Effect of functional composite coating developed via sulphate and chloride process parameter on the UNS G10150 steel for structural and wear mitigation in defence application
2018-07-06OjoSundayIsaacFayomi
Ojo Sunday Isaac Fayomi
a Department of Mechanical Engineering,Covenant University,P.M.B 1023,Ota,Ogun State,Nigeria
bDepartment of Chemical,Metallurgical and Materials Engineering,Tshwane University of Technology,P.M.B.X680,Pretoria,South Africa
1.Introduction
The advent of manufacturing technology over time has signi ficantly help in addressing major complication in sectors such as marine,mecha-electronics system,aerospace,nuclear space,shipbuilding engineering and most especially in defence[1,25].During the world war,composite particulates and materials had been used in the fabrication of artifact and sophisticated component which has light weight with better similar performance with metals for improved corrosion resistance,betterhardness and wear resistance.
The emergence of electroplating in the past decade has enabled several new and exciting incorporation of novel surface-hard and performance improved composite alloy by electrolytic codeposition route[2].Zinc coating has produce an excellent mechanical and electrochemical resistance properties for steel protection in industrial and engineering applications[1-3].However their less becoming popular are due to poor reaction in atmospheric environment and lesser life-span from thermal and mechanical fallout[3,4].Consequently,tremendous approaches from literature survey to improve on this limitation are being made on the use of metalreinforcement composite combination and manufacturing process variables[5].Lately the intention on the choice of composite particulate is due to their significant constituent of solid grains and the novel attention such properties gives in advanced materials[6,7].
The suspended co-deposition of metal composite such as SiO2,Cr2O3,TiO2,ZrO2,Al2O3SnO2CeO2and ZnO had been established to of fer vital functional individual properties[8-12].However[13,14]attested that for excellent application especially in high temperature performance,high surface modifications are required and incorporation of high temperature composite particle had been proven to provide such safeguard.Regrettably,results on modified binary composite alloys through this route are prone to possess possible limitation for high temperature performance, wear vulnerability and electrochemical defects[15-17].
Furthermore the control of formulated variable for advance materials also has been crucial consideration in metal matrix composite co-deposition[18].The wear deformation characteristics,corrosion resistance,thermo-mechanical stability and tribooxidation behavior of binary alloy composite coating have been reported to give stability only at ambient environment[19,20].To the best of our knowledge from literature,there were no works done on quaternary particle reinforced using electrolytic route on Al,TiO2and SnO2especially when subjected to the heat-treatment and wear behavior for multi-facial application in single system.Although,there individual characteristics are known for exceptional properties on zinc blend[21].In the light of this,since bath formulation and process parameter constitute to the kind of coating properties,we’ve attempted a successfully sulphates produced quaternary alloy in our previous work[22]in a view to improve the tribological and poor thermal stability of the binary modified composite.
Our aim in this study is to develop a suitable electrolytic bath from chloride and sulphate framework,fabricate and compare its performance on the basis of thermal stability;improve micromechanical properties,and excellent structural properties with stable interfacial characteristics for extended application in defence engineering.The wear and structural properties of the codeposited alloy was evaluated using sliding wear tester,and their morphological crystal structure/topography was characterized by means[AFM,SEM/EDS and OPM].The phase patterns were examined with the help of X-ray diffractometer(XRD)and Raman.
2.Experimental procedure
2.1.Preparation of substrates
Mild steel was commercially sourced and sectioned into(40mm×20 mm x 1 mm)sheet as cathode and 99.5%zinc plate of(30mm×20 mm x 1 mm)were prepared as anodes.The initial surface preparationwas performed with finer grade of emery paper as describedin our previous studies[1,9].The samplewereproperly cleaned with sodium carbonate,pickled and activated with 10%HCl at ambient temperature for 10 s then followed by instant rinsing in deionized water.The mild steel specimens were obtained from metal sample site in Nigeria.The chemical composition of the sectioned samples is shown in Table 1 as obtained from spectrometer analyzer(see Table 2).
2.2.Processed composition
The electrolytic chemical bath of Zn-Al-Sn-Ti fabricated alloy was performed in a single cell containing two zinc anode and single cathode electrodes.The distance between the anode and the cathode is 15mm.Before the plating,All chemical used are analar grade and de-ionized water were used in all solution admixed.The bath was preheated at 40°C.The processed parameter and bath composition admixed used for the different coating matrix is as follows Zn 75 g/L,Al 30 g/L,KCl 50 g/L,ZnCl 75 g/L,Boric acid 10 g/L,SnO2 7 g/L,TiO2 7 g/L,pH 4.8,time,20min and tempt 40°C.The choice of the deposition parameter is in line with the preliminary study from our previous work[1].
