Catalytic effects of Cu-Co*on the thermal decomposition of AN and AN/KDN based green oxidizer and propellant samples
2018-07-06PrtimKumrPurnChndrJoshiRjivKumrShellyBisws
Prtim Kumr,Purn Chndr Joshi,Rjiv Kumr,Shelly Bisws,*
aDepartment of Space Engineering&Rocketry,Birla Institute of Technology,Ranchi,Jharkhand,India
bDepartment of Mechanical Engineering,Vidya Vihar Institute of Technology,Purnea,Bihar,India
1.Introduction
Solid rocket is the simplest type of rocket system,where fuel and oxidizer called propellant are stored within the combustion chamber itself.In recent years,the requirement of solid rocket has grown up due to the requirement in the exploration of space,advancement of space technology and for conducting space based zero gravity experiments.Mostly solid rocket are used in the booster stages,as solid propellant has the capability to produce sufficient thrust in a very short period of time[1,2].It is mainly because during the rocket take-off,the weight of rocket is maximum,and a rocket has to defy gravity for its upwards movement into the space.
All solid rocket motor employ composite propellants as a source of power for the generation of thrust.A composite propellant contains main ingredients as fuel that also acts as a binder and the oxidizers.The oxidizers are mixed uniformly in the fuel binder to get the heterogeneous composite propellants.Since,a propellant has to sustain self-combustion inside a rocket motor and hence,the presence of both oxidizer and fuel is important.These propellants are stored in the combustion chamber and upon ignition;the decomposition products of both oxidizer and fuel react together in the flame zone to produce combustion products at extremely high temperature and pressure.This high temperature and high pressure combustion products undergoes expansion process in the nozzle and finally thrust is produced[3].
Till date,ammonium perchlorate(AP)has been used as an oxidizer,hydroxyl terminated polybutadiene(HTPB)is used as a fuel binder,and aluminum(Al)is most widely used as metallic fuel in the heterogeneous composite solid propellants.Apart from these,other ingredients used for getting the desired mechanical properties and ballistic properties of solid propellants are plasticizer[di-octyladipate(DOA)],curing agent[Toulene di-isocyanate(TDI)],cross linking agent(Glycerol),and catalyst(ferric oxide,aluminum oxide etc.)[4].Although,AP based propellants shows good ballistic properties,but its combustion products are mainly composed of chlorine and chlorine based various oxides.The chlorine and its oxides have highly detrimental effects on the flora and fauna present near the vicinity of the rocket launch site,and also for the upper ozone layer.Apart from this,the plume signature of AP based propellant is also very clear and dominant.Use of missiles based on AP propellants during war can leave the trail of missile trajectory,which helps enemy to locate the launch site of missile.It is also well known from the fact that during war stealth and surprise attack over enemy is the key for victory.In order to reduce such hazardous effects from AP based propellants,green propellants are being formulated and also being used in some rocket and missile motors.Some of the noteworthy green oxidizers for solid propellants are ammonium nitrate(AN),ammonium dinitramide(ADN),and hydrazinum nitro-formate(HNF)[5].
Among the above mentioned three green oxidizers,AN is the most easily available green oxidizer.Apart from this,combustion products of AN are also non-chlorinated with low molecular weight.Use of oxidizers with low molecular weight combustion products is essential for obtaining high thrust rocket motors[6].Anfind majority of its applications in fertilizers,explosives,and in safety air bags.However,use of AN as an oxidizer entity for solid propellant is still very limited.The reasons for not choosing AN as an oxidizer for solid propellants are its,a)high hygroscopicity,b)incapability to burn metallic fuels,c)low flame temperature,d)crystallographic phase changes near to about room temperature,and e)non-uniform crystals[7].Among the above mentioned five disadvantages,crystallographic phase change at about 32°C is the most problematic.During this phase change,the density of AN crystals increases by 5%which lead to the formation of cracks over solid propellant grain surface[8].There are several methods adopted to resolve these drawbacks.However,majority of works are devoted over the phase stabilization and energy enhancing methods for AN crystals[9-15].
Potassium(K)and copper(Cu)based compounds are primarily used for phase stabilization and as an energy enhancer for AN crystals[12-17].One of the noteworthy potassium based compound that is of great interest for both propellant and for explosive community is potassium dinitramide(KDN).It has been reported in the literature[14,16]that KDN stabilizes the AN and also increases the burn rate of the AN based propellants.The study of the KDN based compound is also interesting because of its complex decomposition mechanism,crystal size,hygroscopicity level of crystal,solid/liquid KDN,pressure,storage condition,storage time etc.These parameters play an important role in governing its decomposition mechanism.Apart from KDN,copper based catalyst also considerably increases the phase transition temperature of AN and in many cases burning rate of AN propellants too.It has also been reported that Copper cobalt based metal oxide increases the burning rate of both AN and AP based composite solid propellants[16,18].
In the present studies,a detailed kinetic analysis has been performed on the catalyzed AN and AN/KDN based oxidizer and propellant samples using Ozawa-Flynn-Wall(OFW)method.The catalyst used here is copper-cobalt based metal oxide(Cu-Co*)as it is effective in the decomposition and also helps in enhancing the burn rate of both AP and AN based oxidizer and propellant samples[16-18].KDN acts as a burn rate enhancer and also the phase stabilizer for AN crystals.Hence,the prime goal of the present paper is to understand the catalytic activity of Cu-Co*on the decomposition mechanism of catalyzed AN and AN/KDN based oxidizer and propellant samples using DSC/TG techniques and kinetic studies.Further,an attempt was also made to understand in detail about the effect of KDN on modifying the decomposition behaviour of AN.
2.Experimental methodology
KDN and copper,cobalt based metal oxide(Cu-Co*)are the ingredients for the preparation of AN/KDN based oxidizer and HTPB based propellants.It was synthesized and its details are also available in Ref.[16]and for better understanding,it has also been explained in the forthcoming section.The procedure adopted for the preparation of oxidizer and propellant samples is explained in Section 2.2.
2.1.KDN and catalyst synthesis and characterization
2.1.1.Synthesis and characterization of KDN
The synthesis of KDN was carried out by using analytical grade Sulfamic acid(93%),Potassium hydroxide(90%)from Sigma-Aldrich,Ethanol,Acetone and Fuming nitric acid(100%)from Merck,and Sulfuric acid(95%)from Acros Organics.Three steps are involved in the synthesis method[16,19].The first step involves potassium sulfamate preparation from sulfamic acid,KOH and C2H5OH.In the second step of the reaction potassium sulfamate is nitrated using nitric and sulfuric acid(ratio of 4:1)at 40°C.The mixture of the three salts potassium nitrate(KNO3),potassium dinitramide,and potassium bisulphate(KHSO4)obtained from nitration is used for the extraction of KDN by using acetone.The final extracted KDN transparent crystals were yellowish white in colour,slightly moist in texture and were stored in vacuum desiccators.
Different analytical techniques were used to characterise KDN analytically,namely a)Differential Scanning Calorimetry(DSC)(Make:TA instruments,Model No.Q 10,USA),b)Thermogravimetric analyzer(TGA)(Make:Shimadzhu,Model No.DTG 60,Japan),c)UV-spectrophotometry(Make:Perkin Elmer,Model No.Lambda 25,USA),d)Fourier transform-infra red(FT-IR)(Make:Make:Shimadzhu,Model No.IR-Prestige 21,Japan).
DSC and TG of synthesized KDN with N2as a purge gas and heating rate of 10°C/min was carried out.The sample of mass was 6mg,and the crucible used for placing the sample was a sealed aluminum pan with a pierced hole in it.The obtained TG and DSC curve is shown in Fig.1(a)and Fig.1(b)respectively.The melting point at 131.6°C and decomposition peak at 202°C was obtained for KDN.An endothermic peak at around 104°C was obtained in the DSC curve.This peak corresponds to the decomposition and partial phase alteration of KDN[20-24].KDN crystals began to crack at around 90°C,and at 97°C a minor weight loss is observed due to partial decomposition to form KNO3.The KNO3formed gets adsorbed on the KDN crystal and the crystal became opaque in appearance[24]and this change takes place at around 104°C.TG curve in Fig.1(a)shows decomposition with two steps,the first correspond tothe first minor weight loss obtained at around 105°C,while the second major decomposition takes place in the temperature range of 200°C-240°C.
