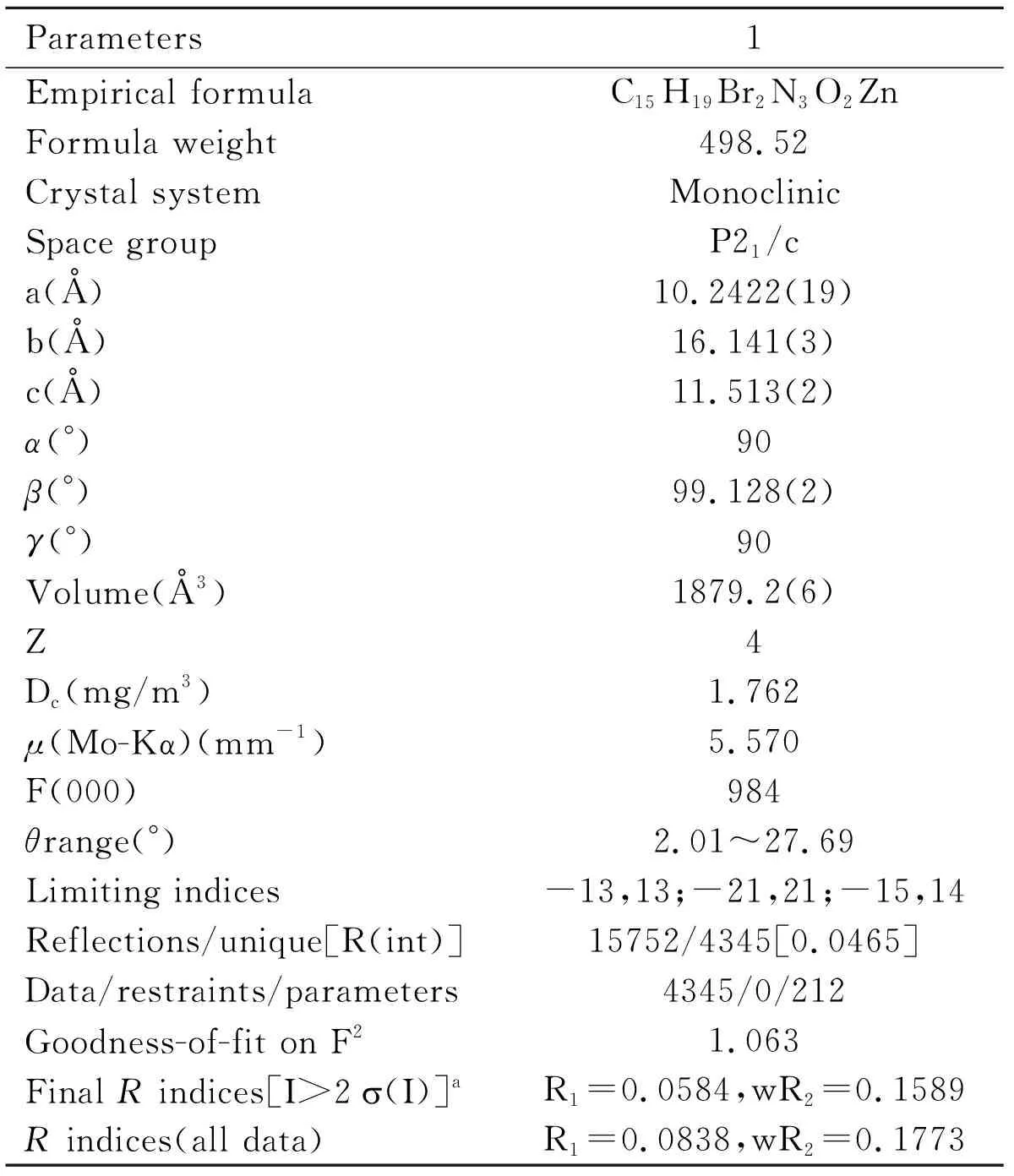
Table 1 Crystallographic data for complex 1
*R1=Σ||Fo|-|Fc||/Σ|Fo|;wR2=[Σw(|Fo2|-|Fc2|)2/Σw|Fo2|2]1/2.