The prepared zinc electrodes were connected to the rectifier at varying applied potential and current density between 0.3 V at 2 A/cm2for 20 min.The distance between the anode and the cathode with the immersion depth were kept constant as described by Ref.[18].The fabricated were rinsed in distilled water and samples air-dried. Portion of the coating were sectioned for characterization.
2.3.Characterization of coating
The structural evolution of the deposited composite coating alloy was characterized with VEGA TESCAN Scanning electron microscope equipped with EDS.The phase change was verified with XRD.Micro-hardness studies were carried out using a Diamond pyramid indenter EMCO Test Dura-scan micro-hardness testers at a load of 10 g for a period of 20 s.The average microhardness trend was measured across the coating interface in an interval of 2 cm using screw gauge attached to the Dura hardness tester.
2.4.Friction and wear tests
The friction and wear properties of the deposited quaternary fabricated alloy were measured using CERT UMT-2 tribological tester at ambient temperature of 25°C.The reciprocating sliding tests was carried out with a load of 5 N,constant speed of 5 mm/s,displacement amplitude of 2 mm in 20 min.A Si3N4 ball(4mm in diameter,HV50g1600)was chosen as counter body for the evaluation of tribological behavior of the coated sample.The dimension of the wear specimen is 2cm by 1.5 cm as prescribed by the specimen holder.After the wear test,the structure of the wear scar and film worn tracks are further examined with the help of scanning electron microscope couple with energy dispersive spectroscopy(VEGAS-TESCAN SEM/EDS).
2.5.Thermo/electro-oxidation test
Isothermal heat treatment(Direct fired furnace atmosphere)of Zn-Al-Sn-Ti composite coating was carried out at 600°C for 1h s to enhance the mechanical stability of the coated samples.
3.Results and discussion
3.1.Structural characterization
SEM/EDS of the as-received mild steel substrate are presented inFig.1.Morphology of fabricated Zn-Al-SnO2-TiO2alloy from sulphate and chloride composite admixed are presented in Fig.2(a)and(b)respectively.From careful comparative study of this microstructure,both deposits with 7 g in 0.3V revealed uniform distribution and a small micro particle inter-link around the major metal lattice.Both produced structures possess adorable and compact grains especially with alloy fabricated from sulphate rich bath.The existence of SnO2and TiO2embedded particles on zinc rich could be observed to influence the micro-crystallites grains without crack initiation seen.The excellent quality of deposit from sulphate produced coating is attributed to the proper control of deposited parameter which is in line with the reported study by Ref.[1]that the nature of the distributed of the crystal on microstructure can be link to particles migration and process parameter.

Ta ble 1Spectrometer chemical composition of mild steel used(wt.%).
Though[2],reported that composite incorporation increased visible particle content in the metal matrix which might result to strong coating adhesion and mass loss reduction as observed in both compared coating but it is important to mention that the stability of coating are sometimes instigated by additive agents,surfactant,brightening agent and carriages.In all the participation of the additive agent could be seen in this study to alter the functional surface properties of sulphate deposited coating than the chloride produced alloy.The porous free and lustrous surface of the sulphate fabricated coating as against the chloride coating could also be trace to the interfacial and absorbing reaction rate capacity of the electrolyte developed on steel plate.Since proper bath conditioning and formation are promising factor in formation of an in situ interfacial/intermetallic phase’s appearance as described by Refs.[22,23].

Table 2Itinerary bath composition of quaternary Zn-Al-Sn-Ti alloy in sulphate and chloride system.

Fig.1.SEM/EDS of mild steel substrate.
3.2.Atomic force microstructural studies
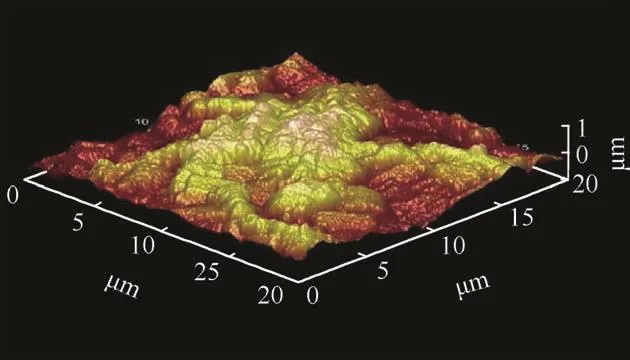
Fig.3.AFM of Chloride coating.