Fig.1(c)shows the UV-spectrum of KDN which have been previously presented in Ref.[16].Obtained molar absorbance coefficient was obtained at 286 nm and 212 nm.The observed result is in agreement with results given in Ref.[19],in which absorbance peaks are observed at 285 and 214nm.The peak in between 284 and 286nm is the characteristic absorption peak of the dinitramide anion[N(NO2)-2][19-26].The FTIR peaks with its characteristic spectrum value are shown in Table 1.Apart from above discussed four analytical methods to characterize KDN,TG-QMS results with KDN mixed propellant samples show the presence of N2O which is the main gaseous decomposition product of KDN[16].This further supports the evidence for the formation of KDN crystals by the present method of synthesis.The characterization results analysis are in agreement with earlier reported research[19-26]and confirms the formation of KDN from the above given method of synthesis.
2.1.2.Catalyst synthesis and characterization
Copper cobalt metal oxides(Cu-Co*)catalyst was synthesized using the procedures described in Refs.[16,18].Citric acid sol-gel methodwas used for synthesizing several mixed metal oxides[27,28]and it has been utilized to synthesize the Copper-cobalt based metal oxide(Cu-Co*)catalyst.Copper(II)nitrate trihydrate[Cu(NO3)2.3H2O], Cobalt(ous) nitrate hexahydrate [Co(NO3)2.6H2O],and Citric acid(C6H8O7.H2O)of analytical grade(make CDH,India)are the precursors used for the Cu-Co*catalyst synthesis.The molar ratio was kept one for Cu/Co in the copper cobalt based metal oxide.The molar ratio was calculated using the weight of the sample divided by the molecular weight of the sample.The mole ratio was 0.00248 mol/dm-3for copper nitrate(MW:241,weight of the sample:0.6 gm),while the mole ratio was 0.00247 mol/dm-3for cobalt nitrate(MW:291,weight of the sample:0.72 gm).The first step involves the homogeneous mixing of 0.6 g of copper nitrate,0.72 g of cobalt nitrate,and 3.15 g of citric acid with 25 mL of distilled water.The second step of the synthesis involves drying of the dissolved solution to a viscous gel at 90°C for 3-4 h.The gel is then dried at 165°C for 2 h to form a bubbly light violet colour dried gel.The dried gel is then calcined at 550°C for 3 h to obtain the black colour catalyst.The synthesized Cu-Co*catalyst was used to prepare oxidizer and propellant samples.The XRD analysis for the prepared catalyst was carried out for the characterization of Cu-Co*catalyst.The instrument used was“Smart Lab diffractometer”supplied by Rigaku,Japan.The patterns were run with Cu-K radiation at 40 kV with a scanning speed of 10omin-1in the 2-theta(2o)range of 10°to 90°.
The XRD results confirmed the presence of CO3O4,CuO,and CoO[29-33]in the prepared Cu-Co*.However,the dominant peak was of Cobalt(II,III)oxide(Co3O4)at the 2-theta value of 36.88.The obtained XRD plot for Cu-Co*catalyst is presented in Fig.2.
There are observances of several peaks in the XRD plotof Cu-Co*catalyst as shown in Fig.2 Out of the peaks,there are three major peaks and seven minor peaks.Three major peaks are at 2-theta value of 36.88,38.66 and 35.58.The peaks at 2-theta value of 32.46 and 36.88 correspond to Co3O4[29-31],peak at 35.58 corresponds to CuO[32],while the peak at 38.66 correspond to CoO[33].Apart from this peaks,severalother minor peaks are also obtained which are for other cobalt and copper oxide present in Cu-Co*.
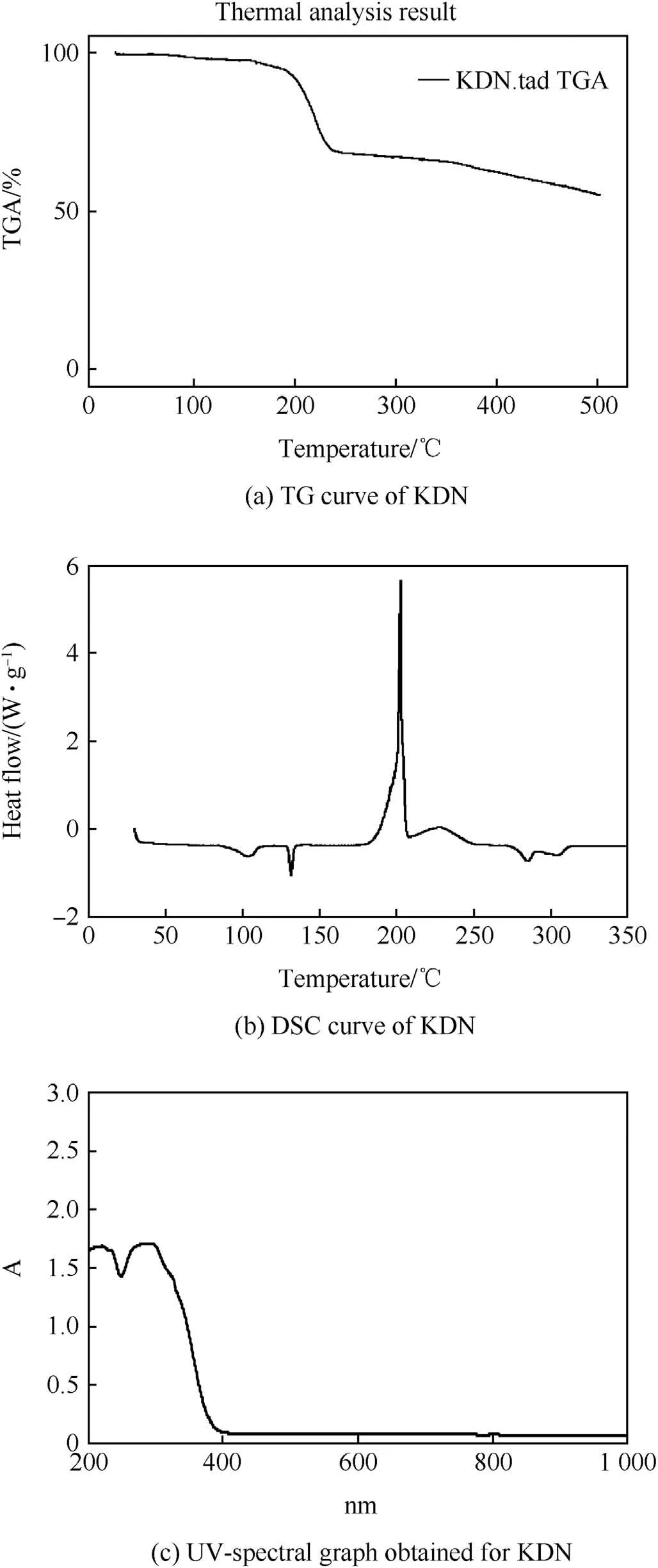
Fig.1.Characterization peaks/curves of KDN.
2.2.Preparation of AN/KDN based oxidizer and propellant
Ammonium nitrate(AN)(purity 99.9%)from Chemical Drug House(CDH,India)was used for the sample preparation.KDN was incorporated in AN crystal by co-cryatallization method to prepare the oxidizer samples.AN/KDN(75/25)oxidizer sample was made by thoroughly mixing a solution of 75 mg of AN and 25 mg of KDN(both dissolved in 2mL of distilled water).The solutionwas dried in a vacuum oven at 60°C to obtain dry crystals.AN/KDN(50/50)oxidizer sample was prepared in a similar manner.An evaporative co-crystallization method was used to prepare oxidizer samples with catalyst[16].Total of 7 oxidizer samples were prepared and it has been presented in Table 2.
For the preparation of propellant samples,a fuel binder Hydroxyl Terminated Poly-Butadiene(HTPB)with,oxidizers AN and AN/KDN mixtures at a constant oxidizer to fuel(O/F)ratio of 80:20wereused.Other ingredients utilized in the preparation of propellant samples are Di-Octyl Adipate(DOA)(as plasticizer),Glycerine(as cross linking agent),Toluene Di-isocyanate(TDI)(curing agent),and Cu-Co*catalyst.For all propellant formulations 14.36 gm of HTPB,4.36 gm of dehumidified DOA and 80 gm of grounded oxidizer particles(AN or AN/KDN)were used.The small quantity of oxidizer was mixed manually with continuous stirring to get a homogeneous mixture in HTPB matrix.Then TDI(1.13gm)and glycerol(0.15gm)was added to the mixture and was continuously mixed.The final step consists of adding the crushed catalyst(2%additional weight),and the mixing was continued till homogeneous slurry obtained.The curing of the homogeneous slurry was carried out at 60°C for 6 days.The prepared propellant samples are listed in Table 3.
2.3.Instrumentation
The Simultaneous Thermal Analyzer(STA Model 409PG luxx)of NETZSCH,Germany were used to analyze the thermal gravimetric properties of each propellant samples.It was used to obtain the Thermo Gravimetric-Differential Thermo Gravimetric(TG-DTG)thermo-grams.The purge gas used was Nitrogen(at a flow rate of 60 mL/min)and the samples were taken in Alumina crucible with lid for placing in the STA sample carrier.The sample weight of 6±0.5 mg for each analysis and indium standard for the heat flow and temperature calibrations were used.Three different heating rates of 3°C/min,5°C/min,and 10°C/min were used for the TGDTG experiments.
