原发干燥综合征肾脏损害临床病理特点及治疗进展
2018-05-03陈丽萌
王 婧,陈丽萌
中国医学科学院 北京协和医学院 北京协和医院肾内科,北京 100730
ActaAcadMedSin,2018,40(2):268-278
原发干燥综合征是一种慢性炎症性自身免疫性疾病[1],其病理基础是自身免疫性上皮炎,表现为导管周围的灶性淋巴细胞浸润,主要累及唾液腺、泪腺等外分泌腺体,也可导致肾小管、肺细支气管和肝脏小胆管等内脏上皮组织炎症损伤[2]。肾脏是干燥综合征的重要靶器官之一,肾脏损害呈现非特异性,肾小管间质和肾小球均可受累[3- 4]。本文主要综述近年原发干燥综合征肾脏损害临床病理特点及治疗的进展。
原发干燥综合征肾脏受累的概况
干燥综合征肾脏受累发生率差异较大,文献报道在0.3%~33.5%[5- 16],而且具有较显著的地区差异(表1)[5- 23]。根据美国Mayo诊所的资料,1967至2007年诊断原发干燥综合征患者7276例,其中24例因显著肾脏受累接受肾穿刺活检,仅占0.3%[11]。大多数欧洲的资料提示其发生率为5%~14%[6- 10,15- 17],干燥综合征肾损害以小管间质受累为主,大多起病隐匿,部分患者早期临床症状不明显。在前瞻性研究中,通过肾小管功能实验能筛查出更多早期亚临床患者,肾损害发生率较回顾性研究高[3]。Gouels等[15]将有临床意义的肾脏损害定义为:禁水试验后尿比重<1.010且尿pH>7,持续半年以上,伴或不伴低钾;肾结石或肾钙化;除外其他原因的Fanconi综合征;血肌酐>1.6 mg/dl或肌酐清除率<50 ml/(min·1.73 m2);尿蛋白>0.5 g/24 h,持续3个月以上;活动性尿沉渣(>10红细胞/高倍视野或红细胞管型);肾活检证实为肾小球肾炎和/或间质性肾炎;其回顾性研究中715例患者的肾损害发生率为4.9%。Both等[20]随机选取57例原发干燥综合征患者进行氯化铵负荷实验,发现完全远端肾小管酸中毒(血碳酸氢根<21 mmol/L,血阴离子间隙正常,伴有尿阴离子间隙阳性和尿酸化功能不全,并且除外药物、高钙血症等其他原因)比例仅5%,但不完全远端肾小管酸中毒(血碳酸氢根正常,氯化铵负荷试验阳性)的比例则高达25%。
而在住院患者中,因为住院的选择性偏倚,肾脏受累的比例明显升高,北京协和医院Lin等[12]2010年报道1985至2006年住院患者中,临床诊断为原发干燥综合征肾脏受累的患者比例高达33.5%(192/573),其中行肾穿刺活检的患者64例,占11.2%。另有研究显示,1993至2015年2770例原发干燥综合征住院患者中,临床表现为肾脏明显受累的患者比例降低21.0%(583/2770),接受肾穿刺活检的比例为5.7%[24]。过去10年收治患者增加,肾穿刺活检指征的变化可能是重要的原因之一。同时,住院患者肾损害的发生率与所住科室有密切关系,但这在各项研究中都少有涉及,可能带来偏倚。
干燥综合征肾小管间质损害
干燥综合征肾脏受累以肾小管间质为主,因干燥综合征肾脏严重受累而接受肾穿刺活检患者中,间质受累比例最高的是上海瑞金医院,达到80.5%[25],其次是美国梅奥诊所,为71.0%[11],而北京协和医院2010年报道的比例为33.0%[12],笔者最新的研究为41.8%[24]。患者肾脏病理表现为肾间质单核细胞、T/B淋巴细胞和浆细胞的灶性浸润,伴有不同程度肾小管萎缩和肾间质纤维化[8]。大多数患者起病隐匿、进展缓慢,逐渐出现肾功能损伤甚至终末期肾病[11,15],但也有少数病例以急性间质性肾炎起病[11,26- 27]。随着病情进展,多出现小分子蛋白尿、肾小管酸中毒和电解质紊乱,根据肾小管受累部位不同,分为远端、近端小管和集合管受累,此外,临床上也常表现为多部位同时受累,如混合性肾小管酸中毒。