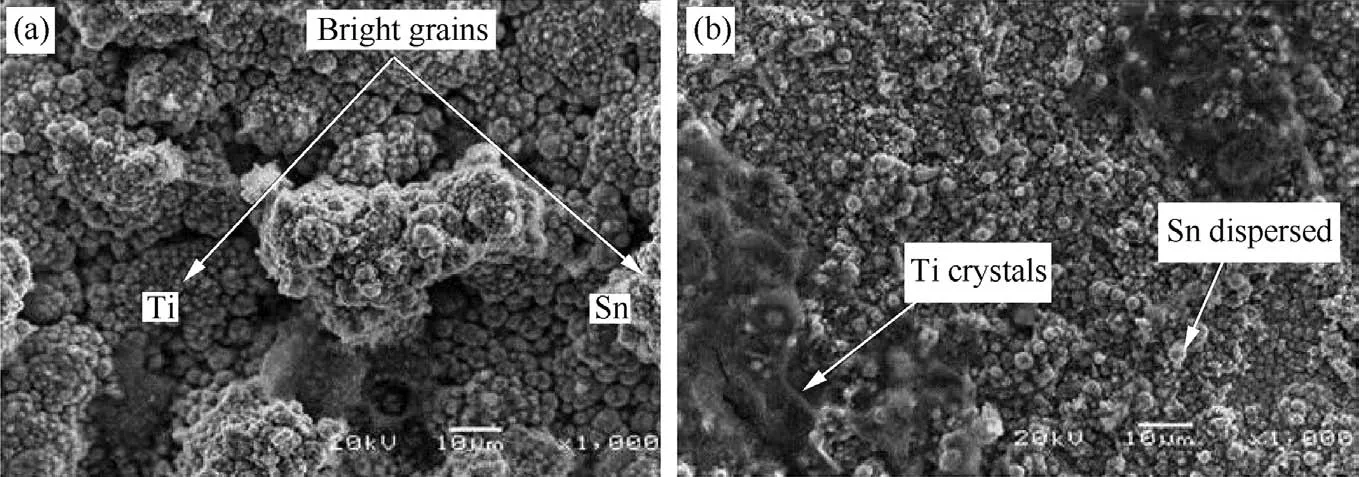
Fig.2.SEM/EDS Spectra showing the Surface Morphology of Zn-Al-7Sn-Ti-0.3V:(a)Sulphate and(b)Chloride Deposition.
Figs.3 and 4 shows the 3D atomic force micrographs image of co-deposited chloride and sulphate structures of Zn-Al-7Sn-Ti-0.3 V alloy with 7 g SnO2 and TiO2 concentration.Both coatings show good topography and distribution in stable form.From all indication,the distributions of the composite particle within the metal matrix were even with the grains.The vast buildup of the crystallites and topography are attributed to the perfect interfacial reaction and dissolving mechanism of sulphate ion rate in response to their weight fraction which was in agreement with the attested results reported by Popoola,Fayomi and Popoola,(2012),Xu et al.(2008)that increasing the concentrations of composite do influence deposition rate and diffusion of the particle into the steel plate.
On the contrary to the speculated results,the similarity in the produced coatings on their physical characteristics are obvious than the likely differences.Firstly,good intermetallic homogeneity at all level of deposition was observed in both chloride and sulphate alloys.The coating formed through Zn-Al-7Sn-Ti-S-0.3Vsulphate coating particles possess exceptional smoother surface and good adhesion than the fabricated chloride coating which exhibit little coarse-grained structure.This observation means that crystallites might take place as results of induced bath process leading to difference in uniform crystal growth.Another view by Ref.[24]affirmed that the effect of the perfect growth and nodule form can be described in term of deposition kinetics of ion diffusion in relation to the volume of particulate in the bath and also charge transfer reaction which is proportional to the diffusion and growth rate.
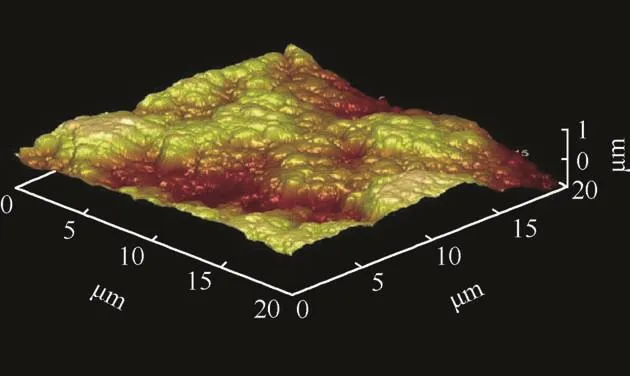
Fig.4.AFM of Sulphate coating.