Kinetic calculations were performed using the guidelines as suggested by International Confederation for Thermal Analysis and Calorimetry(ICTAC)kinetics committee for kinetic computations using thermal analysis data[34].Ozawa-Flynn-Wall(OFW)method was used in the present work for calculating the kinetic parameters of the propellant samples.OFW is a model-free iso-conversional method where the activation energy is calculated as a function of conversion and temperature without considering the reaction model.The rate of solid-state degradation is given as

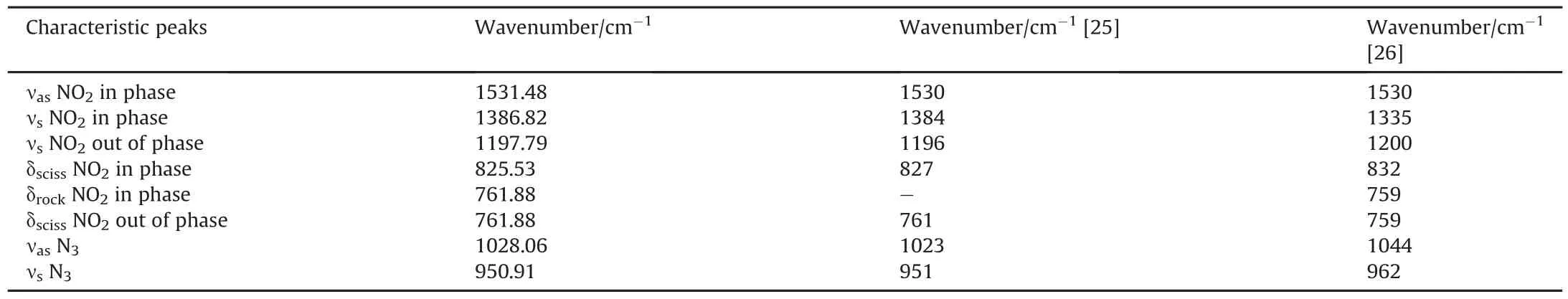
Table 1Characteristic FTIR peaks of KDN.
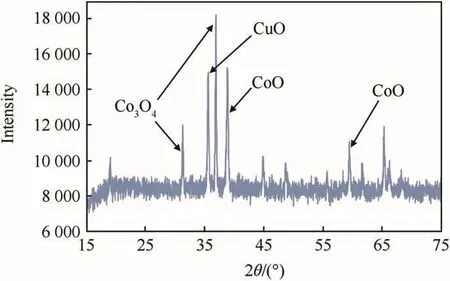
Fig.2.XRD plot of Cu-Co*catalyst.

Table 2List of oxidizer samples with their composition.
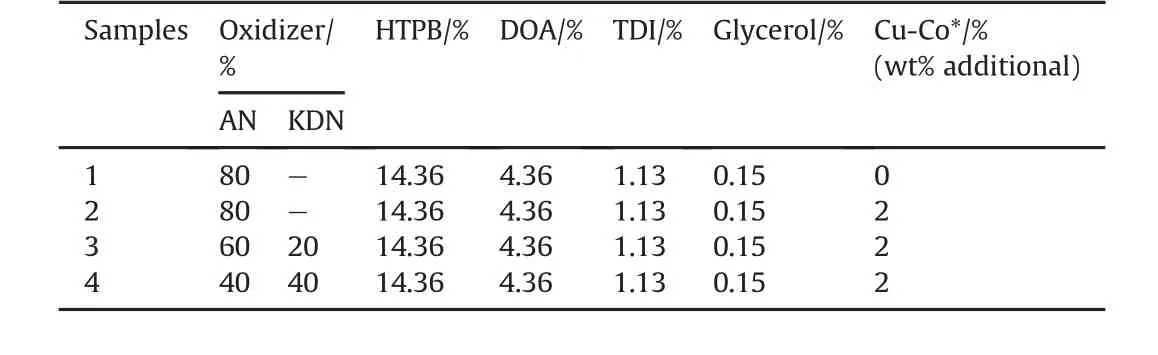
Table 3The ingredients used in the preparation of propellant samples.
Where,αis extent of conversion,Tis temperature andtis the time for the process.The assumption made in Equation(1)states that the rate constant,K(T)is temperature dependent and thus can be separated from the reaction model,f(α).The temperature dependence of the rate constant is presented by replacingK(T)with Arrhenius equation,which gives

Where,Eis the activation energy,Ais the frequency factor,andRis the gas constant.The trivial transformation is used to eliminate the explicit temperature dependence of Eq.(2)
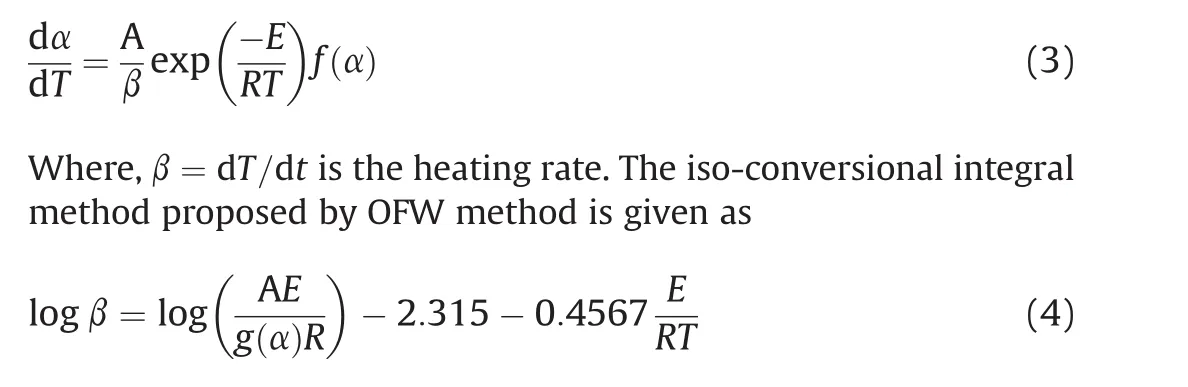
For different heating rates(β),different sets ofTare obtained at differentXlevels and plotting lnβvs.1/T,a straight line with slope of(-E/R)is obtained.Therefore,the variation of activation energy as a function of conversion can be obtained.
3.Results and discussion
TG-DTG curves of all the prepared samples are provided and discussed at the heating rate of 10°C/min and in the temperature range of 50°C-500°C.Only for AN/HTPB propellant sample,the TG-DTG curve was provided in the temperature range of 50°C-600°C.Discussion over TG-DTG and kinetics of all the 11 samples are divided into five groups for enhanced understanding.They are
a)Pure AN,Pure AN propellant
b)Pure KDN
c)AN+Cu-Co*oxidizer,AN+Cu-Co*propellant
d)AN/KDN(75/25)oxidizer,AN/KDN(75/25)+Cu-Co*oxidizer,and AN/KDN(75/25)+Cu-Co*propellant
e)AN/KDN(50/50)oxidizer,AN/KDN(50/50)+Cu-Co*oxidizer,and AN/KDN(50/50)+Cu-Co*propellant
3.1.TG-DTG and kinetics of pure AN,and pure AN propellant
TG-DTG curves of pure AN,pure AN propellant,and HTPB are shown in Fig.3,Fig.4,and Fig.5 respectively.While,the OFW plots of pure AN and pure AN propellant are shown in Fig.6(a)and Fig.6(b)respectively.
TG-DTG curve of pure AN(Fig.3),shows one step decomposition of AN in the temperature range of 200°C-290°C,with corresponding DTG peak at 278°C.While,TG-DTG curve of pure AN propellant(Fig.4)shows two steps decomposition.First step of decomposition in pure AN propellant was observed in the temperature range of 180°C-290°C,which corresponds to AN decomposition,while the second decomposition step was in the temperature range of 400°C-600°C,which corresponds to HTPB decomposition.However,TG-DTG curve of pure HTPB(Fig.5)shows decomposition temperature range of 300°C-500°C with corresponding DTG peak at 450°C.Since,decomposition of AN oxidizer is an endothermic process,and there is large amount of moisture release during AN decomposition,which led to extended decomposition of HTPB in AN propellant by about 100°C[35].