远端肾小管酸中毒干燥综合征导致的肾小管酸中毒以远端肾小管酸中毒(distal renal tubule acidosis,dRTA)更为多见[11,15,28]。dRTA的病因是肾皮质集合管α闰细胞分泌H+功能障碍,酸化尿液能力降低。集合管主细胞的ENaC重吸收管腔中Na+,使得膜外呈负电位,在电位差驱动下,与之相邻的α闰细胞内Ⅱ型碳酸酐酶解离出游离H+,并通过管腔侧质子泵泵出。根据病变程度可以将dRTA分为完全型和不完全型两类[28- 29]。完全型dRTA表现为阴离子间隙正常的代谢性酸中毒,尿液pH>5.3;不完全型dRTA的患者血碳酸氢根水平正常,但在酸负荷条件下仍不能有效酸化尿液[30]。临床上通常用氯化铵负荷实验[31]或呋塞米-氟氢可的松实验[32]诊断不完全型dRTA。dRTA的临床表现主要包括代谢性酸中毒、低钾血症、骨质疏松和骨钙质沉着诱发的肾钙化和肾结石形成等。几项前瞻性研究提示干燥综合征患者发生dRTA的比例在5%~23%[6,8,19],大多数表现隐匿,仅为实验室检查结果异常,但也有少数临床症状明显,出现肌无力[33]、周期性软瘫[34]和骨软化症[35]。干燥综合征发生dRTA的影响因素还不明确。1999年,一项对78例原发干燥综合征患者的横断面研究显示发生合并dRTA组病程更长,血β2微球蛋白水平高,出现蛋白尿和高血压的比例较高[36];而一项近期研究则提示发生dRTA的患者中血清抗La抗体/B型干燥综合征抗体阳性的比例更高[20]。
也有干燥综合征导致继发性Gitelman综合征的报道。Gitelman综合征是常染色体隐性遗传病,由SLC12A3基因突变导致其编码的远曲肾小管上钠氯协同转运蛋白(Na-Cl cotransporter,NCC)失活,导致肾小管髓袢升支粗段盐重吸收能力丧失或重度降低,表现为低血钾、低血镁、代谢性碱中毒、低尿钙和肾素-血管紧张素Ⅱ-醛固酮系统活化[20],但血压正常或偏低。继发Gitelman综合征的本质是非遗传性的远端小管NCC结构和功能异常,目前报道的7例患者中[37- 40],2例行肾活检,1例表现为间质性肾炎[39]、1例为膜性肾病伴轻度肾小管间质损害[38]。免疫组织化学法观察到肾组织NCC表达下降,从外周血提取DNA进行遗传分析提示无SLC12A3变异,但在血清中能够检测出可与小鼠钠氯共转运子蛋白相结合的自身抗体[38],提示干燥综合征累及NCC是导致其继发性功能障碍的原因。
肾性尿崩症肾性尿崩症的病因是远端小管或集合管浓缩功能障碍,可能与主细胞管腔侧水通道蛋白或者基底侧的抗利尿激素受体功能受损有关[41]。患者的主要临床表现是多饮、多尿和夜尿增多,但大多症状较轻,需要行限水实验诊断[28]。几项较大样本的临床研究提示干燥综合征肾脏浓缩功能障碍的发生率为16%~28%[6,8,19](表1),而在合并肾脏受累患者中的发生率可以高达81.9%[25]。集合管浓缩功能障碍可能是干燥综合征肾脏损害的最早期表现,甚至可能出现在口眼干症状之前[8,25]。此外,有研究显示浓缩功能障碍和肾小管酸中毒较少同时发生,提示二者发病机制可能是相互独立的过程[19]。
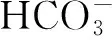
干燥综合征肾小球受累
干燥综合征累及肾小球相对少见。可能由于肾活检病例选择不同,各中心比例存在差异,如美国Mayo诊所为29%[11],欧洲为16.6%~48.6%[15,61],国内上海瑞金医院为19.5%[25],北京协和医院2010年报道为35.9%[12],笔者最近的资料为58.2%[24]。病理类型方面,国外文献报道首位是继发于冷球蛋白血症的膜增性肾小球肾炎,占干燥综合征接受肾活检患者总数的8%~30%[3],该类型在我国的比例较低,上海瑞金医院的数据是4.9%[24]。其病理机制主要由免疫复合物介导,自身免疫炎症反应破坏上皮细胞,导致自身抗原暴露,持续过度活化的B细胞和浆细胞产生大量自身抗体,二者结合形成免疫复合物并进入外周循环,与肾小球毛细血管袢内皮细胞结合后激活补体经典途径,并募集更多的炎症细胞浸润,诱导系膜区和内皮下的炎症损伤和修复[62]。