3.3.XRD/Raman studies
Figs.5 and 6 present the intermediate dispatched composite phases observed from XRD patterns for Zn-Al-7Sn-Ti-Cl-0.3Vand Zn-Al-7Sn-Ti-S-0.3Vcomposite coatings.The main peaks found are Zn,Zn2AlSn,Zn7AlTi,ZnOTiAl3 at 2θBraga angle intensity of=(38.12°,43.10°and 70.22°)for chloride quaternary phase.From observation,remarkable effect of the chloride composite induced coatings identifies are a results of new orientation of the metal particles precipitate.More so,it was observed that Sn content had satisfactory phase above the Ti in their intermetalic patterns,this contributes to the strong character and absorbing tendency of the individual composite[25,26].ForZn-Al-7Sn-Ti-S-0.3V phase compositionpattern,visible Zn-Al as a majorconstituent within the interface is present.The main peaks for the multi-phase coating occur at 2θ=(38.12°,and 43.20°).The zinc rich exist within 2θ=(38.22°,39.98°,and 68.2°).More so,exclusive broad phase consistence three intermetallic deposit were notice at 2θplane between(38.50°-85.50°).With these phases,indication of complete dissolution of the additive participation as against the chloride deposited quaternary phase was seen,this observation is in line with study by Ref.[24].More so,some of the phases with peaks of Zn3Al7.Ti4 for sulphate produced alloy are anticipated to enhance the build-up of strong intermetallic precipitates thereby producing fine grain structure which are tenable to super hard characteristics.Some traceable intermetallic and interfacial growth observed are ZnAl,Zn4TiAl2,Zn2OAlSn,Zn3AlTi as presented in Fig.6.In comparison from literature studies,the height of any peak is considered as an indication of the quantity of its phase in the deposit[9].Hence,an observed multi-phase intensity of sulphate alloys could be seen to be vividly evident than that produced coating from chloride bath possibly due to reactivity of the embedded additive in relation to its process parameter.
More so,it is noteworthy to say from the variance of diffraction pattern that bath formulation and induced particulate especially Al2+,Ti2+and Sn2+has a strong strengthening effect on the deposit which is expected and attested to by Ref.[10].
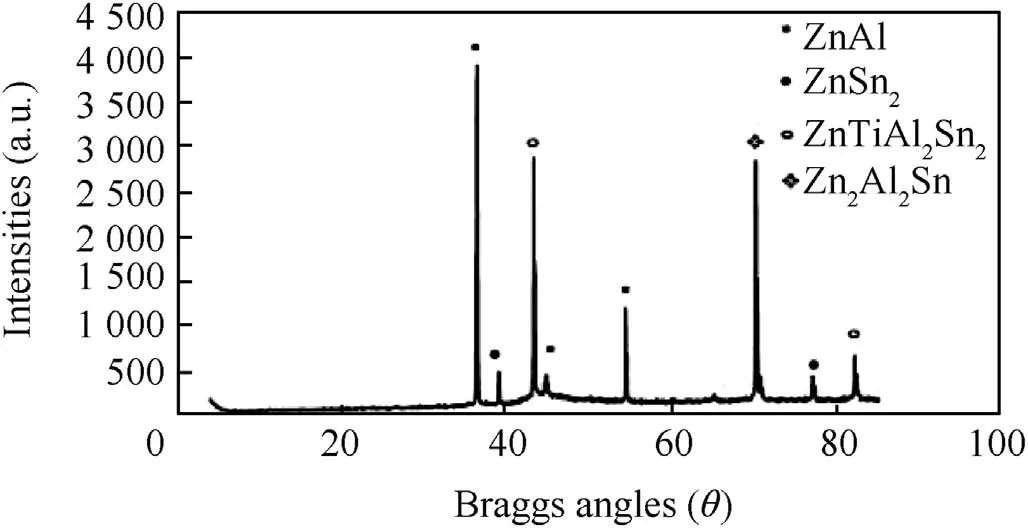
Fig.5.Solid X-Ray diffraction pro file for Zn-Al-7Sn-Ti chloride.
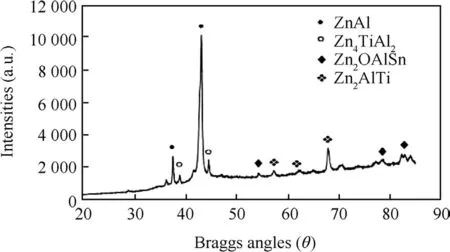
Fig.6.Solid X-Ray diffraction pro file for Zn-Al-7Sn-Ti sulphate.
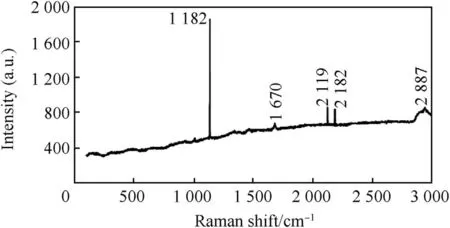
Fig.7.Raman spectrum for chloride deposition.
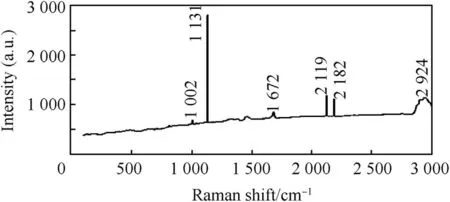
Fig.8.Raman spectrum for sulphate deposition.