OFW plot of pure AN in Fig.6(a)shows that all the decomposition slopes of pure AN are in same pattern at all the three different heating rates used for the study.Such similar pattern of slopes in completeαrange confirmed that pure AN undergoes one step decomposition.The obtained average activation energy(Ea)value of AN decomposition was 108.62 kJ/mol.The reported activation energy of ammonium nitrate was found to be 102 kJ/mol[36].However,the Ea values obtained for AN decomposition generally varies from 107 to 171kJ/mol as reported by different researchers[37].The uncertainty for the obtained activation energy value is 2%.Hence,the obtained Eavalue for AN decomposition is in accordance with the reported literature.OFW plot of pure AN propellant in Fig.6(b)shows the presence of decomposition slopes in twoα(fractional mass loss)ranges.Firstαrange was in between 0.1 and 0.8 for AN decomposition,while the second step decomposition was atαvalue of 0.9 for HTPB decomposition.The average Ea value was 92.24kJ/mol for AN decomposition,while 66.97 kJ/mol for HTPB decomposition.The obtained activation energy of HTPB available in literature was around 75 kJ/mol[38].Hence,the obtained result indicates the accuracy of OFW method for calculating Ea values of AN and AN/HTPB based propellants.
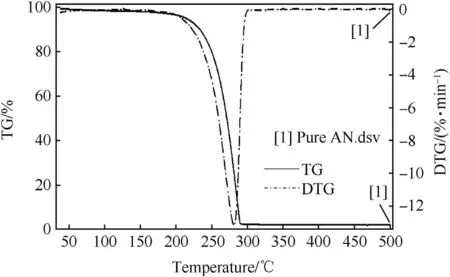
Fig.3.TG-DTG curve of pure AN.
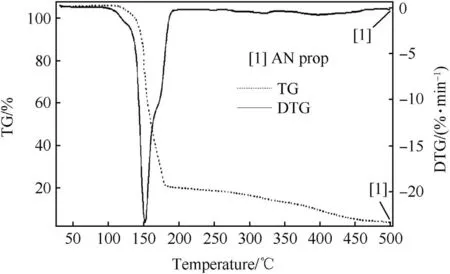
Fig.4.TG-DTG curve of pure AN propellant.

Fig.5.TG-DTG curve of HTPB.
3.2.TG-DTG and kinetics of pure KDN
TG-DTG curve and OFW plot of pure KDN in the temperature range of 50°C-500°C is shown in Fig.7(a)and Fig.7(b)respectively.
TG-DTG curve in Fig.7(a)of pure KDN shows two steps decomposition in the temperature range of 50°C-500°C.First minor decomposition was at just before 100°C,while the second major decomposition was in the temperature range of 180°C-250°C with a corresponding DTG peak at 210°C.First step decomposition was for the partial decomposition of KDN at around 90°C.It is due to the minor decomposition of KDN crystals and its partial phase change to form potassium nitrate(KNO3)[20-24].KDN crystals start to crack at around 90°C,and at 97°C a minor weight loss is observed due to its partial decomposition to form KNO3.The KNO3which is formed at 97°C gets adsorbed over the KDN crystal and the KDN crystal becomes opaque in appearance[24].This transformation takes place at around 90°C.While in the case of freshly prepared KDN sample,minor weight loss was obtained at 104°C as discussed previously in section 2.1.1.Afterwards,majority of KDN decomposes to form KNO3at around 200°C[KN(NO2)2→ KNO3+N2O].KNO3was stable up to 600°C and its decomposition start at or after 600°C[39].
From OFW plot of pure KDN in Fig.7(b),it can be observed that there are three different patterns of decomposition slopes from 50°C to 500°C,where each slope represents one decomposition step.First partial decomposition of KDN with negative Ea value was observed in theαvalue of 0.1,second major decomposition step with net positive Ea value was in theαrange of 0.2-0.8 for KDN decomposition,while the last decomposition slopes with negative Ea value correspond to the initiation of decomposition of KNO3in theαvalue of 0.9.
The reasons for obtaining negative Ea values during various types of decomposition reactions have been observed by several researchers as given in Refs.[40-44].The reason for negative Ea value for the initial decomposition of KDN crystal can be explained on the basis of the decomposition mechanism of KDN as given in Ref.[24].As KDN decomposition proceeds,the lattice defects and cracks over the KDN surface get expands.During this expansion,KNO3gets adsorbed firmly on the KDN surface,and this leads to the development of stress inside the KDN crystal.This stress increases continuously with temperature up to some extent,resulting in decrease of chemical rate constant(k).With further increase in temperature,the KDN crystal surface is completely covered with KNO3and the reaction centre is eliminated;leading to partial or complete termination of the decomposition reactions.This may be one of the reasons for negative activation energy for KDN decomposition in its initial stage.Further,the KDN decomposition is condensed phase decomposition with multiple steps taking place simultaneously,which may further add to complexities and hence negative Ea value was obtained.The average Ea value of the second decomposition step or of major decomposition of KDN was found to be 291.09 kJ/mol.The uncertainty in the calculation of activation energy for pure KDN was 15%.The Ea value obtained for KDN decomposition was much higher as compared to AN decomposition.
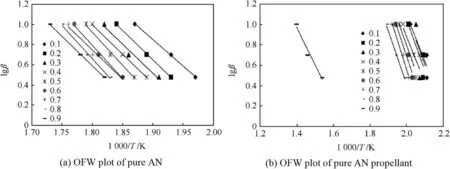
Fig.6.OFW plot of pure AN and pure AN propellant.
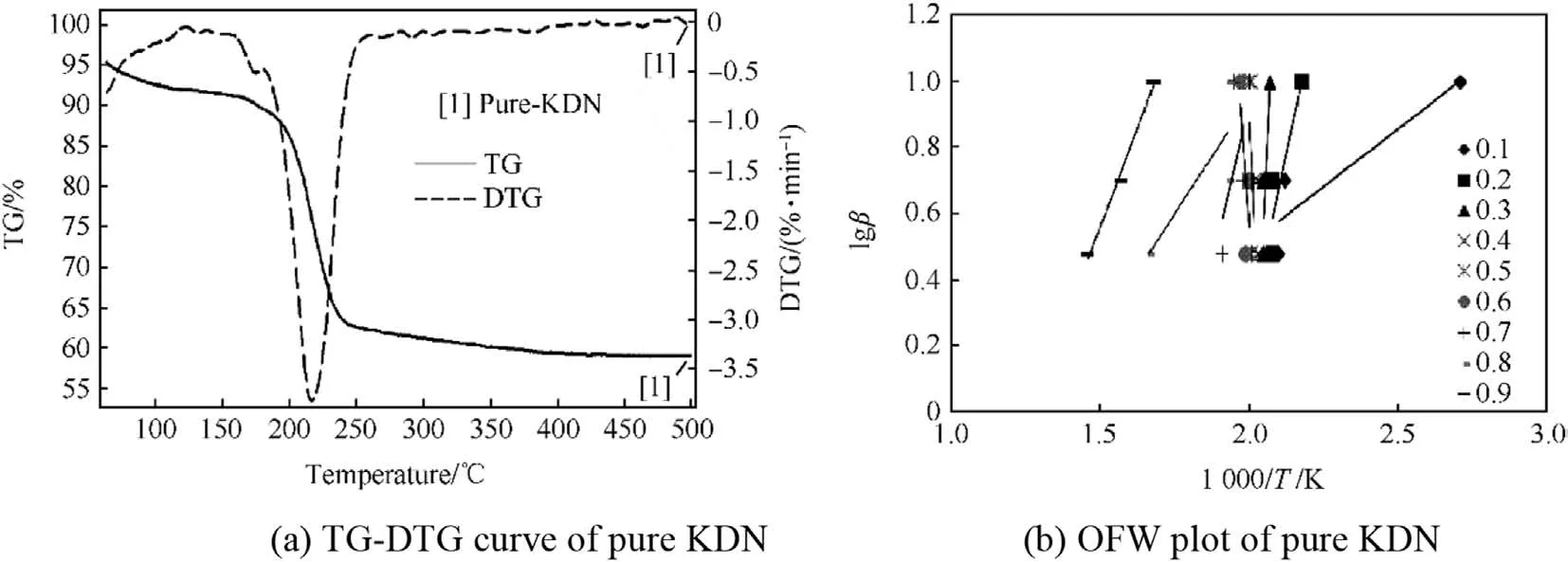
Fig.7.TG-DTG curve of pure KDN and pure KDN.
3.3.TG-DTG and kinetics of AN+Cu-Co*oxidizer and AN+Cu-Co*propellant
TG-DTG curves of AN+Cu-Co*oxidizer and AN+Cu-Co*propellant are shown in Fig.8(a)and Fig.9(a)respectively.While the OFW plots of AN+Cu-Co*oxidizer and AN+Cu-Co*propellant are shown in Figs.8(b)and 9(b)respectively.