系膜细胞的增殖和细胞外基质增加导致肾小球基底膜增厚形成“双轨征”,与毛细血管内冷球蛋白形成的血栓样沉积同为其标志性的病理特征[63],沉积的免疫球蛋白主要是可冷沉淀的单克隆IgMκ型类风湿因子,以及多克隆的IgG和IgA。干燥综合征肾小球损害的其他病理类型还包括膜性肾病(2.4%~15.6%)、IgA肾病(7.3%~21.0%)、局灶节段性肾小球硬化(1.5%~8.0%)、微小病变肾病(4.0%)、分类不明的增生性肾小球肾炎和偶发的新月体性肾炎[3]。此外,少数情况下干燥综合征与抗中性粒细胞细胞质抗体相关性血管炎肾脏损害也可合并存在,目前文献报道11例,均为抗髓过氧化物酶抗体阳性,表现为镜下血尿、蛋白尿(0.5~6.5 g/24 h)和急性肾功能损伤,病理可见毛细血管外增生性病变且免疫荧光染色阴性[64]。但肾小球损害的具体发病机制并不清楚,而IgA肾病与膜性肾病在我国占原发肾小球疾病的比例较高,干燥综合征与这些病理类型是合并存在亦或存在因果关系,还有待探究。与间质小管损害相比,肾小球受累的患者干燥综合征病程更长,血C3降低及发生冷球蛋白血症更多见,出现蛋白尿和活动性尿沉渣的比例也更高;但在多系统受累程度、自身抗体阳性比例、类风湿因子和高球蛋白血症等指标差异均无统计学意义,肾脏生存率更高[15]。
干燥综合征肾损害的治疗
目前,干燥综合征肾脏损害的治疗方案还缺乏大规模循证医学的证据,大多根据临床经验[4],对于肾功能损害和大量蛋白尿的患者治疗较为积极,糖皮质激素是首选的治疗,通常起始剂量0.8~1 mg/(kg·d)(换算为泼尼松剂量),大部分患者同时联合环磷酰胺治疗[15]。也有报道中等剂量糖皮质激素[0.5~0.75 mg/(kg·d)]联合环孢菌素或硫唑嘌呤的治疗方案。其中,环孢菌素作为钙调磷酸酶抑制剂,除了选择性抑制T淋巴细胞活化外,还可以通过促进足细胞骨架稳定性恢复的非免疫机制促使肾病综合征缓解[62],对肾小管间质损伤较轻的患者具有降低尿蛋白作用。近年还有抗CD20单克隆抗体(利妥昔单抗)为代表的B细胞清除靶向治疗的尝试[11,15]。多数患者对糖皮质激素联合免疫抑制治疗反应较好,例如Kidder等[61]报道25例接受肾活检的患者中,60%使用糖皮质激素,32%联合使用免疫抑制剂,随访3年的总体生存率为88%,肾脏生存率为93%,肾脏病变达到完全缓解(尿沉渣检查和血肌酐均正常)的比例为54%。Evans等[65]报道12例肾小管间质受累病例,均使用糖皮质激素联合霉酚酸酯治疗,观察到患者肾功能显著改善,血IgG水平明显降低。利妥昔单抗是抗CD20单克隆抗体,既往在干燥综合征中主要针对进展为B细胞淋巴瘤的病例[65],近年来其在系统性血管炎、系统性红斑狼疮肾损害、原发膜性肾病的成功应用而广受关注,已有将其用于干燥综合征多系统受累的尝试。临床研究结果提示,它对干燥综合征合并冷球蛋白血症引起血管炎并导致周围神经病变的效果较好[65- 66],治疗3个月后有效率可达90%[67]。法国一项前瞻性队列研究中,6例原发干燥综合征合并肾间质损害的患者接受利妥昔单抗治疗,5例达到完全缓解[68]。而对于仅表现为低钾血症或代谢性酸中毒的患者,通常采用补钾和纠正酸中毒等对症治疗方案。
综上,干燥综合征是以自身免疫性上皮炎为病理基础的疾病,肾脏是重要受累靶器官之一,肾小管间质病变较为多见,肾小球病变也并不少见。部分起病隐匿,必要时需行肾小管功能实验等检查发现早期患者。目前影响肾脏预后的因素还不明确,尚需更大病例数、随访时间更长的临床研究。此外,还需要进一步探究肾脏局部免疫反应激活与上皮细胞损伤间的病理机制,寻找其中发挥关键性作用的分子或炎症介质,为靶向治疗提供潜在的干预位点。
[1] 中华医学会风湿病学分会. 干燥综合征诊治指南(草案)[J]. 中华风湿病学杂志,2003,7(7):446- 448.