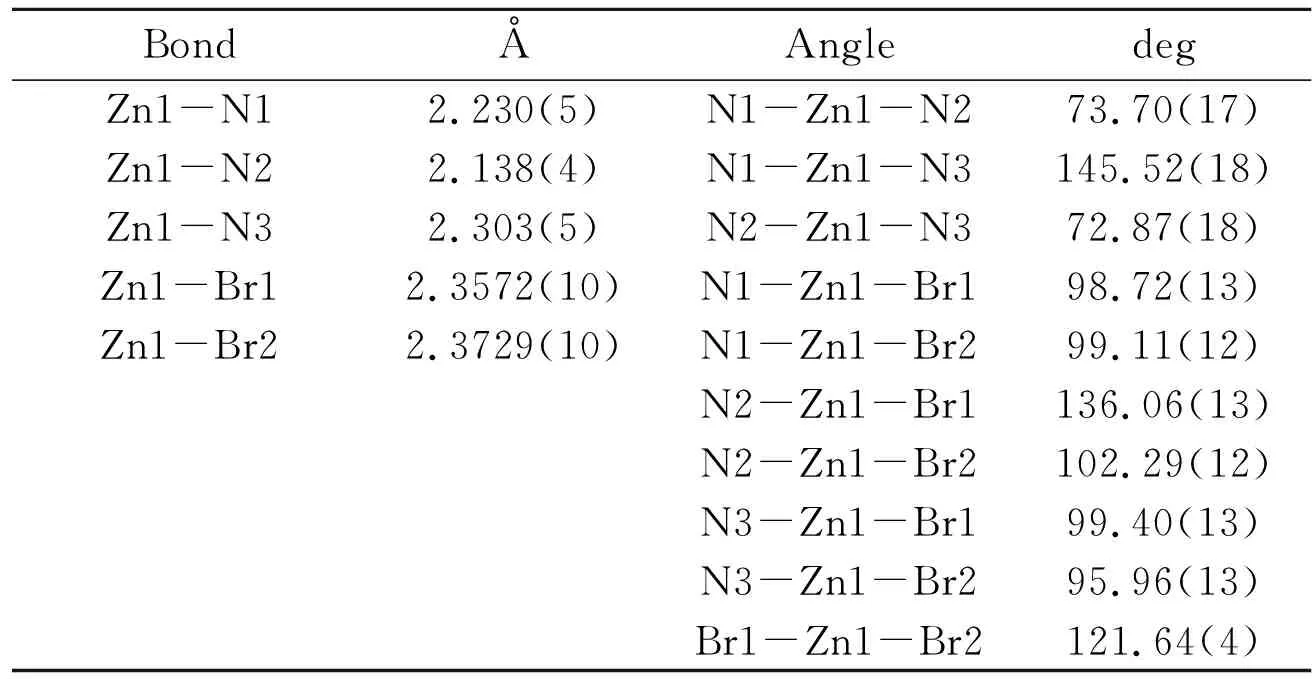
Table 2 Selected Bond Lengths(Å)and Bond Angles(°)
2.2 Crystral structure
The molecular structure ofcomplex 1 was determined by X-ray crystallographic studies.As shown in Figure 1,the pincer ligand coordinated to zinc(II)with three nitrogen atom.The coordination sphere around the Zn(II)atom is best described as midway between trigonal bipyramidal and square pyramidal in structure.The Zn-N distances(2.230(5),2.138(4)and 2.303(5)Å)in 1,which are compatible with a typical single bond length compare to the corresponding ligand precursor with ZnCl2.The Zn-Br distances(2.3572(10)and 2.3729(10)Å)in 1,which are longer than that of in(Dm-Pybox)ZnCl2[13].As seen in Figure 2,there are three kind of intermolecular hydrogen bond between the C atom of pincer ligand and bromide which play a important role for the formation of 3D structure in solid states(Table 3).