Considering the Raman spectra plot of Zn-Al-7Sn-Ti-Cl-0.3V(Fig.7)and Zn-Al-7Sn-Ti-S-0.3 V(Fig.8)composite deposited layer,sulphate fabricated also show a good intensity over the chloride produced coatings.Of Couse the results follows the same trend as XRD pattern with intensity of about 1002-2924cm-1along the deposited interface as shown in Fig.8.The dispatched composite could be seen to have provided a fine inhibitive preferred orientation expected within the matrix.
For chloride 1210 aμ,210 aμ,and 210 aμ,at about 1132 cm-1,2120 cm-1,and2183 cm-1respectively were observed with maximum intensity of 12000aμ.Hexagonal or orthorhombic structures with presence of ZnTiAl3.Sn2buildup were seen to produce a boast for both fabricated coatings.
3.4.Physical characteristics
The composition of Zn-Al-SnO2-TiO2 sulphate and chloride bath matrixes with their corresponding results attained after codeposition were presented in Table 4(see Table 3).The deposition time and voltage were varied during deposition to achieve good physical characteristics.
The physical properties examined after the deposition are the coating thickness,weight gain and coating per unit area.The effect of particles incorporated was seen to significantly alter the physical behaviour of the produced quaternary coatings in term of coating thickness,weight gain and coating per unit area.There are variance in weight gain and coating per unit area at different formulated bath.At fixed time of deposition of 20 min and incorporated composite additive for 7g,the weight gain for sulphate alloy was 0.3658g as when compared to chloride coating with 0.3550g a difference of 0.0108 g.
It is noteworthy to mention that the distribution and increases in the gain weight ahead of the chloride coatings may be as results of interfacial response and absorption of sulphate ions and its activities with incorporated composite materials.For the coating thickness,the same trend was observed with the results obtained for sulphate coating having double thickness value of chloride alloy.Coating thickness of the sulphate fabricated coating was 300μm and for chloride produce quaternaryalloy is 138μm.This behaviour often occuras a results of many factors such as agglomeration of the particulates at the interfaces,fallout after optimum buildup of the crystal at the coating interfaces and the kinetic adsorption and reactivity of the bath formulation.Moreso[11],attested that increases or reduction in incorporated composite in the deposition bath often not show a magnificent coating thickness difference in some case but the activities and contribution of other additive agent and process variable often cause change in surface active or type of coating produced.Hence,sulphate ion in this study was seen to alter a reduction in more number of the active site thereby given a stable migration of particulate to strengthen the active surfaces.
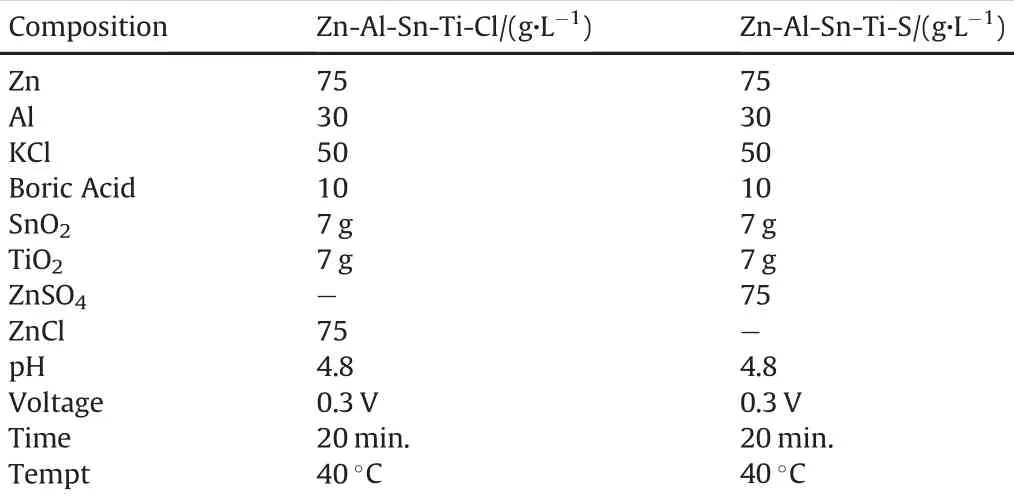
Table 3Summarized bath formulations for comparative matrixes.