[2] Moutsopoulos HM. Sjögren’s syndrome:autoimmune epithelitis[J]. Clin Immunol Immunopathol,1994,72(2):162- 165.
[3] Francois H,Mariette X. Renal involvement in primary Sjögren syndrome[J]. Nat Rev Nephrol,2016,12(2):82- 93.DOI:10. 1038/nrneph. 2015. 174.
[4] Evans R,Zdebik A,Ciurtin C,et al. Renal involvement in primary Sjögren’s syndrome[J]. Rheumatology(Oxford),2015,54(9):1541- 1548.
[5] Goules A,Masouridi S,Tzioufas AG,et al. Clinically significant and biopsy-documented renal involvement in primary Sjögren syndrome[J]. Medicine(Baltimore),2000,79(4):241- 249.
[6] Aasarod K,Haga HJ,Berg KJ,et al. Renal involvement in primary Sjögren’s syndrome[J]. Qjm,2000,93(5):297- 304.




[7] Skopouli FN,Dafni U,Ioannidis JP,et al. Clinical evolution,and morbidity and mortality of primary Sjögren’s syndrome[J]. Semin Arthritis Rheum,2000,29(5):296- 304.
[8] Bossini N,Savoldi S,Franceschini F,et al. Clinical and morphological features of kidney involvement in primary Sjögren’s syndrome[J]. Nephrol Dial Transplant,2001,16(12):2328- 2336.
[9] Garcia-Carrasco M,Ramos-Casals M,Rosas J,et al. Primary Sjögren syndrome:clinical and immunologic disease patterns in a cohort of 400 patients[J]. Medicine(Baltimore),2002,81(4):270- 280.
[10] Ramos-Casals M,Solans R,Rosas J,et al. Primary Sjögren syndrome in Spain:clinical and immunologic expression in 1010 patients[J]. Medicine(Baltimore),2008,87(4):210- 219. DOI:10. 1097/MD. 0b013e318181e6af.
[11] Maripuri S,Grande JP,Osborn TG,et al. Renal involvement in primary Sjögren’s syndrome:a clinicopathologic study[J]. Clin J Am Soc Nephrol,2009,4(9):1423- 1431. DOI:10. 2215/CJN. 00980209.
[12] Lin DF,Yan SM,Zhao Y,et al. Clinical and prognostic characteristics of 573 cases of primary Sjögren’s syndrome[J]. Chin Med J(Engl),2010,123(22):3252- 3257.
[13] Seror R,Ravaud P,Bowman SJ,et al. EULAR Sjögren’s syndrome disease activity index:development of a consensus systemic disease activity index for primary Sjögren’s syndrome[J]. Ann Rheum Dis,2010,69(6):1103- 1109. DOI:10. 1136/ard. 2009. 110619.
[14] Malladi AS,Sack KE,Shiboski SC,et al. Primary Sjögren’s syndrome as a systemic disease:a study of participants enrolled in an international Sjögren’s syndrome registry[J]. Arthritis Care Res(Hoboken),2012,64(6):911- 918. DOI:10. 1002/acr. 21610.
[15] Goules AV,Tatouli IP,Moutsopoulos HM,et al. Clinically significant renal involvement in primary Sjögren’s syndrome:clinical presentation and outcome[J]. Arthritis Rheum,2013,65(11):2945- 2953. DOI:10. 1002/art. 38100.
[16] Gottenberg JE,Seror R,Miceli-Richard C,et al. Serum levels of beta2-microglobulin and free light chains of immunoglobulins are associated with systemic disease activity in primary Sjögren’s syndrome. Data at enrollment in the prospective ASSESS cohort[J]. PLoS One,2013,8(5):e59868. DOI:10. 1371/journal. pone. 0059868.