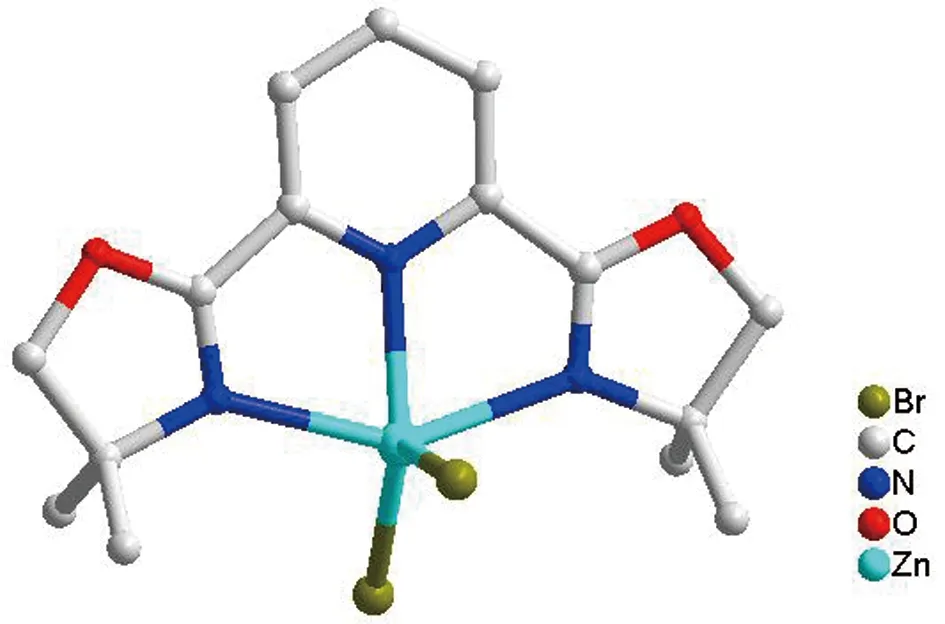
Figure 1 Molecular structure of complex 1
Figure 2 3D structure of 1 mediated by intermolecular hydrogen bonding
Table 3 Hydrogen Bond Lengths(Å)and Bond Angles()
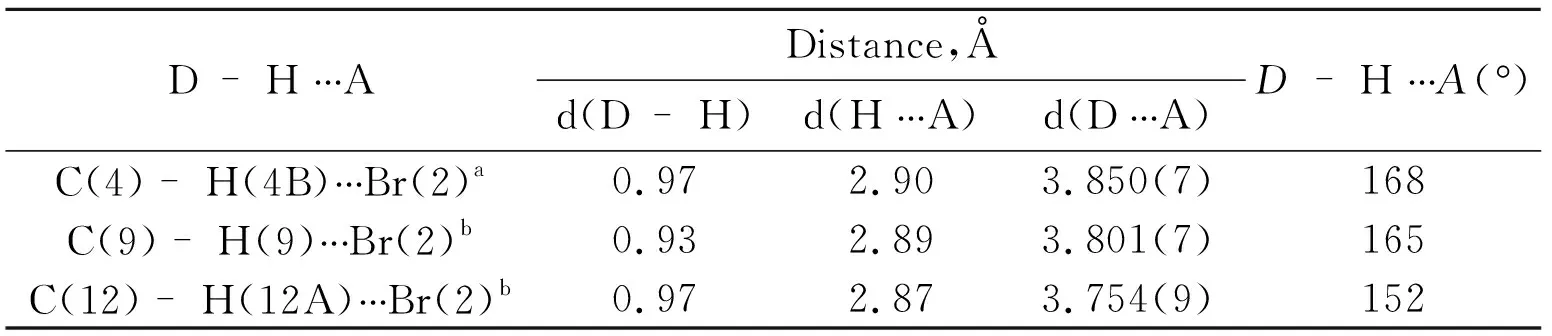
Figure 2 3D structure of 1 mediated by intermolecular hydrogen bonding
D-H〷〷〷ADistance,Åd(D-H)d(H〷〷〷A)d(D〷〷〷A)D-H〷〷〷A(‱)C(4)-H(4B)〷〷〷Br(2)a0.972.903.850(7)168C(9)-H(9)〷〷〷Br(2)b0.932.893.801(7)165C(12)-H(12A)〷〷〷Br(2)b0.972.873.754(9)152
*Symmetry codes:(a)-x,1.5+y,4.5-z;(b)x,1.5-y,2.5+z
2.3 The catalytic activities of complex 1 for Knoevenagel condensation reaction
Knoevenagel condensationreaction between ferrocenecarboxaldehyde and active methylene compounds is often used to test the catalytic activity of obtained zinc complex 1.The ferrocenecarboxaldehyde and different active methylene compounds were performed to give the desired products in high yields using 1 mol% zinc complex 1 catalyst in water at room temperature(Table 4,2a,2b,2c and 2d).The results showed that the complex(Dm-Pybox)ZnBr2was more efficient than that of the complex(Dm-Pybox)ZnCl2[13].The difference in catalytic activity is most likely due to the bromide anion being a better leaving group compared to chloride anion.Knoevenagel condensation reactions are more likely to take place when bromide anion replace with chloride anion in zinc complexes with Dm-pybox ligand.