3.5.Wear rate evaluation
Fig.9 shows variation of the wear rate as a function of time across the coating system of Zn-Al-SnO2-TiO2in sulphate and chloride fabricated coatings.For both compared coating series a remarkable improvement was obtained for the coated system as a result of improved crystal and structural modification as against the control sample.Noticeable severe wear loss was observed for the as-received sample with approximately 2.351 g/min;although this is indeed expected due to lack surface active resilient properties.For the composite strengthen alloys lower plastic dislocation were achieved with wear loss of 0.001 g/min for Zn-Al-SnO2-TiO2 in sulphate system and 0.002 g/min for chloride fabricated coatings.Sulphate produced coating seems to give more wear resilient than chloride bath coatings with attained difference of 0.001 g/min reason for this improvement might be due to the better stability as a result of good movement of particle size distribution and uniformity at the interface.It is evident that Al/SnO2/TiO2strengthening phase lead to the remarkable increase in anti-wear resistance as against the as-received sample.More so,it can be said that the functional activities of multi-grains particulate,additive agents and composite-particle in relation to the ionic migration enhance the change in microstructural of the coatings which help to retard the deformation trend that could have occur with produced coatings.
This observation is in line with[17]report that incorporated solid particle promote wear resistance.Studies by Ref.[12]also revealed that the wear loss of a coating is a function of poor adhesion and mechanical instability of the fabricated coatings.The excellent progression of sulphate alloy is as a result of the activities and the mechanism behind the zinc-rich bath formation.Figs.10 and 11 shows the variation of friction coefficients with sliding time and sliding velocity of the deposited Zn-Al-7Sn-Ti-0.3 V in both sulphate and chloride bath matrix.The effect of functional composite incorporated could be seen to drastically reduced the frictional coefficient behaviour of the two coating system than when compare to the as-received samples[5].Attested that lower friction coefficient and linear wear behaviour reduced majorly through micro-structurally modified ceramics and composite.Although,phenomena of wear stability is attributed to the contribution of the metal matrix composite,their individual solid grain and the process parameter that provide the stable films layer[22].The wear improvement of the sulphate alloy both against the time and sliding velocity can be observed to give lower friction coefficient compare to chloride produced coatings.It is essential to mention that the wear life difference was observed after 200sec.Due to irregularity observed within the coatings.

Table 4Comparison of Zn-Al-Sn-Ti-S and Zn-Al-Sn-Ti-Cl depositions.
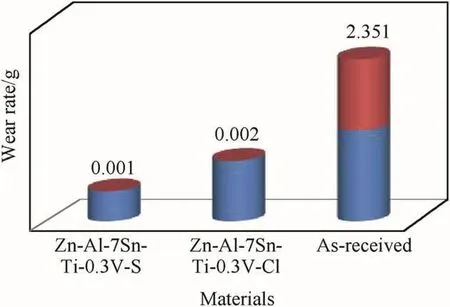
Fig.9.Variation of compared wear rate with time from sulphate and chloride deposition.
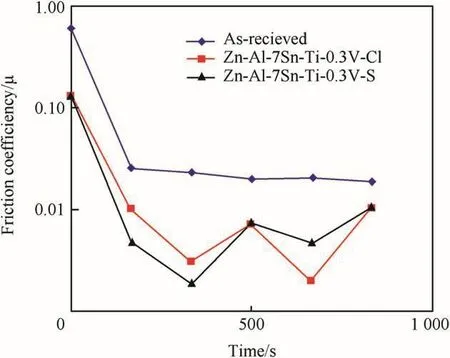
Fig.10.Variation of wear friction co-efficient against time(chloride and sulphate deposition).
Fig.12 show the worn scar surfaces of the control subtract with grooves,debris and severe plastic deformation.Figs.13 and 14 presents Zn-Al-7Sn-Ti-0.3V degree of deformation in both sulphate and chloride coating.
Comparing the coatings,it can be seen from the scars,that little degree of plastic deformation,massive grooves,and pits was observed in both coatings with embedded solid grain visibly seen along the wear track interface.With less pit formation and unseen stress within the interface of the mild steel coatings layer,the strong adhesions of the processed fabricated coating that resist dislocation are justified by the induced film particulate.More so,the anti-wear propagation of the deposited alloy was due to the interference of the composite matrix and the pool of additive agent activities to strengthening intermediate phase expected to cause wear resistance.Although sulphate coated alloy produce less deformation,however,the nature of wear deformation is a function of the conditioning properties.
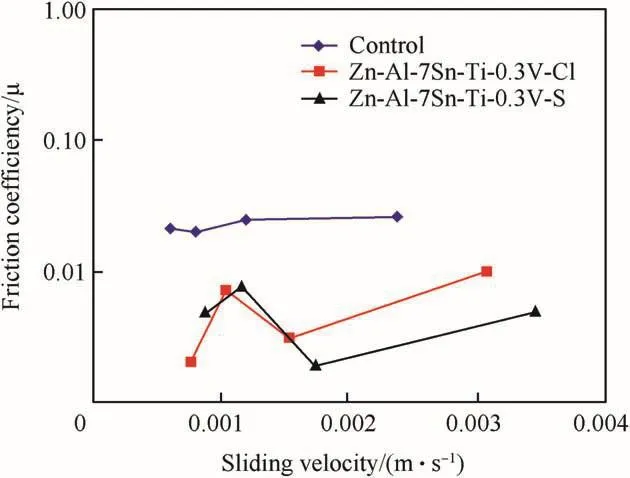
Fig.11.Variation of wear friction co-efficient against sliding velocity(chloride and sulphate deposition).