[17] Ramos-Casals M,Brito-Zeron P,Solans R,et al. Systemic involvement in primary Sjögren’s syndrome evaluated by the EULAR-SS disease activity index:analysis of 921 Spanish patients(GEAS-SS Registry)[J]. Rheumatology(Oxford),2014,53(2):321- 331. DOI:10. 1093/rheumatology/ket349.
[18] Baldini C,Pepe P,Quartuccio L,et al. Primary Sjögren’s syndrome as a multi-organ disease:impact of the serological profile on the clinical presentation of the disease in a large cohort of Italian patients[J]. Rheumatology(Oxford),2014,53(5):839- 844. DOI:10. 1093/rheumatology/ket427.
[19] Duffles Amarante GB,Zotin MC,Rocha E,et al. Renal tubular dysfunction in patients with primary Sjögren syndrome[J]. Clin Nephrol,2014,81(3):185-191. DOI:10. 5414/CN108142.
[20] Both T,Hoorn EJ,Zietse R,et al. Prevalence of distal renal tubular acidosis in primary Sjögren’s syndrome[J]. Rheumatology(Oxford),2015,54(5):933- 939. DOI:10. 1093/rheumatology/keu401.
[21] Yang H,Wang J,Wen Y,et al. Renal involvement in primary Sjögren’s syndrome:a retrospective strudy of 103 biopsy-proven cases from a single center in China[J]. Int J Rheum Dis,2018,21(1):223- 229. DOI:10. 1111/1756- 185X. 13182.
[22] Vitali C,Bombardieri S,Moutsopoulos HM,et al. Preliminary criteria for the classification of Sjögren’s syndrome. Results of a prospective concerted action supported by the European Community[J]. Arthritis Rheum,1993,36(3):340- 347.
[23] Vitali C,Bombardieri S,Jonsson R,et al. Classification criteria for Sjögren’s syndrome:a revised version of the European criteria proposed by the American-European Consensus Group[J]. Ann Rheum Dis,2002,61(6):554- 558.
[24] Wang J,Wen Y,Zhou M,et al. Ectopic germinal center and megalin defect in primary Sjögren syndrome with renal Fanconi syndrome[J]. Arthritis Res Ther,2017,19(1):120. DOI:10. 1186/s13075- 017- 1317- x.
[25] Ren H,Wang WM,Chen XN,et al. Renal involvement and followup of 130 patients with primary Sjögren’s syndrome[J]. J Rheumatol,2008,35(2):278- 284.
[26] Hisahara S,Ohkoshi N,Shoji S,et al. Polymyalgia rheumatica in a patient with acute tubulointerstitial nephritis due to Sjögren’s syndrome[J]. J Intern Med,1998,244(1):83- 86.
[27] Hu DC,Cathro HP,Okusa MD. Polymorphoneutrophilic infiltration in acute interstitial nephritis of Sjögren syndrome[J]. Am J Med Sci,2004,327(5):278- 280.
[28] Ram R,Swarnalatha G,Dakshinamurty KV. Renal tubular acidosis in Sjögren’s syndrome:a case series[J]. Am J Nephrol,2014,40(2):123- 130. DOI:10. 1159/000365199.
[29] Laing CM,Unwin RJ. Renal tubular acidosis[J]. J Nephrol,2006,19(Suppl 9):S46- S52.
[30] Pasternak RC,Thibault GE,Savoia M,et al. Chest pain with angiographically insignificant coronary arterial obstruction. Clinical presentation and long-term follow-up[J]. Am J Med,1980,68(6):813- 817.
[31] Wrong O,Davies HE. The excretion of acid in renal disease[J]. Q J Med,1959,28(110):259- 313.
[32] Walsh SB,Shirley DG,Wrong OM,et al. Urinary acidification assessed by simultaneous furosemide and fludrocortisone treatment:an alternative to ammonium chloride[J]. Kidney Int,2007,71(12):1310- 1316. DOI:10. 1038/sj. ki. 5002220.
[33] Shioji R,Furuyama T,Onodera S,et al. Sjögren’s syndrome and renal tubular acidosis[J]. Am J Med,1970,48(4):456- 463.
[34] Yilmaz H,Kaya M,Ozbek M,et al. Hypokalemic periodic paralysis in Sjögren’s syndrome secondary to distal renal tubular acidosis[J]. Rheumatol Int,2013,33(7):1879- 1882. DOI:10. 1007/s00296- 011- 2322-z.