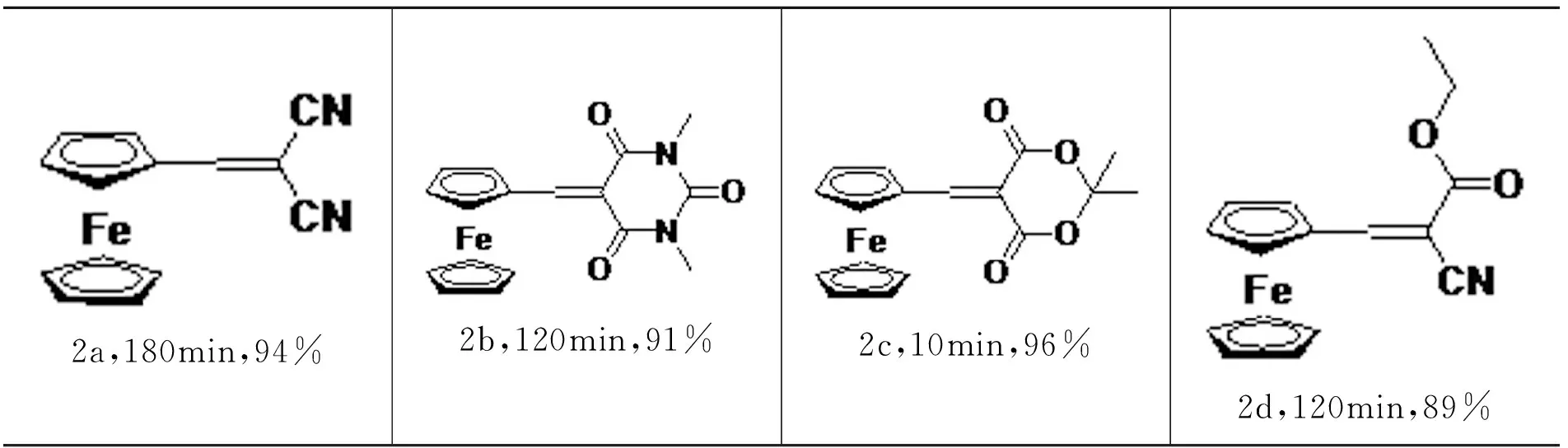
Table 4 Knoevenagel condensation reaction catalyzed by complex 1*
*Isolated yield;Reaction condition:activated methylene compound(0.5 mM,2.0 equiv);ferrocenecarboxaldehyde(0.25 mM),1 mol% Zn catalyst,and 1.0mL H2O,under air.
3 CONCLUSION
In conclusion,we have reported one novel zinc complex with pyridine-based pincer-type ligand.The crystal structure of complex 1 was confirmed through spectroscopic studies and X-ray crystallographic method.And complex 1 is efficient catalyst for the Knoevenagel condensation reaction between ferrocenecarboxaldehyde and activated methylene compounds in water.