TG-DTG curve of AN+Cu-Co*in Fig.8(a)shows one step decomposition in the temperature range of 200-290°C with corresponding DTG peak at 270°C.Inpresence of Cu-Co*catalyst,early decomposition was observed with higher magnitude of decomposition at a lower temperature range.TG-DTG curve of AN+Cu-Co*propellant in Fig.9(a)shows two distinctly visible decomposition steps.In comparison with TG-DTG curve of pure AN propellant,the extent of decomposition in AN+Cu-Co*propellant is much high with slight decrement in decomposition temperature range too.First step decomposition with DTG peak at 220°C corresponds to AN decomposition,second decomposition step with DTG peak at 260°C is for catalytic HNO3decomposition,while third decomposition with DTG peak at 450°C corresponds to HTPB decomposition.In the presence of Cu-Co*catalyst,the range of HTPB decomposition is also much narrower i.e.it lies in between 420°C to 480°C only.
OFW plot of AN+Cu-Co*in Fig.8(b)shows single pattern of decomposition slopes in theαrange of 0.1-0.9.Obtained average Ea values for the AN decomposition in presence of Cu-Co*was 77.87 kJ/mol.Hence,in the presence of Cu-Co*,the activation energy of pure AN dropped to 77.87 kJ/mol from 108.62 kJ/mol.Vergeese et al.[45]had obtained the activation energy(Ea)for AN+CuO as 97 kJ/mol.In this respect,it can be stated that Cu-Co*catalyst is a better decomposition catalyst for AN decomposition as compared to CuO.OFW plot of AN+Cu-Co*propellant in Fig.9(b)shows three different patterns of slopes.First decomposition slope observed in theαrange of 0.1-0.5 corresponds to AN decomposition,while the second decomposition slope at theαvalue of 0.6-0.8 corresponds to the catalytic decomposition of HNO3,while the third decomposition step for theαvalue of 0.9 corresponds to HTPB decomposition.The average Ea value for AN and HTPB decomposition in AN+Cu-Co*propellant are 79.41 kJ/mol and 125.21 kJ/mol respectively.The uncertainty obtained for both the cases are 5%.

Fig.8.TG-DTG curve and OFW plot of AN+Cu-Co*oxidizer.
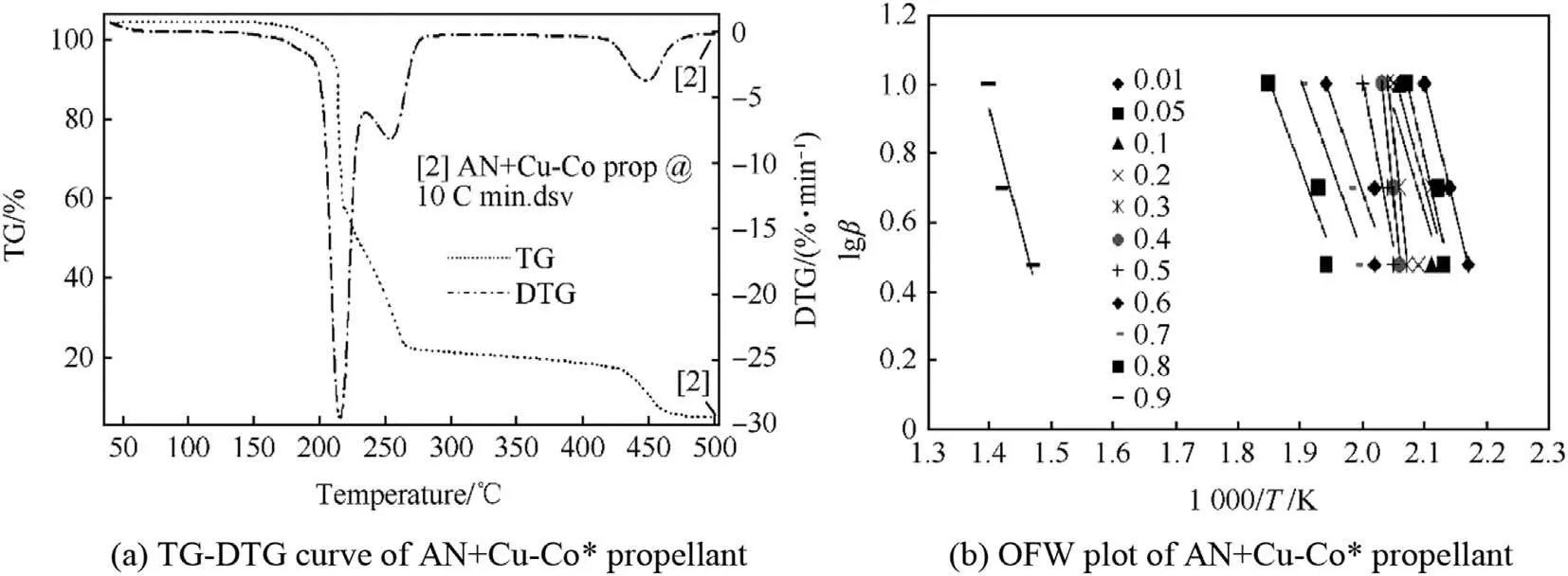
Fig.9.TG-DTG curve and OFW plot of AN+Cu-Co*propellant.
3.4.TG-DTG and kinetics of AN/KDN(75/25)oxidizer,AN/KDN(75/25)+Cu-Co*oxidizer,and AN/KDN(75/25)+Cu-Co*propellant
TG-DTG curves of AN/KDN(75/25)oxidizer,AN/KDN(75/25)+Cu-Co*oxidizer,and AN/KDN(75/25)+Cu-Co*propellant are shown in Fig.10(a),11(a),and 12(a)respectively.While,the OFW plots of AN/KDN(75/25),AN/KDN(75/25)+Cu-Co*,and AN/KDN(75/25)+Cu-Co*propellant are shown in Figs.10(b),11(b),and 12(b)respectively.
TG-DTG curve of AN/KDN(75/25)in Fig.10(a)shows two step decompositions occurring for the temperature range of 50°C-500°C.First step decomposition was for KDN in temperature range of 180°C-220°C with corresponding DTG peak at 205°C,while the second step decomposition was for AN in between 220°C to 300°C with corresponding DTG peak at 270°C.From the results obtained,it has been observed that the onset decomposition temperature for AN reduces in presence of 25%KDN.TG-DTG curve of AN/KDN(75/25)+Cu-Co*in Fig.11(a)shows one minor and two major decomposition steps.Minor decomposition below 90°C was for partial KDN decomposition.Afterwards, first major decomposition step was for KDN decomposition,while 2nd major decomposition step was for AN decomposition.DTG peak of KDN decomposition was at around 190°C,while for AN it was obtained at 273°C.The result indicate that in the presence of Cu-Co*catalyst separate decompositions of KDN and AN takes place.TG-DTG curve for AN/KDN(75/25)+Cu-Co*propellant in Fig.12(a)shows three steps of decomposition in the temperature range of 50°C-500°C.The three decomposition steps are for KDN,AN,and HTPB respectively.The corresponding DTG peaks for decomposition of KDN,AN,and HTPB are 215°C,260°C,and 420°C respectively.The results illustrate that HTPB decomposition occurred much earlier in presence of 25%KDN and 2%Cu-Co*catalyst.One more interesting result obtained from TG-DTG curves is that the initial KDN decomposition stabilizes in presence of Cu-Co*catalyst.TG-DTG curve of AN/KDN(75/25)in Fig.10(a)shows initial decomposition of KDN showed a wavy characteristics curve as compared to stable initial decomposition in presence of Cu-Co*catalyst for the other two catalytic AN/KDN(75/25)oxidizer and propellant samples respectively.
The OFW plot of AN/KDN(75/25)oxidizer in Fig.10(b),shows irregular pattern of slopes,which is due to complex decomposition mechanism of KDN.From TG-DTG curve of AN/KDN(75/25)in Fig.10(a),it was detected that initial decomposition was unstable since the curves are highly wavy in nature.Two different patterns of decomposition slopes are observed for AN/KDN(75/25)in OFW plot.Decomposition slopes in theαrange of 0.1-0.4 are for KDN decomposition and are positive in nature which give apparent negative activation energy for KDN decomposition.The decomposition slopes in theαrange of 0.5-0.9 are for AN decomposition with apparent positive activation energy.The values of Ea for AN decomposition was 140.65 kJ/mol.OFW plot of AN/KDN(75/25)+Cu-Co*catalyst in Fig.11(b)shows two patterns of decomposition slopes with positive Ea values.Such results again confirmed that,Cu-Co*stabilizes the initial onset of KDN decomposition.First decomposition slopes in theαvalue of 0.1-0.4 are for KDN decomposition,while the decomposition slopes in theαrange of 0.5-0.9 are for AN decomposition.Average Ea values of KDN and AN decompositions are 111.29 kJ/mol and 74.13 kJ/mol respectively.