[35] Khandelwal D,Bhattacharya S,Gadodia A,et al. Metabolic bone disease as a presenting manifestation of primary Sjögren’s syndrome:three cases and review of literature[J]. Indian J Endocrinol Metab,2011,15(4):341- 345. DOI:10. 4103/2230- 8210. 85599.
[36] Pertovaara M,Korpela M,Kouri T,et al. The occurrence of renal involvement in primary Sjögren’s syndrome:a study of 78 patients[J]. Rheumatology(Oxford),1999,38(11):1113- 1120.
[37] Schwarz C,Barisani T,Bauer E,et al. A woman with red eyes and hypokalemia:a case of acquired Gitelman syndrome[J]. Wien Klin Wochenschr,2006,118(7- 8):239- 242.
[38] Kim YK,Song HC,Kim WY,et al. Acquired Gitelman syndrome in a patient with primary Sjögren syndrome[J]. Am J Kidney Dis,2008,52(6):1163- 1167. DOI:10. 1053/j. ajkd. 2008. 07. 025.
[39] Hinschberger O,Martzolff L,Ioannou G,et al. Acquired Gitelman syndrome associated with Sjögren’s syndrome and scleroderma[J]. Rev Med Interne,2011,32(8):e96- e98. DOI:10. 1016/j. revmed. 2010. 08. 017.
[40] Kulkarni M,Kadri P,Pinto R. A case of acquired Gitelman syndrome presenting as hypokalemic paralysis[J]. Indian J Nephrol,2015,25(4):246- 247. DOI:10. 4103/0971- 4065. 146031.
[41] Bockenhauer D,Bichet DG. Pathophysiology,diagnosis and management of nephrogenic diabetes insipidus[J]. Nat Rev Nephrol,2015,11(10):576- 588. DOI:10. 1038/nrneph. 2015. 89.
[42] Viergever PP,Swaak TJ. Renal tubular dysfunction in primary Sjögren’s syndrome:clinical studies in 27 patients[J]. Clin Rheumatol,1991,10(1):23- 27.
[43] Fry AC,Karet FE. Inherited renal acidoses[J]. Physiology(Bethesda),2007,22(3):202- 211. DOI:10. 1152/physiol. 00044. 2006.
[44] Mcsherry E,Sebastian A,Morris RC Jr. Renal tubular acidosis in infants:the several kinds,including bicarbonate-wasting,classic renal tubular acidosis[J]. J Clin Invest,1972,51(3):499- 514.
[45] Haque SK,Ariceta G,Batlle D. Proximal renal tubular acidosis:a not so rare disorder of multiple etiologies[J]. Nephrol Dial Transplant,2012,27(12):4273- 4287. DOI:10. 1093/ndt/gfs493.
[46] Shearn MA,Tu WH. Nephrogenic diabetic insipidus and other defects of renal tubular function in Sjoergren’s syndrome[J]. Am J Med,1965,39(2):312- 318.
[47] Walker BR,Alexander F,Tannenbaum PJ. Fanconi syndrome with renal tubular acidosis and light chain proteinuria[J]. Nephron,1971,8(1):103- 107.
[48] Kamm DE,Fischer MS. Proximal renal tubular acidosis and the Fanconi syndrome in a patient with hypergammaglobulinemia[J]. Nephron,1972,9(4):208- 219.
[49] Matsumura R,Kondo Y,Sugiyama T,et al. Immunohistochemical identification of infiltrating mononuclear cells in tubulointerstitial nephritis associated with Sjögren’s syndrome[J]. Clin Nephrol,1988,30(6):335- 340.
[50] Ardiles L,Ramirez P,Calderon S,et al. Life-threatening hypokalemic paralysis and hypophosphatemic myopathy as initial presentations of primary Sjögren’s syndrome[J]. J Clin Rheumatol,2001,7(6):410- 411.
[51] Bridoux F,Kyndt X,Abou-Ayache R,et al. Proximal tubular dysfunction in primary Sjögren’s syndrome:a clinicopathological study of 2 cases[J]. Clin Nephrol,2004,61(6):434- 439.
[52] Kobayashi T,Muto S,Nemoto J,et al. Fanconi’s syndrome and distal(type 1) renal tubular acidosis in a patient with primary Sjögren’s syndrome with monoclonal gammopathy of undetermined significance[J]. Clin Nephrol,2006,65(6):427- 432.