Fig.12.SEM image of the wear scar of the mild steel substrate.
3.6.Microhardness properties
Fig.15 present the micro-hardness(HVN)plot and variable trend of the deposited coatings for Zn-Al-7Sn-Ti-0.3V alloy in both sulphate and chloride bath.The sulphate fabricated hardness coating increased geometrically in hardness from 33.4 HVN to approximately 337 HVN.For chloride produced coating,the microhardness is approximately 299 HVN;i.e a difference of 38 HVN among the fabricated alloys.The observed improvement in the sulphate produced coatings is in par with the results and description attested from Ref.[18]studies.It is good to mention that often when significant improvement is attained they are usually attributed to the formation of multi-facial adhesive phases.The reason for the slight decrease in the micro-hardness properties might not be as a result of over-loading of electrolyte as attested by Ref.[11]but the relative activities of additive agents which determine the migration of ion and adhesion kinetic of any deposits.Although,operating condition is a major factor for enhance structural properties which could also facilitate improved hardness characteristics[19,20].Hence,microstructural evolved in coating depends on the processing parameters which are principal to the buildup of crystal and this is in line with descriptive studies made by Ref.[22].
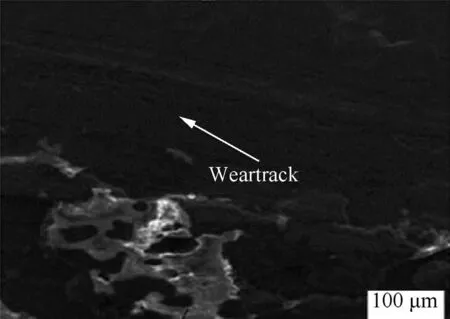
Fig.13.SEM/EDS spectra showing the surface morphology of wear track(Zn-Al-7Sn-Ti-S-0.3 V deposition).

Fig.14.SEM/EDS spectra showing the surface morphology of wear track(Zn-Al-7Sn-Ti-Cl-0.3 V deposition).
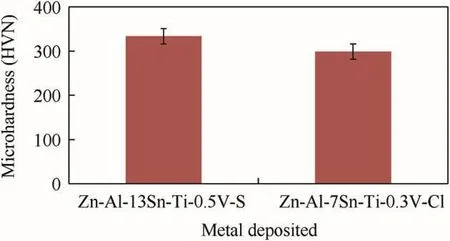
Fig.15.Comparative microhardness chart for Zn-Al-Sn-Ti coatings.
3.7.Thermal studies
Quaternary thermally treated of Zn-Al-7Sn-Ti-0.3V alloy in both sulphate and chloride bath are presented in Fig.16.The hardness characteristics of the deposited quaternary coating before and after heat treatment were access to evaluate their thermal-oxidation behaviour in 600°C.In general,both coatings show preferred response and thermally stable character expected after microhardness indentation except a little fallout of scale at the interface.Sometime mechanical behaviour due to the thermal treatment and porosity within the alloy surface could result in hardness propagation.Few literatures also attested that the compression stress could significantly improve the micro-hardness when it is much less than the ultimate strength of coating but[22]reported that the defect such as porosity,macro-particle at the alloy surface willhave detrimental effect on the mechanical properties.
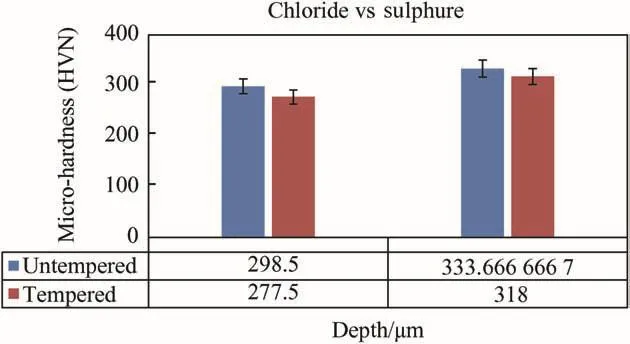
Fig.16.Hardness tempered trend of Zn-Al-Sn-Ti(sulphate&chloride depositions).