OFW plot of AN/KDN(75/25)+Cu-Co*propellant in Fig.12(b)shows positive patterns of slopes for decomposition of all the three entities i.e.of KDN,AN,and HTPB.First decomposition slopes was obtained for KDN decomposition with positive Ea value in theα range of 0.1-0.2,second decomposition slopes for AN was in theα range of 0.3-0.8,while the last decomposition slope was for HTPB was forαvalue of 0.9.The average Ea value for KDN,AN,and HTPB decomposition steps are 42.77 kJ/mol,82.59 kJ/mol,and 112.84 kJ/mol respectively.The error in the calculation Ea value of AN/KDN(75/25)+Cu-Co*oxidizer and AN/KDN(75/25)+Cu-Co*propellants is 10%.
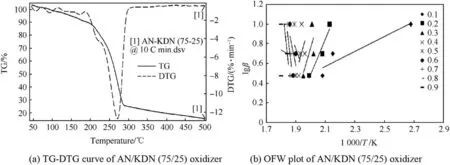
Fig.10.TG-DTG curve and OFW plot of AN/KDN(75/25)oxidizer.
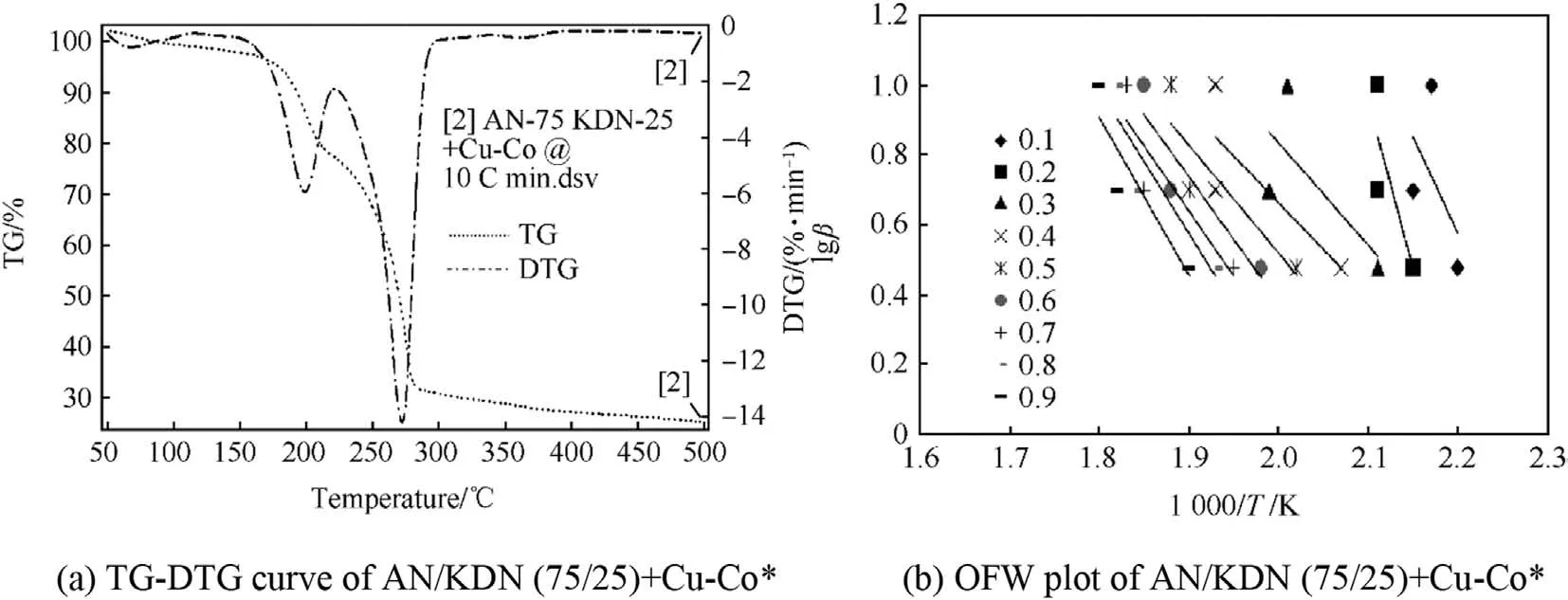
Fig.11.TG-DTG curve and OFW plot of AN/KDN(75/25)+Cu-Co*.

Fig.12.TG-DTG curve and OFW plot of AN/KDN(75/25)+Cu-Co*propellant.
Cu-Co*reduces the decomposition temperature of AN,KDN,and its mixtures for both oxidizer and propellant samples.It may be due to the fine crystal structure of Cu-Co*catalyst as citric-acid sol gel method was used to obtain the crystals of Cu-Co*at high calcination temperature of 550°C.These fine crystals of Cu-Co*get easily settled on various reaction sites of AN/KDN crystals and thus enhances the decomposition of AN/KDN samples uniformly.Apart from this the XRD plot of Cu-Co*also shows the presence of copper,and cobalt oxides in various forms.Cobalt(II,III)oxide(Co3O4)is dominant in Cu-Co*,which acts as an important catalyst to enhance the burn rate of propellant samples.
3.5.TG-DTG and kinetics of AN/KDN(50/50)oxidizer,AN/KDN(50/50)+Cu-Co*oxidizer,and AN/KDN(50/50)+Cu-Co*propellant
TG-DTG curves of AN/KDN(50/50),AN/KDN(50/50)+Cu-Co*,and AN/KDN(50/50)+Cu-Co*propellant are presented in Fig.13(a),14(a),and 15(a)respectively.While,the OFW plots of AN/KDN(50/50),AN/KDN(50/50)+Cu-Co*,and AN/KDN(50/50)+Cu-Co*propellant are shown in Figs.13(b),14(b),and 15(b)respectively.
TG-DTG curve of AN/KDN(50/50)oxidizer in Fig.13(a)shows two decomposition peaks in the temperature range of 50°C-500°C.First minor decomposition peak for the partial KDN decomposition was obtained below 90°C,while the second DTG peak at 205°C was obtained for the simultaneous decomposition of AN and KDN.This result shows that,as we increase the KDN percentage in AN crystals up to 50%,AN decomposes simultaneously with KDN.This may be due to the generation of heat during KDN decomposition,which is sufficient for AN to decompose along with KDN at around 205°C[16].TG-DTG curve of AN/KDN(50/50)+Cu-Co*in Fig.14(a)shows three steps of decomposition.The three decomposition steps correspond for the initial KDN decomposition,simultaneous AN/KDN decomposition,and decomposition of oxide formed between decomposition products of AN/KDN with Cu-Co*.The DTG peaks for these three decompositions steps are at 70°C,200°C,and 260°C respectively.The results shows that in the presence of Cu-Co*catalyst,temperature for partial KDN decomposition and for AN/KDN decomposition further decreases.Four decomposition reaction steps from 50°C to 500°C are observed in the TG-DTG curve of AN/KDN(50/50)+Cu-Co*propellant in Fig.15(a).The four decomposition steps are for the initial KDN decomposition,the AN/KDN simultaneous decomposition(50%),decomposition of oxide formed from KDN and AN/KDN decomposition,and of HTPB decomposition.The DTG peaks for the four decompositions are 100°C,200°C,260°C,and 450°C respectively.
OFW plot of AN/KDN(50/50)in Fig.13(b)shows initial negative activation energy values for KDN decomposition,as previously observed with AN/KDN(75/25)too.Three different patterns of slopes were observed for AN/KDN (50/50)decomposition.Decomposition slope in theαvalue of 0.1 was for initial KDN decomposition,slopes in theαvalue of 0.2-0.8 correspond for simultaneous decomposition of AN/KDN,and last one slope with negative Ea values was for the initiation of KNO3decomposition.Since KNO3decomposition was not completed by 500°C and hence it shows negative Ea value.The observed Ea value for simultaneous AN/KDN decomposition was 148.67 kJ/mol.The OFW plot of AN/KDN(50/50)+Cu-Co*in Fig.14(b)indicate three patterns of slopes.First decomposition slope with positive Ea in theαvalue of 0.1 is for minor KDN decomposition,decomposition slopes in theαrange of 0.2-0.8 are for simultaneous AN/KDN decomposition,and the slope atα=0.9 was for KNO3decomposition(which is again negative in nature).Although,in between theαvalue of 0.6-0.8,the slopes are positive in nature with apparent negative Ea value.This negative Ea value corresponds to the complex decomposition of oxide as discussed previously.Average Ea values of initial KDN decomposition and of simultaneous AN/KDN decomposition are 40.22 kJ/mol and 212.21 kJ/mol respectively.The OFW plot of AN/KDN(50/50)+Cu-Co*propellant in Fig.15(b)displays four patterns of negative slopes with apparent positive activation energy values.The four slopes correspond to the initial KDN decomposition(α =0.1),simultaneous AN/KDN decomposition(α =0.2 to 0.6),oxide decomposition(α =0.7),and of HTPB decomposition(α =0.8 to 0.9).The Ea values for these four decomposition steps are 10.21 kJ/mol,380.2 kJ/mol,421.60 kJ/mol,and 189.64 kJ/mol respectively.The uncertaintyerror in the calculation Eavalue of AN/KDN(50/50)oxidizer,AN/KDN(50/50)+Cu-Co*oxidizer,and AN/KDN(50/50)+Cu-Co*propellant are 8,5 and 2%respectively.