[53] Yang YS,Peng CH,Sia SK,et al. Acquired hypophosphatemia osteomalacia associated with Fanconi’s syndrome in Sjögren’s syndrome[J]. Rheumatol Int,2007,27(6):593- 597. DOI:10. 1007/s00296- 006- 0257- 6.
[54] Nakamura H,Kita J,Kawakami A,et al. Multiple bone fracture due to Fanconi’s syndrome in primary Sjögren’s syndrome complicated with organizing pneumonia[J]. Rheumatol Int,2009,30(2):265- 267. DOI:10. 1007/s00296- 009- 0924- 5.
[55] Wang CC,Shiang JC,Huang WT,et al. Hypokalemic paralysis as primary presentation of Fanconi syndrome associated with Sjögren syndrome[J]. J Clin Rheumatol,2010,16(4):178- 180. DOI:10. 1097/RHU. 0b013e3181df903f.
[56] Ram R,Swarnalatha G,Ashok KK,et al. Fanconi syndrome following honeybee stings[J]. Int Urol Nephrol,2012,44(1):315- 318. DOI:10. 1007/s11255- 010- 9855-z.
[57] Celik G,Ozturk E,Ipekci SH,et al. An uncommon presentation of Sjögren’s syndrome and brucellosis[J]. Transfus Apher Sci,2014,51(1):77- 80. DOI:10. 1016/j. transci. 2014. 03. 011.
[58] Shi M,Chen L. Sjögren’s syndrome complicated with Fanconi syndrome and Hashimoto’s thyroiditis:case report and literature review[J]. J Int Med Res,2016,44(3):753- 759. DOI:10. 1177/0300060515593767.
[59] Saeki T,Nakajima A,Ito T,et al. Tubulointerstitial nephritis and Fanconi syndrome in a patient with primary Sjögren’s syndrome accompanied by antimitochondrial antibodies:a case report and review of the literature[J]. Mod Rheumatol,2016,4:1- 4. DOI:10. 3109/14397595. 2016. 1174422.
[60] Sirac C,Bridoux F,Essig M,et al. Toward understanding renal Fanconi syndrome:step by step advances through experimental models[J]. Contrib Nephrol,2011,169:247- 261. DOI:10. 1159/000313962.
[61] Kidder D,Rutherford E,Kipgen D,et al. Kidney biopsy findings in primary Sjögren syndrome[J]. Nephrol Dial Transplant,2015,30(8):1363-1369. DOI:10.1093/ndt/gfv042.
[62] Sethi S,Fervenza FC. Membranoproliferative glomerulonephritis-a new look at an old entity[J]. N Engl J Med,2012,366(12):1119- 1131. DOI:10. 1056/NEJMra1108178.
[63] D’amico G,Fornasieri A. Cryoglobulinemic glomerulonephritis:a membranoproliferative glomerulonephritis induced by hepatitis C virus[J]. Am J Kidney Dis,1995,25(3):361- 369.
[64] Guellec D,Cornec-Le Gall E,Groh M,et al. ANCA-associated vasculitis in patients with primary Sjögren’s syndrome:detailed analysis of 7 new cases and systematic literature review[J]. Autoimmun Rev,2015,14(8):742- 750. DOI:10. 1016/j. autrev. 2015. 04. 009.
[65] Evans RD,Laing CM,Ciurtin C,et al. Tubulointerstitial nephritis in primary Sjögren syndrome:clinical manifestations and response to treatment[J]. BMC Musculoskelet Disord,2016,17(1):2. DOI:10. 1186/s12891- 015- 0858-x.
[66] Meijer JM,Meiners PM,Vissink A,et al. Effectiveness of rituximab treatment in primary Sjögren’s syndrome:a randomized,double-blind,placebo-controlled trial[J]. Arthritis Rheum,2010,62(4):960- 968. DOI:10. 1002/art. 27314.
[67] Mekinian A,Ravaud P,Hatron PY,et al. Efficacy of rituximab in primary Sjögren’s syndrome with peripheral nervous system involvement:results from the AIR registry[J]. Ann Rheum Dis,2012,71(1):84-87. DOI:10. 1136/annrheumdis- 2011- 200086.
[68] Terrier B,Launay D,Kaplanski G,et al. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis:data from the French autoimmunity and rituximab registry[J]. Arthritis Care Res(Hoboken),2010,62(12):1787- 1795. DOI:10. 1002/acr. 20318.