4.Conclusion
In summary,this work reported a Zn/Al/SnO2/TiO2sulphate and chloride coating in a single-bath via an electrolytic co-deposition technique with comparative analysis of the developed coating system.It was confirmed that zinc rich bath with homogeneously dispersed Al,TiO2and SnO2particles from a sulphate and chloride system significantly impact on the structural build-up of the based metal resulting to reduced plastic deformation upon UNGS 10150 for defence application.Produced coating show good uniform growth,perfect crystal and bright metal matrix composite deposit were obtained.The incorporation and conditioning of the metal composite were seen to greatly improve the thermal stability,microhardness,and wear resistance of both fabricated quaternary oxide coating especially from sulphate electrolyte.No doubt that the impact of the surface active and surface hard characteristics of the developed composite coating will help to improve the fatigue and plastic delimitation found over a short UNGS 10150 steel.
Acknowledgements
This material is based upon work supported financially by the National Research Foundation.The equipment support by Surface Engineering Research Centre(SERC)Tshwane University of Technology,Pretoria is deeply appreciated.The funding received from Covenant University for open access publication is acknowledged.
[1]Chuen-Chang L,Chi-Ming H.J Coat Technol Res 2006;3:99.
[2]Mo JL,Zhu MH,Lei B,Leng YX,Huang N.Depos Phys Vap Deposition Wear 2007;263:1423.
[3]Fayomi OSI,Popool API.Int J Electrochem Sci.2013;8:11502.
[4]Hongmark S,Hedenqvist P,Jacobson S.J Surf Coat&Tech 1997;90:247.
[5]Zum Gahr KH.Wear 1996;200:215.
[6]Volinsky AA,Vella J,Adhihetty IS,Sarihan VL,Mercado L,Yeung BH,Gerberich WW.Mat Res Soc 2001;649:6.
[7]Popoola API,Fayomi OSI,Popoola OM.Int J Electrochem Sci.2012;7:4860.
[8]Xu R,Wang J,Guo Z,Wang H.J Rare Earth 2008;26:579.
[9]Abou-Krisha MM,Assaf FH,El-Naby SA.J Res Coat Technol 2009;6:391.
[10]Yang G,Chai S,Xiong X,Zhang S,Yu L,Zhang P.Trans Nonferr Met Soc China 2012;22:366.
[11]Vathsala K,Venkatesha TV.Zn-ZrO2J Applied Surf Sci 2011;257:8929.
[12]Wang TG,Jeong D,Liu Y,Lyengar S,Melin S,Kim KH.J Surf Coat Technol 2012;206:2638.
[13]Hammami O,Dhouibi L,Bercot P,Rezrazi EM,Triki E.Int J Corros Sci 2012;8:1.
[14]Popoola API,Fayomi OSI,Popoola OM.Int J Electrochem Sci.2012;7:4898.
[15]Praveen BM,Venkatesha TV.Appl Surf Sci 2008;254:2418.
[16]Kazimierezak H,Ozga P.Surf Sci 2013;607:33.
[17]Blejan D,Muresan LM.Mat and Corrosion 2012;63:1.
[18]Sancakoglu O,Culha O,Toparli M,Agaday B,Celik E.J Mater Des 2011;32:4054.
[19]Xuli X,Igor Z,Joseph RM.J Mat Sci Tech 2009;209:2632.
[20]Fayomi OSI,Abdulwahab M,Popoola API.J Ovonic Res 2013;9:123.
[21]Zhang W,Liu W,Wang C.J Eur Ceram Soc 2002;22:2869.
[22]Fayomi OSI,Popoola API,Aigbodion VS.J alloys and Comp 2014;617:455.
[23]Rahman MJ,Sen SR,Moniruzzaman M,Shorowordi KM.J Mech Eng 2009;40:9.
[24]Arici M,Nazir H,Aksu A.J Alloys Comp 2011;509:1534.
[25]P.Nieslony,P.Cichosz,G.M.Krolczyk,S.Legutko,D.Smyczek,M.Kolodziej,Measurement 78 129-137.
[26]Nieslony P,Krolczyk GM,Zak K,Maruda RW,Legutko S.Precision engineering.J Inter Soc Precis Eng Nanotech 2017;47:104-10.
杂志排行
Defence Technology的其它文章
- Effect of body material and temperature variation on the performance of the time delay pyrotechnic compositions
- Catalytic effects of Cu-Co*on the thermal decomposition of AN and AN/KDN based green oxidizer and propellant samples
- Dynamic and ballistic impact behavior of biocomposite armors made of HDPE reinforced with chonta palm wood(Bactris gasipaes)microparticles
- Pitting and stress corrosion cracking studies on AISI type 316N stainless steel weldments
- Virtual ballistic impact testing of Kevlar soft armor:Predictive and validated finite element modeling of the V0-V100probabilistic penetration response
- Optimizing submerged arc welding using response surface methodology,regression analysis,and genetic algorithm