Present study reveals some interesting results on the catalytic action of Cu-Co*on AN,KDN,and AN/KDN based oxidizer and propellant samples.The studies shows that,a)Cu-Co*helps in stabilizing KDN decomposition,and b)KDN plays the main role in governing the decomposition mechanism of AN/KDN based oxidizer and propellant samples.Further,KDN modifies the decomposition behavior of AN oxidizer.Cu-Co*catalyst also decreases the Ea values for AN/KDN(75/25)based oxidizer and propellant samples.However,with the increase of KDN percentage in AN crystals Ea values increases.The suitable reason for such behaviour is due the complex decomposition mechanism of KDN and the high Ea value(291.09 kJ/mol)for pure KDN decomposition.As the KDN percentage is increased in the AN crystals,more amount of KNO3will be formed during the initial KDN decomposition that blocks the reaction sites of KDN.Further in the catalyzed AN/KDN(50/50),AN and Cu-Co*were also present.Because of the presence of three entities i.e.KNO3,AN,and Cu-Co*the Ea values increases in cases of catalyzed AN/KDN(50/50)based oxidizer and propellant samples.

Fig.13.TG-DTG curve and OFW plot of AN/KDN(50/50)oxidizer.
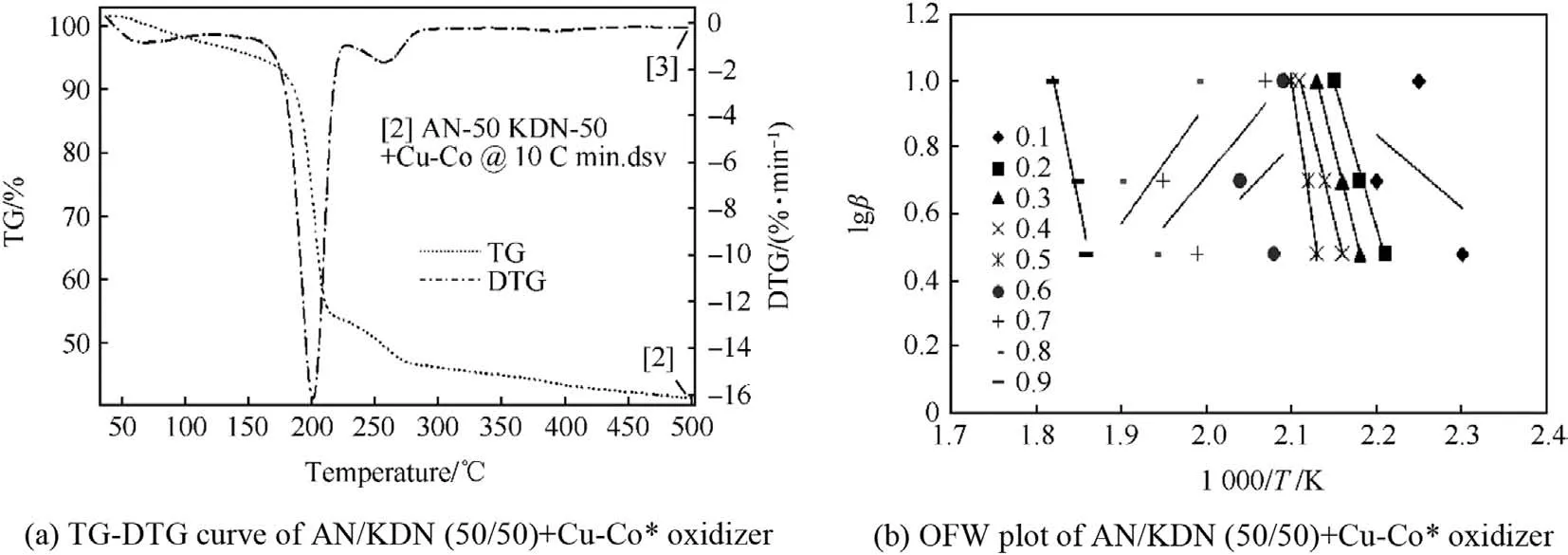
Fig.14.TG-DTG curve and OFW plot of AN/KDN(50/50)+Cu-Co*oxidizer.

Fig.15.TG-DTG curve and OFW plot of AN/KDN(50/50)+Cu-Co*propellant.
4.Conclusion
Present study primarily focused on the TG-DTG and kinetic studies using OFW method over AN,KDN,and its mixtures in presence of Cu-Co*as a catalyst.As previous studies suggested,Cu-Co*not only helps in increasing the burn rate drastically,but it also makes AN propellant to sustain its combustion even at sub atmospheric pressure.Results obtained from the present study with Cu-Co*as a catalyst for oxidizer and propellant samples also revealed some interesting effects.In presence of Cu-Co*catalyst,there is great reduction in decomposition temperatures,net high exothermic decomposition,and smoothness in decomposition patterns.Following conclusions can be drawn from the present studies:
a)Catalyst Cu-Co*reduces the decomposition temperature of AN,KDN,and its mixtures for both oxidizer and propellant samples.
b)In presence of Cu-Co*catalyst,early decomposition of HTPB,and KNO3were also observed.Decomposition temperature of KNO3is at and above 600°C,however,in presence of Cu-Co*catalyst decomposition of KNO3starts below 500°C in case of 50%KDN samples.Such result also confirms the efficacy of Cu-Co* as a superior catalyst for KNO3decomposition.
c)Cu-Co*decreases the activation energy value for pure AN.The obtained Ea value for pure AN was 108.62 kJ/mol,while for AN+Cu-Co*it was 77.87 kJ/mol.
d)Cu-Co*catalyst also decreases the Ea values for AN/KDN(75/25)based oxidizer and propellant samples.However,with the increase of KDN percentage in AN crystals Ea values increases.
e)From OFW plot,it was observed that the initial KDN decomposition was erroneous in nature with negative Ea values in AN/KDN(75/25&50/50)samples.But with the addition of Cu-Co*catalyst in the samples,the negative activation energy slowly changed into positive values(although positive Ea value was also much less).Also,the wavy decomposition patterns of initial KDN decomposition in AN/KDN(75/25)get suppressed in presence of Cu-Co*.
f)Second major decomposition of KDN was stable with net positive Ea values in all cases of the sample tested in presence of catalyst Cu-Co*.
It can be concluded that,Cu-Co*is a catalyst of interest and it stabilizes the KDN decomposition mechanism,lowers the Ea values for AN and for AN/KDN(75/25)decomposition,and also promotes the decomposition of all entities used in present work i.e.AN,KDN,KNO3,and HTPB.
Acknowledgement
Authors are highly thankful to the Central Instrumentation Facility and Chemistry Department of BIT,Mesra,Ranchi for providing all the essential facilities required for carrying out this research work.
[1]Sutton GP.Solid propellants.In:Rocket propulsion elements.seventh ed.New York:John Wiley&,Sons Inc;2001.p.474-519.
[2]Sutton GP.Solid propellant rocket fundamentals.In:Rocket propulsion elements.seventh ed.New York:John Wiley&Sons Inc;2001.p.417-73.
[3]Lyon JM.Introduction to rocket propulsion.Technical report RD-PR-91-17,U.S.Army missile command,Redstone arsenal,Alabama.December 1991.
[4]Thakre P,Yang V.Solid propellants.In:Encyclopedia of aerospace engineering.John Wiley Publications;2010.p.1-10.
[5]Talawar B,Sivabalan R,Mukundan T,Muthurajan H,Sikder AK,Gandhe BR,Rao AS.Environmentally compatible next generation green energetic materials(GEMs).J Hazard Mater 2009;161:589-607.
[6]Chan Jr ML,Reed R,Ciaramitaro DA.Advances in solid propellant formulations.In:Brill TB,Ren WZ,Yang V,editors.Progress in Astronautics and Aeronautics:Solid propellant chemistry,combustion,and motor interior ballistics,AIAA;2000.p.185-206.
[7]Oommen C,Jain SR.Ammonium nitrate:a promising rocket propellant oxidizer.J Hazard Mater 1999;67:253-81.
[8]Dellien IA.DSC study of the phase transformations of ammonium nitrate.Thermochim Acta 1982;55:181-91.
[9]Oxley JC,Smith JL,Rogers E,Yu M.Ammonium nitrate:thermal stability and explosivity modifiers.Thermochim Acta 2002;384:23-45.
[10]Golovina N,Nechiporenko G,Nemstev G,Zyuzin I,Manelis GB,Lembert D.Ammonium nitrate phase stabilization with small amounts of some organic compounds.Cent Eur J Energ Mater 2009;6(1):45-56.
[11]Rubstov YI,Kazakov AI,Lembert DB,Manelis GB.Kinetic regularities of the heat release for the interaction of some organic compounds with ammonium nitrate.Propellants,Explos Pyrotech 2006;31(6):421-34.
[12]Vargeese AA,Joshi SS,Krishnamurthy VN.Use of potassium ferrocyanide as habit modifier in the size reduction and phase modification of ammonium nitrate crystals in slurries.J Hazard Mater 2010;180:583-9.
[13]Sudhakar AOR,Mathew S.Thermal behavior of CuO doped phase stabilized ammonium nitrate.Thermochim Acta 2006;451:5-9.
[14]Borman S.Advanced energetic materials emerge for military and space applications.Chem Eng News 1994;72:18-21.
[15]Klyakin GF,Taranushich VA.Phase stabilization of ammonium nitrate with binary additives consisting of potassium nitrate and complexone salts.Russ J Appl Chem 2008;81:748-51.
[16]Kumar P,Joshi PC,Kumar R.Thermal decomposition and combustion studies of catalyzed AN/KDN based solid propellants.Combust Flame 2016;166:316-32.
[17]Kumar P,Joshi PC,Kumar R.Thermal decomposition and kinetics studies of AN,KDN and their mixtures with and without catalysts.Cent Eur J Energ Mater 2017;14(1):184-200.
[18]Rao DCK,Yadav N,Joshi PC.Cu-Co-O nano-catalysts as a burn rate modifier for composite solid propellants.Defence Technol 2016;12:297-304.
[19]Golo fit T,Maksimowski P,Biernacki A.Optimization of potassium dinitramide preparation.Propellants,Explos Pyrotech 2013;38:261-5.
[20]Berger BP,Matheiu J,Folly P.Alkali-dinitramide salts part 2:oxidizers for special pyrotechnic applications.Propellants,Explos Pyrotech 2006;31:269-77.
[21]Cliff MD,Smith MW.Thermal characteristics of alkali metal dinitramide salts.J Energetic Mater 1999;17(1):69-86.
[22]Lei M,Zhang Z,Kong Y,Liu Z,Zhu C,Shao Y,Zhang P.The thermal stability of potassium dinitramide.Part 1.Thermal stability.Thermochim Acta 1999;335:105-12.
[23]Lei M,Liu Z,Kong Y,Yin C,Wang B,Wang Y,Zhang P.The thermal stability of potassium dinitramide.Part 2.Mechanism of thermal decomposition.Thermochim Acta 1999;335:113-20.
[24]Yin C,Liu Z,Kong Y,Zhao F,Wang Y,Lei M,Luo Y,Zhang P,Shao Y,Li S.Thermal decomposition of potassium dinitramide at elevated pressures.In:Brill TB,Ren WZ,Yang V,editors.Progress in Astronautics and Aeronautics:Solid propellant chemistry,combustion,and motor interior ballistics,AIAA,vol.185;2000.p.425-37.
[25]Christe KO,Wilson WW,Petrie MA,Michels HH,Bottaro JC,Gilardi R.The dinitramide anion,N(NO2)2-Λ.Inorg Chem 1996;35:5068-71.
[26]Santhosh G.Synthesis and evaluation of energetic materials,Ph.D.Thesis.India:Mahatma Gandhi University;2005.p.83-123.Publication Number:AAI3251372.
[27]Hwang BJ,Santhanam R,Liu DG.Characterization of nanoparticles of LiM-n2O4synthsized by citric acid sol-gel method.J Power Sources 2001;97-98:443-6.
[28]Hao Y,Lai Q,Lui D,Xu Z,Ji X.Synthesis by citric acid sol-gel method and electrochemical properties of Li4Ti5O12anode material for lithium-ion battery.Mater Chem Phys 2005;94:382-7.
[29]Porta P,Dragone R,Fierro G,Inversi M,Jacono ML,Moretti G.Preparation and characterization of cobalt-copper hydroxysalts and their oxide products of decomposition.J Chem Soc Faraday Trans 1991;88(3):311-9.
[30]Deraz NM,Fouda MMG.Fabrication and magnetic properties of cobalt-copper nano-composite.Int J Electrochem Sci 2013;8:2682-90.
[31]Manigandan R,Giribaru K,Suresh R,Vijaylakhsmi L,Stephen A,Narayanan V.Cobalt oxide nanoparticles:characterization and its electrocatalytic activity towards nitrobenzene.Chem Sci Trans 2013;2(S1):S47-50.
[32]Tur KM,Ippolito SJ,Sabri YM,Tardio J,Bhargava SK.Investigations of oxides of cobalt and copper as potential catalysts for mercury oxidation,Paper No.1254.CHEMECA,September 28-October 1,2014;Perth,Australia.
[33]Porta P,Dragone R,Fierro G,Inversi M,Jacono ML,Moretti G.Copper-cobalt hydroxysalts and oxysalts:bulk and surface characterization.J Mater Chem 1991;1(4):531-7.
[34]Vyazovkin S,Burnham AK,Criado JM,Perez-Maqueda LA,Popescu C,Sbirrazzouli N.ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data.Thermochim Acta 2011;520:1-19.
[35]Kondrikov BN,Annikov VE,DeLuca LT.Combustion mechanism of ammonium nitrate based compositions,in 29th international annual conference of ICT,June 30-July 3,1998;Karlsruhe,Germany,p.163-171.
[36]Boyars C,Holdan JR,Bertram AL,Minol IV.A new explosive composition containing ammonium nitrate-potassium nitrate solid solution,Part I.Technical Report No.AD-763332.White Oak,Maryland:Naval Ordnance Laboratory;1973.
[37]Chuiko SV,Sokoloskii FS.Effect of small additives on the combustion of composite solid propellants.Russ J Phys Chem B 2009;3(6):926-35.
[38]Mahanta AK,Pathak DD.HTPB-polyurethane:a versatile fuel binder for composite solid propellant.In:Zafar F,editor.Polyurethane;2012.p.229-62.https://doi.org/10.5772/47995.InTech.
[39]Hosseini SG,Eslami A.Thermoanalytical investigation of relative reactivity of some nitrate oxidants in tin-fueled pyrotechnic systems.J Therm Anal Calorim 2010;101:1111-9.
[40]Oliveberg M,Tan Y,Fersht AR.Negative activation enthalpies in the kinetics of protein folding.Proc Natl Acad Sci 1995;92:8926-9.
[41]Vyazovkin S.A time to search: finding the meaning of variable activation energy.Phys Chem Chem Phys 2016;18(28):18643-56.
[42]Mozurkewich M,Benson SW.Negative activation energies and curved Arrhenius plots.1.Theory of reactions over potential wells.J Phys Chem 1984;88:6429-35.
[43]Mozurkewich M,Benson SW.Negative activation energies and curved Arrhenius plots.2.OH+CO.J Phys Chem 1984;88:6435-41.
[44]McCarty J.Introduction to Differential Scanning Calorimetry(effects of self heating on kinetics).Phoenix,Arizona,USA:TLN Systems Inc;2002.http://www.TechLearningNow.com.
[45]Vargeese AA,Mija SJ,Muralidharan K.Effect of copper oxide,titanium dioxide,and lithium fluoride on the thermal behavior and decomposition kinetics of ammonium nitrate.J Energetic Mater 2014;32(3):146-61.
杂志排行
Defence Technology的其它文章
- Effect of body material and temperature variation on the performance of the time delay pyrotechnic compositions
- Dynamic and ballistic impact behavior of biocomposite armors made of HDPE reinforced with chonta palm wood(Bactris gasipaes)microparticles
- Pitting and stress corrosion cracking studies on AISI type 316N stainless steel weldments
- Virtual ballistic impact testing of Kevlar soft armor:Predictive and validated finite element modeling of the V0-V100probabilistic penetration response
- Optimizing submerged arc welding using response surface methodology,regression analysis,and genetic algorithm
- Effect of functional composite coating developed via sulphate and chloride process parameter on the UNS G10150 steel for structural and wear mitigation in defence application
